Abstract
We present the first case (male, 35 years old) of a mammary analogue secretory carcinoma occurring in a submandibular gland and document findings on fine needle aspiration cytology. On histology, the tumor displayed characteristic features: circumscribed nodules composed of bland, pink to light red neoplastic cells with low proliferative/mitotic activity arranged in tubular, vaguely cribriform, and microcystic structures containing Periodic acid Schiff-positive, diastase-resistant secretory material. Immunohistochemistry showed strong and diffuse positivity for cytokeratin 7, S100 protein, and vimentin, as well as moderate to strong immunoreactivity for c-kit in the majority of tumor cells. A rearrangement of the ETV6 gene on fluorescence in situ hybridization was documented. The patient underwent an ipsilateral selective (levels I–IV) neck dissection which showed metastasis in 3 out of 36 lymph nodes (levels 1–3). Adjuvant radiotherapy was administered. No local recurrence or metastatic disease has been detected during a follow up period of 28 months.
Keywords: Salivary gland, Carcinoma, Submandibular gland, Secretory carcinoma, Fine needle aspiration cytology
Introduction
Recently, a new entity in neoplastic salivary gland pathology has been described. In a series comprising 16 cases, Skalova et al. [1] characterized the mammary analogue secretory carcinoma of salivary glands (MASC), a neoplasm which shows identical histological as well as molecular features to its counterpart in the breast. In the seminal paper, most tumors (13/16 cases) arose in the parotid gland and three cases originated in the minor salivary glands. In this paper, we present the first reported case of a MASC arising in a submandibular gland and present for the first time the cytological features of this neoplasm.
Case Report
A previously healthy 35-year-old male presented with a painless lump in the left submandibular region. Based on the fine needle aspiration cytological (FNAC) findings, a possible pleomorphic adenoma with oncocytic features was suggested, but no definitive diagnosis was given. A subsequent resection of the left submandibular gland was performed. Thereafter, the patient underwent an ipsilateral selective (levels I–IV) neck dissection which showed metastasis in 3 out of 36 lymph nodes (levels 1–3) with extranodal extension in a level 1 lymph node. The largest metastasis was 4 mm in size. Adjuvant radiotherapy was administered. No local recurrence or metastatic disease has been detected during a follow up of 28 months.
Materials and Methods
The specimen was fixed in formalin, embedded in paraffin and 4 μm thin sections were cut and stained with hematoxylin and eosin and Periodic acid Schiff (PAS) with diastase digestion. Immunohistochemical studies were performed, according to the manufacturer’s instructions, using commercially available antibodies, including: cytokeratin (CK)7, p63, gross cystic disease fluid protein (GCDFP) 15, S100 protein, vimentin, c-kit, and Ki-67.
A molecular genetic study (RT-PCR) and fluorescence in situ hybridization (FISH) was performed. The RecoverAll Total Nucleic Acid Isolation Kit (Ambion, Austin, Texas) was used for RNA extraction and cDNA was synthesized using the Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturers’ protocols. For testing of the RNA quality, amplification of 105-bp and 133-bp products of the β2-microglobulin gene and a 247-bp product of the phosphoglycerkinase (PGK) gene was performed. For the FISH study, LSI ETV6 (TEL) (12p13) Dual Color, Break Apart Rearrangement Probe (VYSIS/Abbott, Abbot Park, IL) was used. Scoring was done counting the number of fluorescent signals in 100 randomly selected non-overlapping tumor cell nuclei.
Results
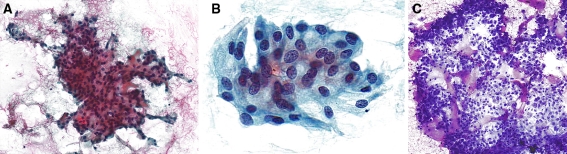
On FNA, the smears showed low to moderate cellularity featuring loosely cohesive small sheets and clusters of neoplastic cells with low-grade nuclear features forming vague acinar structures (Fig. 1a). The nuclei had mostly smooth contours with only focal nuclear membrane irregularities and finely dispersed chromatin with occasional minute nucleoli. The cytoplasm was moderately abundant with a slightly bubbly appearance and the cell borders were indistinct (Fig. 1b). Round to irregular elongated secretory (“colloid-like”) material with variable staining intensity was intimately admixed with the tumor cells on the Giemsa-stained smear (Fig. 1c).
Fig. 1.
a FNA smear shows small loosely cohesive sheets of neoplastic cells forming vague acinar structures in the periphery (a; Papanicolau). On high power, the tumor cells display smooth contours with only focal nuclear membrane irregularities and finely dispersed chromatin with occasional minute nucleoli and vaguely bubbly cytoplasm (b; Papanicolau). Round to irregular elongated secretory (“colloid”-like) material with a variable staining intensity is intimately admixed with the tumor cells (c; Giemsa)
The gross resected specimen was a submandibular gland (4 × 3.5 × 3 cm) with a solid white–grey multinodular, well-delineated tumor, measuring 2.5 cm in maximum dimension.
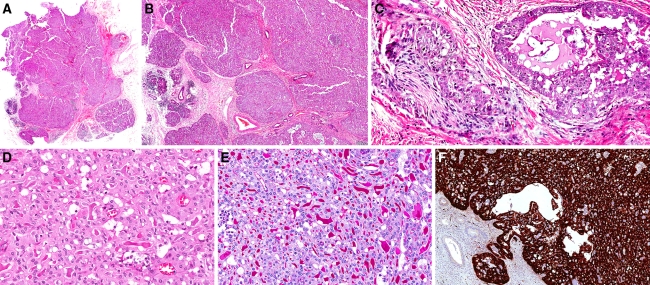
Low power histological examination revealed a multinodular, unencapsulated tumor with an “eosinophilic” appearance (Fig. 2a). The different tumor nodules were of varying sizes and were relatively well-circumscribed and divided by hypocellular connective tissue septae with a mild sprinkling of lymphocytes. Definitive infiltrative growth into the surrounding salivary gland parenchyma in several areas and focal perineural growth were identified (Figs. 2b, c). The tumor nodules displayed a rather uniform appearance and were composed of medium-sized neoplastic cells arranged in tubular, microcystic and acinar structures imparting a vague cribriform appearance. The nuclei were round to ovoid, rather isomorphic and had fine, pale chromatin and inconspicuous to small nucleoli. The cytoplasm was light red to pink with a vague granular and focally slightly vacuolated appearance (Fig. 2d). The lumina contained faint basophilic and/or variably distinct/thick eosinophilic secretory material which was PAS-positive and diastase-resistant (Fig. 2e). Occasional mitotic figures were discerned but the overall mitotic activity was less than 1/10 high power fields (1 hpf = 0.238 mm²). A few PAS-positive, diastase-resistant cytoplasmic granules were identified in occasional neoplastic cells, but no zymogen granules were identified on the hematoxylin and eosin-stained sections. Scattered calcifications were noted, but no necrosis was present.
Fig. 2.
A multinodular, unencapsulated tumor with an “eosinophilic” appearance (a). The tumor is composed of nodules of varying sizes featuring infiltrative growth into the surrounding salivary gland parenchyma (b; left) and focal perineural growth (c; left). The neoplastic cells are medium sized with round to ovoid isomorphic nuclei harbouring fine—pale chromatin and inconspicuous to small nucleoli (d). The secretory material was faintly basophilic or variably eosinophilic, and PAS-positive, diastase resistant (e). The tumor is strongly positive for S100-protein on immunohistochemistry (f)
The immunohistochemical work-up showed that the neoplastic cells were strongly positive for CK7, S100 protein (Fig. 2f), and vimentin. No immunoreactivity for p63 or GCDFP15 was discerned (data not shown). Moderate to strong, mainly membranous immunoreactivity for c-kit was found in the majority of tumor cells (data not shown). The proliferative activity was low with less than 5% of cells showing nuclear immunoreactivity for Ki-67 (data not shown).
The FISH study with ETV6 break apart probe revealed split signals in 47% of nuclei (Fig. 3). In the RT-PCR study, we failed to detect the 110 bp long t(12; 15) (ETV6-NTRK3) fusion transcript. (The assay was repeated two times in duplicates.) The amplicons (105 and 133 bp) from the control (β2-microglobulin) gene were positive, whereas the amplicon (247 bp) from the other control (PGK) gene was negative.
Fig. 3.
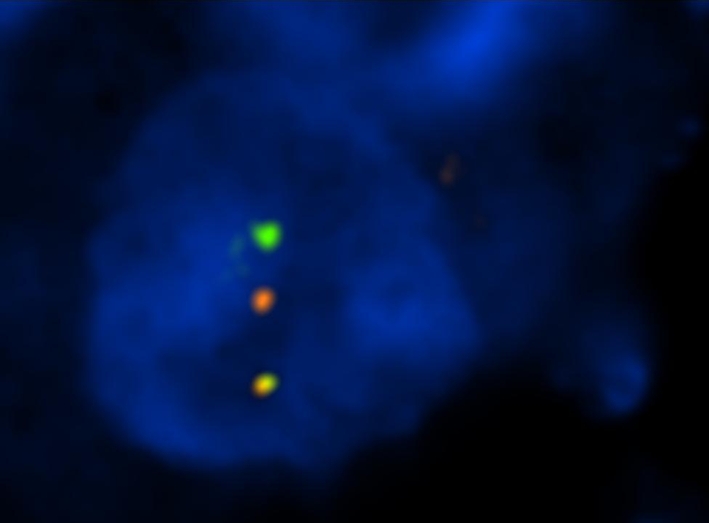
FISH using LSI ETV6 (TEL) (12p13) Dual Color, Break Apart Rearrangement Probe shows green and red split signals indicating break of the ETV6 gene
Discussion
Secretory carcinoma (SC) of the breast is a rare tumor with a characteristic histologic appearance that was first described in a series of young patients in 1966 by McDivitt and Stewart. SC has been shown to harbor a specific genetic alteration, t(12; 15) (p13; q25) which leads to the fusion of the ETV6 gene on chromosome 12 with the NTRK3 gene on chromosome 15 encoding for a chimeric tyrosine kinase. Recently, a series of a new salivary gland carcinoma with identical histological features and which harbors the aforementioned genetic change, was published by Skalova et al. [1]. The authors chose to christen this new salivary gland neoplasm “mammary analogue secretory carcinoma of salivary glands” (MASC). This seminal paper on MASC comprised 16 adult patients (both male and female) with an age range of 21–75 years. The salient histological features (circumscribed nodules, bland pink to light red neoplastic cells with low proliferative/mitotic activity, in tubular, vaguely cribriform and microcystic arrangements with secretory material) and immunohistochemical findings, as described by the authors, are virtually identical to those displayed by the tumor we present. All but three tumors appeared in the parotid gland (the other 3 occurred in minor salivary glands—buccal mucosa, upper lip, and palate). Hence, the tumor presented herein is the first documented case of MASC occurring in the submandibular gland. Skalova et al. performed an exhaustive immunohistochemical characterization of MASC; we selectively reduplicated a few of the salient immunohistochemical markers for our study. Highly characteristic immunohistochemical features of MASC include strong positivity for cytokeratin 7, S100 protein, and vimentin (all cases positive in the study by Skalova et al.) and likewise, our tumor displayed distinct immunoreactivity for these markers. Similarly, we found no positivity for p63. Immunoreactivity for GCDFP-15 was seen in 8/11 examined cases, but our tumor was negative.
We performed both FISH and RT-PCR for the molecular genetic analysis, similar to Skalova et al. In Skalova’s study, all but one examined cases (13/14) were positive for the t(12;15) (ETV6-NTRK3) fusion transcript and all examined cases (11/11) were positive for the ETV6 gene rearrangement on FISH. The RT-PCR negative case (no. 8) was not included in the FISH study. Using the same methodology and protocols, our case was positive for the ETV6 gene rearrangement on FISH, but the RT-PCR was (repeatedly) negative. RNA degradation is a possible but unlikely explanation for this observed negative RT-PCR result, since both amplicons from the control β2-microglobulin gene were positive. Since the sections taken for the FISH and RT-PCR studies were from the same blocks, sampling is highly unlikely to account for the negative RT-PCR result either. At least three possible explanations are: (1) a different fusion partner to the ETV6 gene or (2) a break in the NTRK3 gene not recognized by the primers or (3) a deletion or insertion in the gene segments in question during translocation. We have not investigated this further. However, reportedly, the frequency of detection of the ETV6-NTRK3 fusion in SC of the breast is not 100%. In four studies comprising 29 cases of SC of the breast [2–5], 27 contained this genetic change. Moreover, in two secretory carcinomas of the skin (with identical histological features), the ETV6-NTRK3 fusion transcript was not identified [6, 7].
The cytological features of mammary SC (but not MASC) have been characterized in previous publications comprising a few small series (up to five cases) and a number of case reports [8–17]. The salient FNAC features of SC include “grapelike” clusters and slightly discohesive neoplastic cells featuring uniform, minimally atypical nuclei and bubbly to variably vacuolated cytoplasm, including the presence of signet ring-like cells in some cases. The cytological findings of the case presented herein conform to the previous descriptions. Although the cytological characteristics of the neoplastic cells in MASC are not pathognomonic, the presence of secretory material in conjunction with the cytomorphology would invoke this differential diagnostic possibility, distinguishing MASC from other benign/low-grade malignant salivary gland neoplasms, especially acinic cell carcinoma. Moreover, the secretory material in MASC differs from the metachromatric (fibrillary and/or vascular) stroma or sharply demarcated, hyalinized (stromal) cords and globules, which may be seen in pleomorphic adenoma, basal cell adenoma/basal cell adenocarcinoma, polymorphous low-grade adenocarcinoma, epithelial-myoepithelial carcinoma and adenoid cystic carcinoma.
That certain mammary gland neoplasms have morphological/biological counterparts in the salivary gland is not surprising given the shared embryological (ectodermal) origin and histological (exocrine) organization of the mammary and salivary glands. Such examples include adenoid cystic carcinoma, mucoepidermoid carcinoma, acinic cell carcinoma and pleomorphic adenoma. Of note is that even though salivary-type carcinomas may appear very similar histologically to their mammary counterparts, their clinical behaviors may differ. For example, adenoid cystic carcinomas of the salivary glands are tumors that carry a significant long-term (15 years) mortality risk, whereas the mammary counterpart displays a very low-grade biological behaviour. Whether this is due to inherent biological differences or is a site-dependent phenomenon (increased risk of intractable disease due to perineural spread in the complex anatomy of the head and neck region), is currently not known. Furthermore, breast tumors such as micropapillary and cribriform carcinoma, when occurring in the salivary glands are classified within the spectrum of salivary duct carcinoma. Other shared entities are mucinous (colloid) carcinoma, cystadenocarcinoma, and sebaceous carcinoma. In addition, the mammary tubular adenoma and adenomyoepithelioma share significant histomorphological features with both epithelial-myoepithelial carcinoma and the tubular variant of basal cell adenoma of salivary glands. However, polymorphous low-grade adenocarcinomas have not been definitively documented in the breast and only very rare cases of mammary oncocytic carcinoma have been reported.
The degree of malignancy in MASC is difficult to firmly establish at this stage. In the series by Skalova et al., follow up was available for 15 patients with a range of 3 months to 10 years. Two patients died of disease and three experienced recurrences. Metastases and local recurrences appeared to be related to the dimension of the tumours. After adjuvant radiotherapy, the patient presented herein has been free from disease for 28 months. However, as documented in the seminal series, the risk of late recurrences and metastasis, especially when the primary tumor is large, is not insignificant.
The identification of the ETV6-NTRK3 gene fusion allows for a targeted therapeutics-oriented tumor classification approach and paves the way for future studies into possible targeted therapeutics for this group of tumors. NTRK3, the neurotrophic tyrosine kinase, receptor, type 3, belongs to the NTRK (Trk) family of neurotrophin receptors which are central to normal development, particularly of the nervous system [18]. Various members have also been implicated in oncogenesis, such as NTRK1 (TrkA) and NTRK2 (TrkB) in neuroblastomas [19]. Trk kinase inhibitors have been explored as potential anti-cancer therapeutics with apparent preclinical and clinical efficacy; as previously mooted by Uren and Toretsky [20], future studies investigating the utility of such targeted agents in tumors with the ETV6-NTRK3 fusion are warranted.
In summary, we herein present the cytological features of a mammary analogue secretory carcinoma occuring in the submandibular gland. The histological features, immunohistochemical findings and genetic change are highly characteristic for this neoplasm which appears to have a more aggressive clinical behavior than its mammary counterpart.
Acknowledgments
We thank Tomas Vanecek, PhD, for skillful assistance with the molecular genetic study.
References
- 1.Skalova A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34:599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 2.Lae M, Freneaux P, Sastre-Garau X, et al. Secretory breast carcinomas with ETV6-NTRK3 fusion gene belong to the basal-like carcinoma spectrum. Mod Pathol. 2009;22:291–298. doi: 10.1038/modpathol.2008.184. [DOI] [PubMed] [Google Scholar]
- 3.Makretsov N, He M, Hayes M, et al. A fluorescence in situ hybridization study of ETV6-NTRK3 fusion gene in secretory breast carcinoma. Genes Chromosomes Cancer. 2004;40:152–157. doi: 10.1002/gcc.20028. [DOI] [PubMed] [Google Scholar]
- 4.Reis-Filho JS, Natrajan R, Vatcheva R, et al. Is acinic cell carcinoma a variant of secretory carcinoma? A FISH study using ETV6’split apart’ probes. Histopathology. 2008;52:840–846. doi: 10.1111/j.1365-2559.2008.03046.x. [DOI] [PubMed] [Google Scholar]
- 5.Tognon C, Knezevich SR, Huntsman D, et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell. 2002;2:367–376. doi: 10.1016/S1535-6108(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 6.Kazakov DV, Hantschke M, Vanecek T, et al. Mammary-type secretory carcinoma of the skin. Am J Surg Pathol. 2010;34:1226–7; author reply 1228. [DOI] [PubMed]
- 7.Brandt SM, Swistel AJ, Rosen PP. Secretory carcinoma in the axilla: probable origin from axillary skin appendage glands in a young girl. Am J Surg Pathol. 2009;33:950–953. doi: 10.1097/PAS.0b013e31819c2628. [DOI] [PubMed] [Google Scholar]
- 8.Gupta RK, Kenwright D, Naran S, et al. Fine needle aspiration cytodiagnosis of secretory carcinoma of the breast. Cytopathology. 2000;11:496–502. doi: 10.1046/j.1365-2303.2000.00283.x. [DOI] [PubMed] [Google Scholar]
- 9.Shinagawa T, Tadokoro M, Kitamura H, et al. Secretory carcinoma of the breast. Correlation of aspiration cytology and histology. Acta Cytol. 1994;38:909–914. [PubMed] [Google Scholar]
- 10.Shinagawa T, Tadokoro M, Takeuchi E, et al. Aspiration biopsy cytology of secretory carcinoma of the breast. A case report. Acta Cytol. 1992;36:189–193. [PubMed] [Google Scholar]
- 11.Nonomura A, Kimura A, Mizukami Y, et al. Secretory carcinoma of the breast associated with juvenile papillomatosis in a 12-year-old girl. A case report. Acta Cytol. 1995;39:569–576. [PubMed] [Google Scholar]
- 12.Vesoulis Z, Kashkari S. Fine needle aspiration of secretory breast carcinoma resembling lactational changes. A case report. Acta Cytol. 1998;42:1032–1036. doi: 10.1159/000331954. [DOI] [PubMed] [Google Scholar]
- 13.Jena M, Shariff S. Cytodiagnosis of secretory carcinoma of the breast: a report on two cases. Diagn Cytopathol. 2010;38:921–924. doi: 10.1002/dc.21342. [DOI] [PubMed] [Google Scholar]
- 14.Mardi K, Sharma J. A rare case of secretory breast carcinoma in an elderly woman: correlation of aspiration cytology and histology. Indian J Pathol Microbiol. 2007;50:865–867. [PubMed] [Google Scholar]
- 15.Oh YH, Jang KS, Song YS, et al. Secretory carcinoma of the breast diagnosed by fine needle aspiration. Acta Cytol. 2005;49:343–344. doi: 10.1159/000326162. [DOI] [PubMed] [Google Scholar]
- 16.Alenda C, Aranda FI, Segui FJ, et al. Secretory carcinoma of the male breast: correlation of aspiration cytology and pathology. Diagn Cytopathol. 2005;32:47–50. doi: 10.1002/dc.20157. [DOI] [PubMed] [Google Scholar]
- 17.Buchino JJ, Moore GD, Bond SJ. Secretory carcinoma in a 9-year-old girl. Diagn Cytopathol. 2004;31:430–431. doi: 10.1002/dc.20173. [DOI] [PubMed] [Google Scholar]
- 18.Klein R, Smeyne RJ, Wurst W, et al. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell. 1993;75:113–122. [PubMed] [Google Scholar]
- 19.Nakagawara A, Azar CG, Scavarda NJ, et al. Expression and function of TRK-B and BDNF in human neuroblastomas. Mol Cell Biol. 1994;14:759–767. doi: 10.1128/mcb.14.1.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uren A, Toretsky JA. Pediatric malignancies provide unique cancer therapy targets. Curr Opin Pediatr. 2005;17:14–19. doi: 10.1097/01.mop.0000147904.84978.ae. [DOI] [PubMed] [Google Scholar]