Abstract
We report a case of crystal storing histiocytosis (CSH) of the upper lip and cheek in a 51-year-old woman and review the clinicopathologic features of 80 cases in the literature. These occurred in 41 men and 39 women with a respective mean age of 59 and 61 years (range 17–81 years). Forty-six patients (58%) had localized CSH, and, of these, 16 (35%) occurred in the head and neck, with the most common site being the eye/orbit. The remaining 34 patients (42%) had generalized CSH primarily involving bone marrow, liver, lymph nodes, spleen and/or kidney. Regardless of whether the CSH was localized or generalized, the vast majority of patients (90%) had an underlying lymphoproliferative or plasma cell disorder, especially multiple myeloma, lymphoplasmacytic lymphoma, or monoclonal gammopathy of undetermined significance. In 7 cases (8.8%), the CSH was associated with a variety of benign disorders, often with an inflammatory background, and no evidence of a clonal lymphoproliferative or plasma cell disorder. Treatment and prognosis varied according to the underlying disease. A classification of CSH based on etiology and/or associated disease and chemical composition of the crystal is proposed, rare non-immunoglobulin variants of CSH are discussed, and a differential diagnosis of other potentially confusing lesions is provided.
Keywords: Crystal-storing histiocytosis, Intracellular crystals, Immunoglobulin crystals, Histiocytes
Introduction
Crystal-storing histiocytosis (CSH), a rare condition in which crystalline material accumulates in the cytoplasm of histiocytes, is typically associated with disorders that express monoclonal immunoglobulins, such as multiple myeloma (MM), lymphoplasmacytic lymphoma (LPL), and monoclonal gammopathy of undetermined significance (MGUS) [1, 2]. With few exceptions, the crystalline material within the histiocytes is of kappa light chain origin without a consistent affiliation with any specific heavy chain [2, 3]. More recently, other variants of CSH have also been described in which the crystalline material is not an immunoglobulin. Among these include clofazimine-induced CSH, Charcot-Leyden crystal-associated CSH, and CSH associated with hereditary cystinosis (Table 1) [4–6].
Table 1.
Proposed classification of CSH
| According to etiology and/or associated disease | According to crystal |
|---|---|
| 1. Hematopoietic | 1. Immunoglobulin |
| A. Multiple myeloma | A. Type |
| B. Extramedullary plasmacytoma | (1) Heavy chain |
| C. Lymphomas | (2) Light chain |
| B. Clonality | |
| 2. MGUS-Amyloid | (1) Monoclonal |
| (2) Polyclonal | |
| 3. Drugs | (3) Indeterminate |
| A. Clofazimine | |
| 2. Clofazimine | |
| 4. Allergic-autoimmune | |
| A. Rheumatoid arthritis | 3. Charcot-Leyden |
| B. Eosinophilic colitis | |
| C. Mastocytosis | 4. Other |
| D. Hypereosinophilic syndrome | A. Cystine |
| B. Silica | |
| 5. Metabolic | |
| A. Cystinosis | |
| 6. Inflammatory-reactive | |
| A. Pulmonary infections | |
| B. Plasma cell granuloma | |
| C. Crohn’s disease | |
| D. Helicobacter pylori | |
| 7. Other | |
| A. Silica |
CSH crystal-storing histiocytosis, MGUS monoclonal gammopathy of undetermined significance
We report a case of CSH (immunoglobulin variant) that occurred in the left upper lip and cheek of a 51-year-old woman that was difficult to diagnose histologically and totally unexpected clinically. We also: (1) review the literature regarding the clinicopathologic features of CSH; (2) propose a classification of CSH; (3) discuss treatment and prognosis; (4) comment on the non-immunoglobulin variants; and (5) provide a differential diagnosis.
Case Report
Clinical History
A 51-year-old Caucasian woman presented to her local physician with a 1.5 cm submucosal swelling of the left upper lip and cheek of 2 weeks duration. There was no lymphadenopathy. Her past medical history included osteoarthritis, hypothyroidism, elevated platelet count, and an unknown pulmonary infection treated with tetracycline. She also indicated that she had a “pseudotumor of the brain and papillary edema” but no official diagnosis was otherwise given. A whole-body scan performed 7 months prior to presentation showed multifocal degenerative joint changes with no evidence of metastatic disease. The mass was thought to be a pleomorphic adenoma and was subsequently excised.
Pathology
Gross
The specimen consisted of a 1.5 × 1.3 × 1.0 cm firm, yellow–brown, poorly demarcated soft tissue mass that varied on cross section from grey–white to yellow–tan. It was entirely submitted for microscopic evaluation. After an initial diagnosis of “Fragments of fibroadipose tissue, nerve bundles and minor salivary glands infiltrated by spindle and epithelioid cells” was made by the local pathologist, the case was referred to the Division of Head and Neck Pathology at the University of Pittsburgh Medical Center-Presbyterian Hospital, Pittsburgh, Pennsylvania for a second opinion.
Microscopic
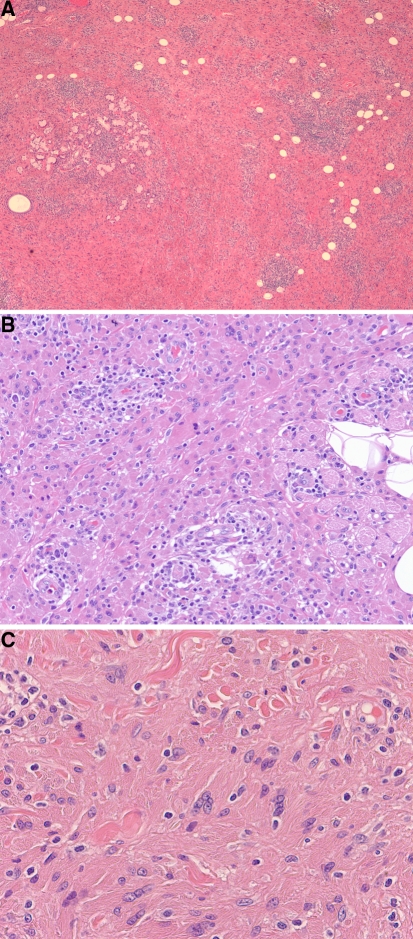
Hematoxylin and eosin stained sections revealed diffuse sheets of polygonal and a few spindle-shaped histiocytes associated with interspersed lymphoplasmacytic aggregates infiltrating the submucosa and intermingling between minor salivary glands, adipose tissue, and skeletal muscle fibers (Fig. 1a). The histiocytes had abundant opaque, deeply eosinophilic cytoplasm and round to ovoid nuclei with fine, pale chromatin and occasional small conspicuous nucleoli (Fig. 1b). A few multinucleated giant cells were also observed. The lymphocytes and plasma cells appeared mature and were free of Dutcher bodies. Eosinophils, necrosis, cellular pleomorphism, granulomas, “xanthoma cells”, and mitoses were not apparent. Because of the dense, opaque cytoplasm, the histiocytes were thought to be devoid of inclusions. However, on closer re-examination, some of the cells were focally found to contain linear, non-polarizable cytoplasmic crystal-like striations which raised the possibility of CSH (Fig. 1c).
Fig. 1.
a Diffuse sheets of eosinophilic histiocytes with admixed lymphoplasmacytic aggregates infiltrate minor salivary glands, adipose tissue, and skeletal muscle (×40 magnification). b Histiocytes have abundant opaque, deeply eosinophilic cytoplasm, round to ovoid nuclei and fine, pale chromatin. Lymphocytes and plasma cells are mature (×200 magnification). c Linear, non-polarizable cytoplasmic crystal-like striations within histiocytes are focally apparent (×400 magnification)
Histochemistry and Immunohistochemistry
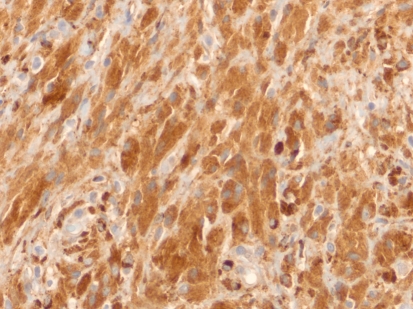
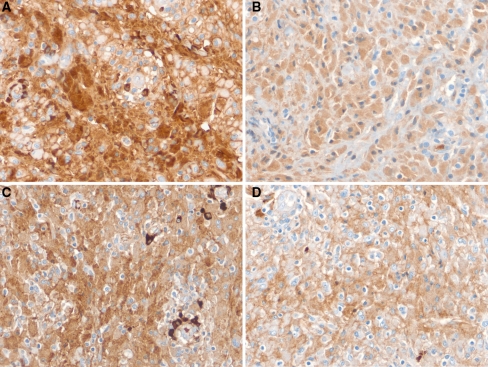
The tissue was nonreactive with the periodic acid-Schiff (PAS) stain and negative for fungi (Gomori methenamine silver) and acid fast bacilli. The histiocytes were strongly positive for CD68 (Fig. 2), alpha-1-antitrypsin, and alpha-1-antichymotrypsin and negative for desmin, myoglobin, S-100 protein, CD1a, langerin, and cytokeratin AE1/AE3. The intracytoplasmic crystal-like striations were strongly positive for IgM heavy chain and weakly positive for IgG heavy chain (Fig. 3a, b, respectively). They were negative for IgG4 and IgA. The crystal inclusions were also strongly immunoreactive for lambda light chain and weakly reactive for kappa light chain (Fig. 3c, d, respectively). The surrounding lymphoplasmacytic infiltrate showed IgM lambda light chain restriction (further confirmed by a hematopathologist in our Department). The Ki-67 proliferation index was less than 2%. Since no tissue remained in the paraffin block, stains for amyloid could not be done. None, however, was apparent on review of the existing hematoxylin and eosin stained slides.
Fig. 2.
Histiocytes are strongly positive for CD68 (×400 magnification)
Fig. 3.
Immunohistochemical stains for immunoglobulins confirm the nature of the crystalline inclusions within the histiocytes. a Immunostain for IgM heavy chain is strong and diffuse. b IgG heavy chain is diffuse but relatively weak. c Immunoreactivity for lambda light chain is strong within the histiocytes as well as in the plasma cells, which demonstrate IgM lambda light chain restriction. d Kappa light chain is weakly positive (×400 magnification)
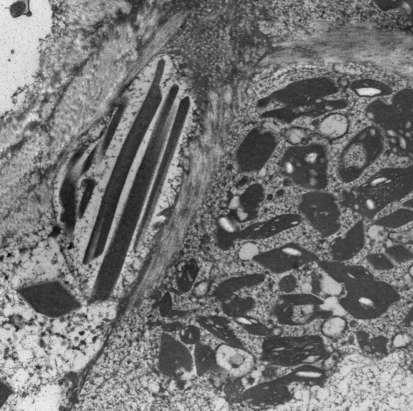
Electron Microscopy
Electron microscopy performed on formalin-fixed, paraffin-embedded tissue demonstrated numerous elongated and rhomboid-shaped dense crystals within the cytoplasm of the histiocytes (Fig. 4).
Fig. 4.
Electron microscopy reveals elongated and rhomboid shaped dense crystals within the cytoplasm of histiocytes. Some of the crystals contain clear vacuoles (×11,500 magnification)
Final Pathologic Diagnosis
Based on the above studies, a diagnosis of “Crystal-storing histiocytosis, immunoglobulin variant, associated with a mild lymphoplasmacytic infiltrate exhibiting IgM lambda light chain restriction” was made with a comment that the patient should be evaluated for an underlying lymphoproliferative or plasma cell disorder.
Follow-up
With a tissue diagnosis of CSH, the patient was re-evaluated by her local physician. No additional foci of CSH were found and there was no evidence of lymphadenopathy or hepatosplenomegaly. Her peripheral blood count was normal but her serum protein analysis was significant for slightly increased total protein of 8.2 g/dL (normal range = 6.2–8.0 g/dL), increased gamma globulins of 1.7 g/dL (normal range = 0.6–1.6 g/dL) and beta-microglobulin of 3.32 mg/L (normal range = 0.00–2.51 mg/L). Urinalysis, however, failed to reveal monoclonal bands and blood work-up was negative for monoclonal proteins. A bone marrow biopsy was normocellular. Plasma cells were within normal limits, no lymphoid infiltrates were seen, and no additional foci of CSH were detected in the marrow. A repeat complete skeletal imaging survey was negative for blastic or lytic lesions or osteopenia. Although a monoclonal protein was not identified, a working clinical diagnosis of “possible monoclonal gammopathy of undetermined significance (MGUS)” was nevertheless made by her local physician based on the borderline to slightly elevated serum proteins and histologic findings. Since she was otherwise asymptomatic, no treatment was recommended other than periodic follow-up. At last examination (8 months since her diagnosis of CSH), there has been no significant change in her physical condition or laboratory data.
Literature Review
Eighty acceptable cases of CSH were identified through a PubMed search of the English literature from 1950 to 2010 (until September) (Table 2). Only cases describing immunoglobulin crystals within histiocytes were considered for this review. Non-immunoglobulin variants are addressed separately in the “Discussion” section. The cases were subdivided into two categories: (1) localized CSH (L-CSH), defined as a single deposit involving only one organ or site (for example, CSH involving the cornea and conjunctiva of the same eye would still be classified as L-CSH) and (2) generalized CSH (G-CSH), defined as involving two or more distant organs or sites (for example, bone marrow and kidney). The following data, if available, were tabulated for each case: gender, age, site(s) of CSH, localized versus generalized, association with other diseases (MM, LPL, MGUS, etc.), symptoms, treatment, prognosis, and type and clonality of immunoglobulin within the histiocytes (Tables 2,3, 4).
Table 2.
Review of 80 cases of CSH from the literature
| Men (n = 41) | |
| Age, average (range) | 59 (38–75) |
| Women (n = 39) | |
| Age, average (range) | 61 (17–81) |
| Number of cases (%) | |
|---|---|
| 1. CSH with underlying LP-PCD | 72 (90%) |
| A. Multiple myeloma | 23 (31.9%) |
| B. Lymphoplasmacytic lymphoma | 17 (23.6%) |
| C. Paraproteinemia/MGUS | 15 (20.8%) |
| D. Plasma cell dyscrasia/neoplasm, not further specified | 4 (5.6%) |
| E. B-cell lymphoma | 11 (15.3%) |
| MALT/EMZL | 6 |
| MZL | 2 |
| B-cell lymphoma (not classified) | 2 |
| F. Other LP-PCDa | 2 (2.8%) |
| 2. CSH with unknown history | 1 (1.2%) |
| 3. CSH without underlying LP-PCD | 7 (8.8%) |
| Lung | 3 |
| Stomach | 1 |
| Brain | 1 |
| Base of tongue | 1 |
| Upper lip/cheek | 1 |
LP-PCD lymphoproliferative or plasma cell disorder, MALT mucosa-associated lymphoid tissue lymphoma, EMZL extranodal marginal zone lymphoma, MZL marginal zone lymphoma
aIncluding diagnoses of reticuloendotheliosis and purpura hemorrhagica
Table 3.
Sites of localized CSH cases
| Localized CSH (N = 46) | |
|---|---|
| Region/organ/tissue | Number (%) |
| 1. Head and neck | 16 (35%) |
| Eye/orbita | 6 |
| Oral, pharyngeal and sinonasal mucosa | 3 |
| Cervical lymph nodes | 2 |
| Soft tissue | 2 |
| Parotid and periparotid lymph nodes | 1 |
| Skin | 1 |
| Brain | 1 |
| 2. Lung and pleura | 11 (24%) |
| 3. Bone marrow | 5 (11%) |
| 4. Kidney | 5 (11%) |
| 5. Lymph nodesb | 2 |
| 6. Gastrointestinal mucosa | 2 |
| 7. Skinb | 2 |
| 8. Body fluids | 2 |
| 9. Heart | 1 |
aIncluding the ocular adnexa
bOther than head and neck region
Table 4.
Sites of generalized CSH Cases
| Generalized CSH (N = 34) | |
|---|---|
| Organ/tissue site | Number (%) |
| Bone marrow | 33 (97%) |
| Liver | 16 (47%) |
| Lymph nodes | 15 (44%) |
| Spleen | 15 (44%) |
| Kidney | 13 (38%) |
| Gastrointestinal mucosa | 7 (21%) |
| Lung | 4 (12%) |
| Adrenalsa | 4 (12%) |
| Hearta | 3 (9%) |
| Pleuraa | 3 (9%) |
| Peritoneum | 3 (9%) |
| Bone | 3 (9%) |
| Skin | 2 (6%) |
| Pancreasa | 2 (6%) |
| Testisa | 2 (6%) |
| Thyroida | 1 (3%) |
| Dura and pia matera | 1 (3%) |
| Mesenterya | 1 (3%) |
| Thymus | 1 (3%) |
| Pericardiuma | 1 (3%) |
| Bladdera | 1 (3%) |
| Parotid | 1 (3%) |
| Conjunctivaa | 1 (3%) |
| Tonguea | 1 (3%) |
| Sinonasal mucosa | 1 (3%) |
| Connective tissue | 1 (3%) |
| Adipose tissue | 1 (3%) |
| Ascites | 1 (3%) |
aOrgans involved by generalized CSH at autopsy only
Discussion
The accumulation of crystalline material within the cytoplasm of histiocytes is an uncommon condition known as CSH. Although the crystals are most often of immunoglobulin origin and associated with an underlying lymphoproliferative or plasma cell disorder (LP-PCD) other non-immunoglobulin, non-hematologic associated types of CSH exist and are addressed later in the discussion (Table 1). Based on etiology and/or associated disease and the chemical composition of the crystal, CSH can be classified as shown in Table 1.
Of the 80 cases of CSH of the immunoglobulin type retrieved from the literature, 41 (51%) occurred in men 38–75 years of age (mean 59) and 39 (49%) in women 17–81 years of age (mean 61) (Table 2) [1–3, 7–57]. Fifty-eight percent (N = 46/80) of the patients presented with L-CSH and of these, 35% (N = 16/46) occurred in the head and neck, with the most common site being the eye/orbit (Table 3). The second most common site for L-CSH was the lung and pleura (N = 11/46 or 24%). The remaining 42% (N = 34/80) of patients had G-CSH. Besides the bone marrow which was involved in all but one case, the most frequently involved sites in G-CSH were the liver, lymph nodes, spleen and kidney (Table 4).
Regardless of whether CSH was localized or generalized, 90% of cases were associated with an underlying LP-PCD, and most of these patients had either MM, LPL, or MGUS (Table 2). Notable among the 7 cases (7/80 or 8.8%) that were not related to a clonal LP-PCD was a female predominance and an association with diseases with an inflammatory background (Table 5). Although most cases of CSH occur in patients with a previous well-established diagnosis of MM, LPL, or MGUS, we have identified at least seven cases in which the diagnosis of CSH led to the discovery of a simultaneous, previously unrecognized LP-PCD and/or paraproteinemia [1, 15, 18, 31, 41, 43, 53]. In another three cases, CSH preceded the diagnosis of a LP-PCD by “a few months”, 7 months, and 4 years, respectively [3, 36, 39].
Table 5.
CSH without underlying clonal lymphoproliferative or plasma cell disorder
| Author | Age/sex | Patient history/underlying disease | Duration of symptoms | Symptoms/indication for work up | Organ(s) involved by CSH |
|---|---|---|---|---|---|
| Bosman [7] | 73 F | Rheumatoid arthritis, polyclonal hypergammaglobulinema | Unknown | Mass at the base of tongue | Base of tongue and hypopharynx |
| Jones [3] | 54 F | Plasma cell granuloma | Unknown | None/incidental finding | Lung |
| Ionescu [20] | 50 F | Rheumatoid arthritis | Unknown | None/incidental finding | Lung |
| Joo [21] | 56 F | H. pylori + gastritis, polyclonal plasma cell proliferation | 2 weeks | Dyspepsia, gastric pain | Stomach |
| Lee [29] | 64 M | Lung abscesses, possible tuberculosis (?), history of asbestos exposure | 7 months | Cough, fever, unresolved lung nodules | Lung |
| Kaminsky [22] | 27 F | Crohn’s disease | Unknown | Localized neurologic deficits | Brain |
| Present case, 2010 | 51 F | Arthritis, hypothyroidism, pulmonary infection treated with tetracycline | Unknown | Upper lip/cheek swelling | Upper lip/cheek mucosa and submucosa |
Although the majority of patients with CSH present clinically with an asymptomatic mass or swelling, often associated with a yellow or tan hue, there are exceptions. De Alba Campomanes et al. [10] describe an orbital CSH in a 66-year-old man that was associated with progressive ptosis, proptosis, and external ophthalmoplegia. Sailey et al. [44] discuss a cardiac CSH in a 64-year-old man that was responsible for recurrent atrial arrhythmias and dizziness, and Kapadia et al. [1] mention an 18-year-old woman with a “symptomatic” CSH of the right lateral wall of the nasopharynx extending to the soft palate (specific symptoms not indicated).
Most CSH range in size from microscopic to 4 cm and are composed of sheets of eosinophilic epithelioid to spindle-shaped histiocytes with poorly defined margins. The nuclei are bland, round to ovoid and often contain small nucleoli. Lymphocytes and plasma cells of varying proportions and maturity are commonly observed in the background, arranged either diffusely and/or as small aggregates. The histiocytes are strongly positive for CD68 and negative for desmin, muscle-specific actin, myoglobin, S-100 protein and CD1a. The crystals typically stain blue with phosphotungstic acid hematoxylin (PTAH) and are variably positive with the PAS stain. They are usually not birefringent. On immunohistochemical analysis, the crystals are typically monoclonal but in some instances may be polyclonal or even fail to stain. Failure of the immunoglobulin crystals to stain may be due to: (1) suboptimal tissue fixation, (2) antigen masking resulting from the crystalline structure of the protein, (3) altered molecular configuration of the protein with decreased antigenicity, or (4) the fact that the crystals are truly not of immunoglobulin origin and represent one of the other CSH variants (Table 1) [1, 58]. Ultrastructurally, the crystals are dense, membrane bound and elongated, rectangular and/or rhomboid in configuration. Some may also contain small vacuoles [1, 2, 44].
The immunoprofile of the 80 cases of CSH described in the literature was often not indicated. The specific type of heavy chain was mentioned in only 37 cases, and of these, 14 were IgM, 10 IgG, 6 IgA, and 7 polyclonal. The light chain component was documented in 51 cases, and of these, 33 were kappa, 8 lambda, and 10 polyclonal.
The exact mechanism for crystal formation in CSH is not well understood and may involve multiple factors, ranging from simple overproduction to abnormal secretion to impaired excretion of immunoglobulins. Circumstantial evidence indicates that crystallogenesis is more related to the type of light chain (particularly kappa) rather than to a specific heavy chain [2, 3]. CSH occurring in some patients with proximal renal tubular dysfunction (Fanconi syndrome) does add credence to the hypothesis that in some instances CSH may be due to decreased excretion of immunoglobulins [1, 2]. In a very elaborate study, Lebeau et al. examined the molecular configuration of a stored kappa light chain in a 73-year-old man with G-CSH associated with monoclonal gammopathy and observed that the light chain was structurally altered by several amino acid substitutions. They postulated that conformational alteration induced by the abnormal amino acid sequences was a probable crucial factor in the pathogenesis of CSH, promoting crystallization of the protein or adversely affecting its intralysosomal degradation or both [2]. Whether chemotherapy might have a similar structural affect on proteins and elicit the formation of CSH in some patients with a LP-PCD is open to speculation.
Pathologists should be aware of several problematic issues in evaluating cases of CSH. Not infrequently the cytoplasm of the histiocytes is so deeply eosinophilic and opaque that it will obscure any inclusions, crystals, or striations on microscopic examination resulting in a missed diagnosis (as was a potential issue in our case). Likewise, in most cases of CSH, the histiocytic component is dominant and, as such, may mask the neoplastic nature of any background lymphocytes or plasma cells. And lastly, in exceptional cases, there may be discordance between the clonality of the crystals and the serum; for example, the crystals may appear polyclonal on immunostaining while a monoclonal protein is apparent in the serum [2, 44].
Once the diagnosis of CSH is established, attention should be directed to the underlying cause. As in our review of the literature, 90% of individuals will have an underlying LP-PCD. A minimal workup should include a thorough history and physical examination looking for additional foci of CSH, lymphadenopathy, and hepatosplenomegaly; a complete peripheral blood count; bone marrow aspirate; serum and urine protein studies; serum free light chain analysis; and a complete skeletal survey for blastic-lytic lesions and osteopenia. Clinicians must be aware that in a few instances CSH may coincide with or even antedate the diagnosis of a LP-PCD, and accordingly, despite initial negative evaluation, the patient must be kept under periodic surveillance for this possibility. Not all cases, however, are associated with a neoplastic disease. In our review, 8.8% of cases occurred in patients with a variety of benign disorders, often associated with an inflammatory component, such as rheumatoid arthritis, pulmonary infections, and Crohn’s disease (Table 5).
Treatment and prognosis of patients with CSH vary according to the associated disease. With the exception of a few reports, there is very little information available regarding the specific response of CSH following chemotherapy or simple excision. Jones et al. [3], however, do mention that four of their myeloma patients with follow-up biopsies showed persistence of CSH after chemotherapy or bone marrow transplantation. Jones et al. [3] also describe a 54-year-old woman without a clonal LP-PCD disorder who presented with a solitary asymptomatic focus of CSH of the lung that was initially excised but subsequently recurred 10 years later. There is also data, in need of further verification, indicating that the number of foci of CSH may also influence prognosis; patients with G-CSH tend to have a worst prognosis than those with L-CSH [2]. Interestingly, many myeloma patients with CSH have reported survivals of 5–15 years after diagnosis, which is longer than the median survival for this entity [2, 3]. This extended survival, according to Lebeau et al. [2], may be related to the fact that myeloma patients with CSH commonly present at an early stage with low paraprotein levels, hypogammaglobulinemia, and minimal plasma cell infiltrates and that the symptoms of immunoglobulin crystallization might lead to diagnosis at an earlier stage of disease than would otherwise occur.
Although the immunoglobulin variant of CSH is by far the most frequent, other rare variants exist (Table 1). Most of these are probably best recognized by a detailed clinical history rather than by biopsy. One of these variants is related to clofazimine, a drug used to treat leprosy and some mycobacterial infections [6]. A side effect is gastrointestinal toxicity, especially if given in high dosage over a prolonged interval. These patients typically exhibit a red discoloration of the skin and experience severe abdominal distress, often prompting an exploratory laparotomy. Imaging studies characteristically demonstrate coarsening of the mucosal folds of the small intestines often associated with regional lymphadenopathy, raising the possibility of a lymphoma and the need for biopsy. The clofazimine crystals are localized to the macrophages in the lamina propria and lymph nodes and appear bright red on frozen sections and show prominent red birefringence under polarized light. However, in formalin-fixed, paraffin embedded tissue, the elongated crystals are colorless and negative for both PAS and immunoglobulin stains.
At least three examples of CSH associated with massive deposits of Charcot-Leyden crystals have been recognized. One case involved a 78-year-old woman with eosinophilic colitis and a remote history of cutaneous mastocytosis. Surgical resection of the ascending and transverse colon demonstrated multiple 0.1–0.7 cm mucosal polyps composed of macrophages filled with Charcot-Leyden crystals [5]. Another case involved the bone marrow of a 91-year-old woman with aggressive systemic mastocytosis [59]. The third example involved the skin of a patient with hypereosinophilic syndrome (no further details available) [60]. In all three cases, the histiocytes were distended with deeply eosinophilic needled-shaped crystals with jagged ends and were associated with an intense infiltrate of eosinophils.
Weiss et al. have described seven patients who developed tissue masses following injection of a sclerosing agent (silica) for repair of a hernia. Microscopically, the lesions consisted of broad sheets of histiocytes separated by collagen. Intra- and extra-cellular birefringent crystals, identified by X-ray diffraction as silica, were identified in all cases [61]. Interestingly, the lesions were often confused with a benign or malignant fibrous histiocytoma. Gebrail et al. [4] describe a unique case of crystalline histiocytosis in a 23-year-old man with hereditary cystinosis. Because of an abnormal peripheral blood count, a bone marrow biopsy was performed which showed numerous macrophages containing clusters of hexagonal, tubular, and rectangular cystine crystals that were birefringent with polarized light and first-order compensator filters.
The differential diagnosis of histiocytic reactions such as CSH can be long. In this discussion eight entities will be covered: adult rhabdomyoma (ARM), granular cell tumor, Langerhans cell histiocytosis (LCH), fibrous histiocytoma, xanthogranuloma, Gaucher’s disease, malakoplakia, and mycobacterial spindle cell pseudotumor. Although CSH and the ARM are both composed of large eosinophilic, epithelioid cells and contain crystals and striations, they are usually easily distinguished [1]. The crystals in ARM (so called “Jack straw” crystals), if seen at all, are very focal and represent hypertrophic “Z” bands (tropomyosin) rather than immunoglobulins. Striations in CSH, due to parallel arrays of crystals, are typically linear rather than crossed or perpendicular. More importantly, ARM, in contrast to CSH, is positive for muscle makers, such as desmin, muscle specific actin, and myoglobin, and negative for immunoglobulins. A granular cell tumor is positive for S-100 protein and does not contain crystals or immunoglobulins. In contrast to the round to ovoid nuclei seen in the histiocytes of CSH, the nuclei of the histiocytic cells in LCH are folded or grooved resembling a coffee bean and are immunoreactive for CD1a. LCH is also commonly associated with a component of eosinophils which are absent in CSH. Fibrous histiocytoma, in contrast to CSH, exhibits a storiform pattern, possesses a more fibrous matrix, and lacks crystals. Although xanthogranuloma is more common in children, it may occur in adults as well [62, 63]. The presence of xanthoma cells, positive immunoreactivity for factor XIIIa, and absence of immunoglobulins separate this lesion from CSH. The striated appearance of some cells seen in CSH (so called pseudo-Gaucher cells or pseudo-pseudo-Gaucher cells as some prefer in order not to confuse them with the pseudo-Gaucher cells seen in chronic myelogenous leukemia) may lead one to suspect Gaucher’s disease [2, 45]. In Gaucher’s disease the striated appearance is due to the deposition of glucocerebroside not immunoglobulins. Moreover, the Gaucher cell is usually strongly positive for iron as opposed to the histiocytes in CSH. In cases of doubt, assays for beta-glucocerebrosidase activity should be obtained [64]. Although malakoplakia is found most often in the urinary tract, it has been observed in many other sites, including the head and neck [65]. Pathologically it is composed of sheets of CD68-positive histiocytes (von Hansemann histiocytes) and scattered pathognomonic Michaelis-Gutmann bodies and may contain bacteria on Gram stain. It also lacks immunoglobulins. CSH with a prominent component of spindle-shaped histiocytes might invite suspicion for a mycobacterial spindle cell pseudotumor. Stains for acid fast bacilli will readily resolve this dilemma.
In summary, CSH is an uncommon lesion that occurs over a broad age range (17–81 years) with an equal gender distribution. It may be either localized or generalized and involve almost any anatomic site. Its importance lies in the fact that in 90% of cases it is associated with a serious LP-PCD, especially MM, LPL, and MGUS. Not all cases, however, are hematologically related; 8.8% in our review occurred in patients with a variety of benign diseases, often associated with an inflammatory background. Rare, non-immunoglobulin variants of CSH also exist.
Acknowledgments
We thank Raymond E. Felgar, MD, PhD of the Division of Hematopathology, University of Pittsburgh Medical Center, Presbyterian Hospital for reviewing this case, Jay Arlick, DMD, oral surgeon, Clearfield Hospital, Clearfield, Pennsylvania, who removed the lesion and provided some evaluation and follow-up and Jacinthe Chenevert, MD, Division of Endocrine and Head and Neck Pathology, University of Pittsburgh Medical Center, Presbyterian Hospital for collecting some published data.
Contributor Information
Snjezana Dogan, Email: dogans@mskcc.org.
Leon Barnes, Email: barnesleon41@yahoo.com.
Wilhelmina P. Cruz-Vetrano, Email: wpcruzmd@atlanticbb.net
References
- 1.Kapadia SB, Enzinger FM, Heffner DK, Hyams VJ, Frizzera G. Crystal-storing histiocytosis associated with lymphoplasmacytic neoplasms. Report of three cases mimicking adult rhabdomyoma. Am J Surg Pathol. 1993;17:461–467. doi: 10.1097/00000478-199305000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Lebeau A, Zeindl-Eberhart E, Muller E-C, Muller-Hocker J, Jungblut PR, Emmerich B, Lohrs U. Generalized crystal-storing histiocytosis associated with monoclonal gammopathy: molecular analysis of a disorder with rapid clinical course and review of literature. Blood. 2002;100:1817–1827. [PubMed] [Google Scholar]
- 3.Jones D, Bhatia VK, Krausz T, Pinkus GS. Crystal-storing histiocytosis : a disorder occurring in plasmacytic tumors expressing immunoglobulin kappa light chain. Hum Pathol. 1999;30:1441–1448. doi: 10.1016/S0046-8177(99)90166-1. [DOI] [PubMed] [Google Scholar]
- 4.Gebrail F, Knapp M, Perrotta G, Cualing H. Crystalline histiocytosis in hereditary cystinosis. Arch Pathol Lab Med. 2002;126(9):1135. doi: 10.5858/2002-126-1135-CHIHC. [DOI] [PubMed] [Google Scholar]
- 5.Lewis JT, Candelora JN, Hogan RB, Briggs FR, Abraham SC. Crystal-storing histiocytosis due to massive accumulation of Charcot-Leyden crystals: a unique association producing colonic polyposis in a 78-year old woman with eosinophilic colitis. Am J Surg Pathol. 2007;31:481–485. doi: 10.1097/01.pas.0000213420.46127.9c. [DOI] [PubMed] [Google Scholar]
- 6.Sukpanichnant S, Hargrove NS, Kachintorn U, Manatsathit S, Chanchairujira T, Siritanaratkul N, Akaravipuyh T, Thakerngpol K. Clofazimine-induced crystal-storing histiocytosis producing chronic abdiminable pain in a leprosy patient. Am J Surg Pathol. 2000;24:129–135. doi: 10.1097/00000478-200001000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Bosman C, Camassei FD, Boldrini R, Piro FR, Saponara M, Romeo R, Corsi A. Solitary crystal-storing histiocytosis of the tongue in a patient with rheumatoid arthritis and polyclonal hypergammaglobulinemia. Arch Pathol Lab Med. 1998;122:920–924. [PubMed] [Google Scholar]
- 8.Colby TV, Koss MN, Travis WD. Tumors of the lower respiratory tract. Washington, DC: Armed Forces Institute of Pathology; 1995. Atlas of tumor pathology, 3rd series, fascicle 13.
- 9.Coupland SE, Foss HD, Hummel M, Stein H. Extranodal marginal zone B-cell lymphoma of the lacrimal gland associated with crystal-storing histiocytosis. Ophthalmology. 2002;109:105–110. doi: 10.1016/S0161-6420(01)00837-5. [DOI] [PubMed] [Google Scholar]
- 10.Alba Campomanes AG, Rutar T, Crawford JB, Seiff S, Goodman D, Grenert J. Crystal-storing histiocytosis and crystalline keratopathy caused by monoclonal gammopathy of undetermined significance. Cornea. 2009;28(9):1081–1084. doi: 10.1097/ICO.0b013e318199f73b. [DOI] [PubMed] [Google Scholar]
- 11.Lastours V, Papo T, Cazals-Hatem D, Eden A, Feydy A, Belmatoug N, Chauveheid MP, Lidove O, Fantin B. Bone involvement in generalized crystal-storing histiocytosis. J Rheumatol. 2006;33(11):2354–2358. [PubMed] [Google Scholar]
- 12.El Hamel C, Thierry A, Trouillas P, Bridoux F, Carrion C, Quellard N, Goujon JM, Aldigier JC, Gombert JM, Cogné M, Touchard G. Crystal-storing histiocytosis with renal Fanconi syndrome: pathological and molecular characteristics compared with classical myeloma-associated Fanconi syndrome. Nephrol Dial Transplant. 2010;25(9):2982–2990. doi: 10.1093/ndt/gfq129. [DOI] [PubMed] [Google Scholar]
- 13.Fairweather PM, Williamson R, Tsikleas G. Pulmonary extranodal marginal zone lymphoma with massive crystal storing histiocytosis. Am J Surg Pathol. 2006;30:262–267. doi: 10.1097/01.pas.0000178093.99889.f7. [DOI] [PubMed] [Google Scholar]
- 14.Farooq U, Bayerl MG, Abendroth CS, Verma C, Talamo G. Renal crystal storing histiocytosis in a patient with multiple myeloma. Ann Hematol. 2009;88:807–809. doi: 10.1007/s00277-008-0660-z. [DOI] [PubMed] [Google Scholar]
- 15.Friedman MT, Molho L, Valderrama E, Kahn LB. Crystal-storing histiocytosis associated with a lymphoplasmacytic neoplasm mimicking adult rhabdomyoma. Arch Pathol Lab Med. 1996;120:1133–1136. [PubMed] [Google Scholar]
- 16.Galed-Placed I. Immunoglobulin crystal-storing histiocytosis in a pleural effusion from a woman with IgA kappa multiple myeloma: a case report. Acta Cytol. 2006;50:539–541. doi: 10.1159/000326010. [DOI] [PubMed] [Google Scholar]
- 17.Garcia JF, Sanchez E, Lloret E, Martin J, Piris MA. Crystal-storing histiocytosis and immunocytoma associated with multifocal fibrosclerosis. Histopathology. 1998;33:459–464. doi: 10.1046/j.1365-2559.1998.00531.x. [DOI] [PubMed] [Google Scholar]
- 18.Grossniklaus HE, Stulting RD, L’Hernault N. Corneal and conjunctival crystals in paraproteinemia. Hum Pathol. 1990;21:1181–1183. doi: 10.1016/0046-8177(90)90156-Y. [DOI] [PubMed] [Google Scholar]
- 19.Harada M, Shimada M, Fukayama M, Kaneko T, Kitazume K, Weiss SW. Crystal-storing histiocytosis associated with lymphoplasmacytic lymphoma mimicking Webel Christian disease: immunohistochemical, ultrastructural, and gene-rearrangement studies. Hum Pathol. 1996;27(1):84–87. doi: 10.1016/S0046-8177(96)90143-4. [DOI] [PubMed] [Google Scholar]
- 20.Ionescu DN, Pierson DM, Qing G, Li M, Colby TV, Leslie KO. Pulmonary crystal-storing histiocytoma. Arch Pathol Lab Med. 2005;129:1159–1163. doi: 10.5858/2005-129-1159-PCH. [DOI] [PubMed] [Google Scholar]
- 21.Joo M, Kwak JE, Chang SH, Kim H, Chi JG, Moon YS, Kim KM. Localized gastric crystal-storing histiocytosis. Histopathology. 2007;51(1):116–119. doi: 10.1111/j.1365-2559.2007.02710.x. [DOI] [PubMed] [Google Scholar]
- 22.Kaminsky IA, Wang AM, Olsen J, Schechter S, Wilson J, Olson R. Central nervous system crystal-storing histiocytosis: neuroimaging, neuropathology, and literature review. AJNR Am J Neuroradiol. 2011;32(2):E26–E28. doi: 10.3174/ajnr.A1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufmann O, Hansen A, Deicke P, Burmester GR, Dietel M. Subcutaneous crystal-storing histiocytosis associated with lymphoplasmacytic lymphoma (immunocytoma) Pathol Res Pract. 1996;192:1148–1151. doi: 10.1016/S0344-0338(96)80036-7. [DOI] [PubMed] [Google Scholar]
- 24.Kazzaz B, Dewar A, Corrin B. An unusual pulmonary plasmacytoma. Histopathology. 1992;21:285–287. doi: 10.1111/j.1365-2559.1992.tb00391.x. [DOI] [PubMed] [Google Scholar]
- 25.Keane C, Gill D. Multi-organ involvement with crystal-storing histiocytosis. Br J Haematol. 2008;141(6):750. doi: 10.1111/j.1365-2141.2008.07131.x. [DOI] [PubMed] [Google Scholar]
- 26.Kornstein MJ, deBlois GG, Williams ME. Unusual biclonal plasma cell dyscrasia with crystallike inclusions producing colonic tumors (plasma cell polyposis) and terminating in T-cell lymphoma. Arch Pathol Lab Med. 1992;116(2):168–172. [PubMed] [Google Scholar]
- 27.Kusakabe T, Watanabe K, Mori T, Iida T, Suzuki T. Crystal-storing histiocytosis associated with MALT lymphoma of the ocular adnexa: a case report with review of literature. Virchows Arch. 2007;450(1):103–108. doi: 10.1007/s00428-006-0323-1. [DOI] [PubMed] [Google Scholar]
- 28.László R, Degrell P, Kellermayer M, Bollmann D, Egyed M, Seress L, Pajor L. Crystal-storing histiocytosis associated with only one of two consecutive, but genetically unrelated B-cell lymphomas. Pathol Res Pract. 2009;205(4):273–278. doi: 10.1016/j.prp.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Lee WS, Kim SR, Moon H, Choe YH, Park SJ, Lee HB, Jin GY, Chung MJ, Lee YC. Pulmonary crystal-storing histiocytoma in a patient without a lymphoproliferative disorder. Am J Med Sci. 2009;338(5):421–424. doi: 10.1097/MAJ.0b013e3181ad3feb. [DOI] [PubMed] [Google Scholar]
- 30.Lesesve JF, Bronowicki JP, Galed-Placed I. Crystal-storing histiocytosis in ascites from a patient with IgM kappa lymphoplasmacytic lymphoma. Cytopathology. 2011;22(3):207–208. doi: 10.1111/j.1365-2303.2010.00823.x. [DOI] [PubMed] [Google Scholar]
- 31.Llobet M, Castro P, Barcelo C, Trull JM, Campo E, Bernadó L. Massive crystal-storing histiocytosis associated with low-grade malignant B-cell lyanphoma of MALT-type of the parotid gland. Diagn Cytopathol. 1997;17:148–152. doi: 10.1002/(SICI)1097-0339(199708)17:2<148::AID-DC12>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 32.Martin AW, Carstens PH, Yam LT. Crystalline deposits in ascites in a case of cryoglobulinemia. Acta Cytol. 1987;31(5):631–636. [PubMed] [Google Scholar]
- 33.Mennemeyer R, Hammar SE, Cathey WJ. Malignant lymphoma with intracytoplasmic IgM crystalline inclusions. N EnglJ Med. 1974;291:960–963. doi: 10.1056/NEJM197410312911809. [DOI] [PubMed] [Google Scholar]
- 34.Mullen B, Chalvardjian A. Crystalline tissue deposits on a case of multiple myeloma. Arch Pathol Lab Med. 1981;105(2):94–97. [PubMed] [Google Scholar]
- 35.Padmalatha C, Warner TF, Hafez GR. Pseudo-Gaucher cell in IgMk plasmacytoid lymphoma. Am J Surg Pathol. 1981;5(5):501–505. doi: 10.1097/00000478-198107000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Papla B, Spolnik P, Rzenno E, Zduńczyk A, Rudzki Z, Okoń K, Szczepański W, Dabroś W, Stachura J. Generalized crystal-storing histiocytosis as a presentation of multiple myeloma: a case with a possible pro-aggregation defect in the immunoglobulin heavy chain. Virchows Arch. 2004;445:83–89. doi: 10.1007/s00428-004-1031-3. [DOI] [PubMed] [Google Scholar]
- 37.Pinkerton RM, Robertson DM. Corneal and conjunctival changes in dysproteinemia. Invest Ophthalmol. 1969;8:357–364. [PubMed] [Google Scholar]
- 38.Pitman SD, Wang J, Serros ER, Zuppan C. A 70-year-old woman with acute renal failure. Crystal-storing histiocytosis. Arch Pathol Lab Med. 2006;130:1077–1078. doi: 10.5858/2006-130-1077-AYWWAR. [DOI] [PubMed] [Google Scholar]
- 39.Pock L, Stuchlik D, Hercogova J. Crystal storing histiocytosis of the skin associated with multiple myeloma. Int J Dermatol. 2006;45:1408–1411. doi: 10.1111/j.1365-4632.2006.02919.x. [DOI] [PubMed] [Google Scholar]
- 40.Prasad MJ, Charney DA, Sarlin J, Keller SM. Pulmonary immunocytoma with massive crystal storing histiocytosis. Am J Surg Pathol. 1998;22:1148–1153. doi: 10.1097/00000478-199809000-00015. [DOI] [PubMed] [Google Scholar]
- 41.Rao NA, Font RL. Plasmacytic conjunctivitis with crystalline inclusions. Immunohistochemical and ultrastructural studies. Arch Ophthalmol. 1980;98(5):836–841. doi: 10.1001/archopht.1980.01020030830004. [DOI] [PubMed] [Google Scholar]
- 42.Robak T, Urbanska-Rys H, Jerzmanowski P, Bartkowiak J, Liberski P, Kordek R. Lymphoplasmacytic lymphoma with monoclonal gammopathy-related pseudo- Gaucher cell infiltration in bone marrow and spleen—diagnostic and therapeutic dilemmas. Leuk Lymphoma. 2002;43:2343–2350. [PubMed] [Google Scholar]
- 43.Rossi G, Morandi U, Nannini N, Fontana G, Pifferi M, Casali C. Crystal-storing histiocytosis presenting with pleural disease. Histopathology. 2010;56(3):403–405. doi: 10.1111/j.1365-2559.2010.03481.x. [DOI] [PubMed] [Google Scholar]
- 44.Sailey CJ, Alexiev BA, Gammie JS, Pinell-Salles P, Stafford JL, Burke A. Crystal-storing histiocytosis as a cause of symptomatic cardiac mass. Arch Pathol Lab Med. 2009;133(11):1861–1864. doi: 10.5858/133.11.1861. [DOI] [PubMed] [Google Scholar]
- 45.Schaefer HE. Gammopathy-related crystal-storing histiocytosis, pseudo- and pseudo-pseudo-Gaucher cells. Critical commentary and mini-review. Pathol Res Pract. 1996;192(11):1152–1162. doi: 10.1016/S0344-0338(96)80037-9. [DOI] [PubMed] [Google Scholar]
- 46.Sethi S, Cuiffo BP, Pinkus GS, Rennke HG. Crystal-storing histiocytosis involving the kidney in a low-grade B-cell lymphoproliferative disorder. Am J Kidney Dis. 2002;39:183–188. doi: 10.1053/ajkd.2002.29914. [DOI] [PubMed] [Google Scholar]
- 47.Stokes MB, Aronoff B, Siegel D, D’Agati VD. Dysproteinemia related nephropathy associated with crystal-storing histiocytosis. Kidney Int. 2006;70:597–602. doi: 10.1038/sj.ki.5001903. [DOI] [PubMed] [Google Scholar]
- 48.Sun Y, Tawfiqul B, Valderrama E, Kline G, Kahn LB. Pulmonary crystal-storing histiocytosis and extranodal marginal zone B-cell lymphoma associated with a fibroleiomyomatous hamartoma. Ann Diagn Pathol. 2003;7:47–53. doi: 10.1053/adpa.2003.50008. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi K, Naito M, Takatsuki K, et al. Multiple myeloma, IgA kappa type, accompanying crystal-storing histiocytosis and amyloidosis. Acta Pathol Jpn. 1987;37:141–154. doi: 10.1111/j.1440-1827.1987.tb03142.x. [DOI] [PubMed] [Google Scholar]
- 50.Terashima K, Takahashi K, Kojima M, Imai Y, Tsuchida S, Migita S, Ebina S, Itoh C. Kappa-type light chain crystal storage histiocytosis. Acta Pathol Jpn. 1978;28:111–138. doi: 10.1111/j.1440-1827.1978.tb01254.x. [DOI] [PubMed] [Google Scholar]
- 51.Tholouli E, Krebs M, Reeve R, Houghton JB. Crystal-storing histiocytosis in a patient with IgGK multiple myeloma. Br J Haematol. 2005;128:412. doi: 10.1111/j.1365-2141.2004.05362.x. [DOI] [PubMed] [Google Scholar]
- 52.Thorson P, Hess JL. Transformation of monocytoid B-cell lymphoma to large cell lymphoma associated with crystal-storing histiocytes. Arch Pathol Lab Med. 2000;124:460–462. doi: 10.5858/2000-124-0460-TOMBCL. [DOI] [PubMed] [Google Scholar]
- 53.Todd WU, Drabick JJ, Benninghoff MG, Frauenhoffer EE, Zander DS. Pulmonary crystal-storing histiocytosis diagnosed by computed tomography-guided fine-needle aspiration. Diagn Cytopathol. 2010;38(4):274–278. doi: 10.1002/dc.21193. [DOI] [PubMed] [Google Scholar]
- 54.Tomioka M, Ueki K, Nakahashi H, Isoda A, Kuroiwa T, Kaneko Y, Hiromura K, Nojima Y. Widespread crystalline inclusions affecting podocytes, tubular cells and interstitial histiocytes in the myeloma kidney. Clin Nephrol. 2004;62:229–233. doi: 10.5414/cnp62229. [DOI] [PubMed] [Google Scholar]
- 55.Weichman K, Dember LM, Prokaeva T, Wright DG, Quillen K, Rosenzweig M, Skinner M, Seldin DC, Sanchorawala V. Clinical and molecular characteristics of patients with non-amyloid light chain deposition disorders, and outcome following treatment with high-dose melphalan and autologous stem cell transplantation. Bone Marrow Transplant. 2006;38(5):339–343. doi: 10.1038/sj.bmt.1705447. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto T, Hishida A, Honda N, Ito I, Shirasawa H, Nagase M. Crystal-storing histiocytosis and crystalline tissue deposition in multiple myeloma. Arch Pathol Lab Med. 1991;115:351–354. [PubMed] [Google Scholar]
- 57.Zioni F, Giovanardi P, Bozzoli M, Artusi T, Bonacorsi G, Sighinolfi P. Massive bone marrow crystal-storing histiocytosis in a patient with IgA-lambda multiple myeloma and extensive extramedullary disease. A case report. Tumori. 2004;90:348–351. doi: 10.1177/030089160409000318. [DOI] [PubMed] [Google Scholar]
- 58.Stewart CJR, Spagnolo DV. Crystalline plasma cell inclusions in helicobacter-associated gastritis. J Clin Pathol. 2006;59:851–854. doi: 10.1136/jcp.2005.033233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alayed KM, Alabdulaali MK, Alkhairy KS, Elnour S, Alhajjaj A. Aggressive systemic mastocytosis with Charcot-Leyden crystals-associated crystal storing histiocytosis in bone marrow. Pathology. 2010;42(1):85–87. doi: 10.3109/00313020903434652. [DOI] [PubMed] [Google Scholar]
- 60.Dvorak AM, Weller PF, Monahan-Earley RA, Letourneau L, Ackerman SJ. Ultrastructural localization of Charcot-Leyden crystal protein (lysophospholipase) and peroxidase in macrophages, eosinophils, and extracellular matrix of the skin in the hypereosinophilic syndrome. Lab Invest. 1990;62(5):590–607. [PubMed] [Google Scholar]
- 61.Weiss SW, Enzinger FM, Johnson FB. Silca reaction simulating fibrous histioctoma. Cancer. 1978;42:2738–2743. doi: 10.1002/1097-0142(197812)42:6<2738::AID-CNCR2820420632>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 62.Dehner LP. Juvenile xanthogranulomas in the first two decades of life: a clinicopathologic study of 174 cases with cutaneous and extracutaneous manifestations. Am J Surg Pathol. 2003;27(5):579–593. doi: 10.1097/00000478-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 63.Rodriguez J, Ackerman AB. Xanthogranuloma in adults. Arch Dermatol. 1976;112(1):43–44. doi: 10.1001/archderm.1976.01630250015004. [DOI] [PubMed] [Google Scholar]
- 64.Chen M, Wang J. Gaucher disease: review of the literature. Arch Pathol Lab Med. 2008;132(5):851–853. doi: 10.5858/2008-132-851-GDROTL. [DOI] [PubMed] [Google Scholar]
- 65.Yousef GM, Naghibi B, Hamodat MM. Malakoplakia outside the urinary tract. Arch Pathol Lab Med. 2007 Feb;131(2):297-300. Review. Erratum in: Arch Pathol Lab Med. 2009 Jun;133(6):850. Hamodat, Mowafak M [added].