Abstract
Many dermal fillers have been used for reducing facial skin lines and for providing lip augmentation, and hyaluronic acid (HA) is one of the most widely used agents. One of the main commercial forms of HA is Restylane (Q Med, Sweden) produced by microbiological engineering techniques. Although HA is non-immunogenic, hypersensitivity and Granulomatous foreign body reactions have been reported. Herein, we report three female patients (average age 56 years) who presented with firm nodular lesions of the lip and a history of injection with HA (Restylane, Q Med, Sweden). Histopathologically, all cases showed pools of amorphous hematoxyphilic material surrounded by bands of densely collagenized connective tissue with no inflammation or foreign body reaction. Histochemical stains confirmed the presence of acid mucopolysaccharides such as hyaluronic acid. We conclude HA (Restylane, Q Med, Sweden) is an inert filler that may persist at an injection site, resulting in a tumor-like nodule.
Keywords: Cosmetic filler, Hyaluronic acid, Restylane, Lip nodule
Introduction
Many dermal fillers have been used for reducing facial skin lines and wrinkles, and for providing lip augmentation and hyaluronic acid (HA) is one the most widely used agents [1]. This was first used as an injectable dermal filler by Balazs et al. in [2] and it was introduced on the market in Europe in 1996 as a biodegradable product [3].
In 2003, the hyaluronic acid filler Restylane (Q Med, Uppsala, Sweden) was approved by the FDA in the US for soft tissue augmentation and since then its use has increased by 70% [4]. Although HA fillers are non-toxic and non-immunogenic, hypersensitivity and granulomatous foreign body reaction have been reported [5–15].
Here we report three new patients who developed lip nodules after injection with Restylane (Q Med, Uppsala, Sweden).
Report of the Cases
All three cases were females, with an average age of 56 years, who each presented with a painless discrete nodule on the labial mucosa. All patients had a history of Restylane (Q Med, Uppsala, Sweden) injection into their lips and had undergone biopsy. The characteristics of the current cases are presented in Table 1.
Table 1.
Clinical features of current cases and similar previously reported cases with lip nodules and history of Restylane injection
| Cases | Gender/age | Site/size (cm) | Clinical diagnosis | Period of time between restylane injection and biopsy |
|---|---|---|---|---|
| Current case #1 | F/55 | Upper lip/0.5 | Adenoma/fibrous hyperplasia | 4 months |
| Current case #2 | F/57 | Lower lip/1.0 | Sclerotic/inflamed minor salivary gland | 24 months |
| Current case #3 | F/56 | Lower lip/0.8 | Fibroma | Unknown |
| Bennett et al. [16] | F/61 | Upper lip/0.6 | Basal cell carcinoma | 23 months |
| Anatelli et al. [17] | F/80 | Upper lip/0.3 | Basal cell carcinoma | Not specified |
Histopathologic Findings
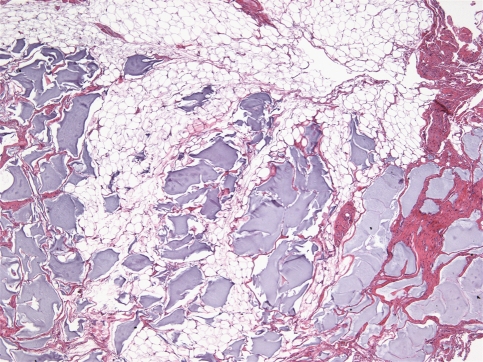
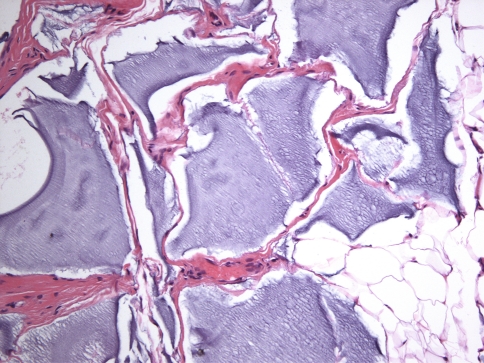
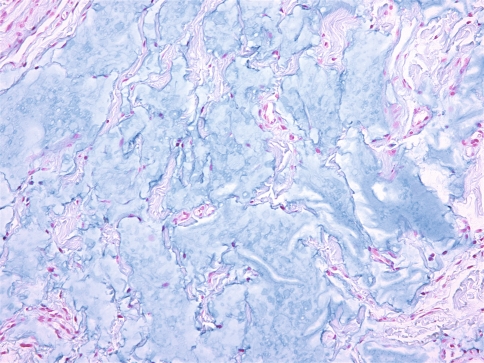
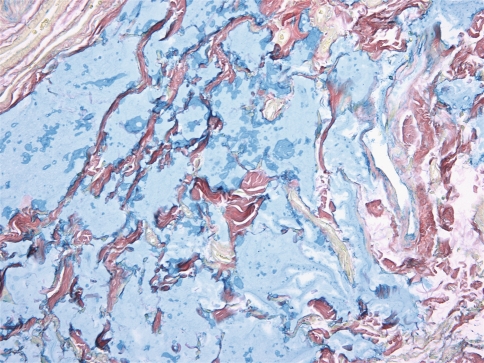
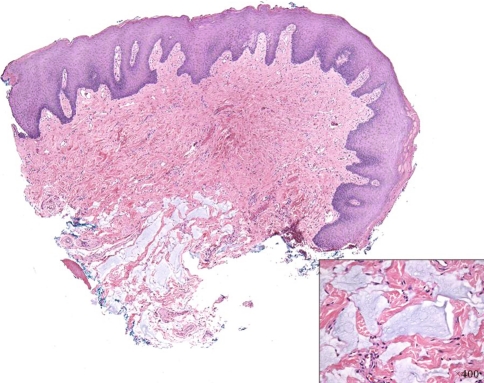
All cases were identical histopathologically. They consisted of pools of amorphous hematoxyphilic material surrounded by collagenized connective tissue and mature adipose tissue without inflammation or foreign body reaction (Figs. 1, 2). Alcian blue and colloidal iron stains confirmed the presence of acid mucopolysaccharides such as HA (Figs. 3, 4). In case 2, salivary gland lobules were also noted and these exhibited very mild chronic obstructive sialadenitis.
Fig. 1.
Pools of hematoxyphilic material surrounded by fibro-fatty tissue (H&E, ×40)
Fig. 2.
Amorphous hematoxyphilic material surrounded by densely collagenized connective tissue (H&E, ×200)
Fig. 3.
Pools of alcianophilic acid mucopolysaccharides (Alcian blue, ×200)
Fig. 4.
Pools of acid mucopolysaccharides positive for colloidal iron (Colloidal iron, ×200)
Case 3 demonstrated a nodule of densely collagenized fibrovascular tissue covered by hyperkeratotic stratified squamous epithelium typical for a fibroma (Fig. 5) Pools of basophilic and alcianophilic material, surrounded by thin septa of fibrous tissue and unassociated with inflammation or giant cell reaction, identical to what was seen in cases 1 and 2, were noted as an incidental finding at the base of the fibroma.
Fig. 5.
Case 3 showing a nodule of densely collagenized fibrovascular tissue with pools of basophilic material at the base (inset) and overlying hyperkeratotic squamous epithelium (H&E, ×40)
All cases were diagnosed as “inert foreign material consistent with hyaluronic acid filler (Restylane; Q Med, Uppsala, Sweden) unassociated with foreign body reaction”.
Discussion
The use of cosmetic dermal fillers is on the rise [18]. The ideal filler should be efficacious in reducing wrinkles and plumping the tissues without looking unnatural, be easy and safe to introduce into the tissues, have a long duration of action, be relatively inert and not incite a painful or bulky tissue response, and have acceptable cost [19].
Dermal fillers may be classified as permanent or degradable [19, 20]. Permanent fillers tend to be synthetic or alloplastic and show a very low rate of breakdown or may even remain permanently in the dermis. Examples of these include silicone and polymethylmethacrylate [19, 21].
Non-permanent or degradable fillers are generally made up of naturally-occurring biological agents such as collagen or hyaluronic acid that undergo degradation at variable rates [19, 21]. Collagen dermal fillers are available in the form of bovine collagen and human-based collagen [14, 22].
HA is a non-sulfated glycosaminoglycan polysaccharide composed of repeating disaccharide units of glucoronic acid and N-acetylglucosamine. HA is a major and natural component of the extracellular matrix in all animal tissues produced by mesenchymal cells with no organ or species specificity; as such, there is no risk for immunogenicity and it is non-toxic and biocompatible [19, 23]. It is highly hydrophilic and this property helps it to retain water and occupy larger volumes relative to its mass [22].
HA fillers have been used to eliminate photoaging wrinkles such as are often present on the nasolabial fold and lip, as well as peri-oral rhytides, and marionette lines. Such fillers may also be used for lip filling or contouring and chin and cheek augmentation [24]. Other indications for HA are noted in otologic surgery for regeneration of perforated tympanic membrane, in ophthalmic surgery for production of artificial tears, and in orthopedics as an anti-inflammatory lubricant [19]. Its use should be avoided in pregnant/breast feeding women, in patients younger than 18 and patients with history of allergy or anaphylaxis to the filler or one of its components [25].
HA fillers may be obtained from both animal and non-animal sources and there are two main commercial forms of HA. One is Hyaloform (Biomatrix, USA), an extract derived from rooster combs and the other is Restylane (Q Med, Uppsala, Sweden) which is a cross-linked HA produced by microbiologic engineering techniques (generated by streptococcus equi) [5, 19]; this latter product is more resistant to early degradation by hyaluronidase and rendered more water-insoluble because of cross-linkage [19, 24].
Adverse reactions to HA consisting primarily of localized hypersensitivity reactions and injection site inflammation are uncommon and occur in 0.05–0.15% of cases (Friedman et al. [26]). The most common early or immediate, non-allergic, local, and transient side effects are injection site reactions such as pain, mild to moderate edema, ecchymoses and hematoma; palpability, hypercorrection, and bluish discoloration may also occur secondary to superficial injection of the implant and inappropriate technique [19, 27]. The incidence of such reactions has been noted in approximately 2% of treatments for Hylaform [28]. Delayed adverse reactions include hypersensitivity and granulomatous foreign body reaction that occur in up to 0.6% of cases [3, 5]. This may result from reactivity of some patients to the protein residues of bacterial or avian origin, or impurities from the cross-linking process [19]. Uncommon non-allergic adverse reactions include bacterial infection, aseptic or cold abscess, herpes reactivation [29], generalized scleromyxedema [30], scar sarcoidosis [31], interferon-induced systemic sarcoidosis in patients with chronic hepatitis C [32], and necrosis and livedoid pattern due to accidental arterial embolization [33]. Migration of the filler into a tunnel or track created by a large needle or cannula may manifest as a single and well-defined nodule 1–2 months after injection and may persist until resorption [34, 35].
HA is an inert and non-immunogenic filler and is usually resorbed within 4–6 months with no evidence of filler at nine months [19, 36]. However, a few cases of hypersensitivity and foreign body reaction have been documented, usually developing within 6–24 months [5–15, 35].
In this paper, we report three patients with a history of Restylane (Q Med, Uppsala, Sweden) injection to their lips who presented with lip nodules. Two other previously reported cases included female patients who presented similarly (Table 1). Histologic sections revealed pools of basophilic amorphous material within the fibro-fatty tissue of the lip typical for HA, unassociated with a foreign body reaction; one of our three cases was a fibroma with the HA present as an incidental finding at the base. The time period to development in the first two cases was 4 and 24 months, respectively. Case 2 was unusual in its exceptionally long period of persistence, similar to one other case in the literature [16]. Such persistence may be related to the cross-linking process, which makes Restylane more resistant to breakdown.
The basophilic nature of HA may be mistaken for the myxoid fibroplasias often seen around tumor islands in basal cell carcinomas [16, 17].
Conclusion
This is a report of three cases of lip nodules with a history of Restylane (Q Med, Uppsala, Sweden) injection into the lip. Such nodules may clinically resemble other conditions such as mucocele, fibroma, benign salivary gland lesions/tumors, and other benign soft tissue tumors. Histologically, HA filler, which is inert, is seen as pools of amorphous basophilic material unassociated with a foreign body reaction.
References
- 1.Redbord KP, Busso M, Hanke CW. Soft-tissue augmentation with hyaluronic acid and calcium hydroxyl apatite fillers. Dermatol Ther. 2011;24:71–81. doi: 10.1111/j.1529-8019.2010.01380.x. [DOI] [PubMed] [Google Scholar]
- 2.Balazs EA, Denlinger JL. Clinical uses of hyaluronan. Ciba Found Symp. 1989;143:265–275. doi: 10.1002/9780470513774.ch16. [DOI] [PubMed] [Google Scholar]
- 3.Piacquadio D, Jarcho M, Goltz R. Evaluation of hylan b gel as a soft tissue augmentation implant material. J Am Acad Dermatol. 1997;36:544–549. doi: 10.1016/S0190-9622(97)70241-X. [DOI] [PubMed] [Google Scholar]
- 4.Cosmetic Surgery National Data Bank Statistics 2004. Garden Grove, California: American Society for Aesthetic Plastic Surgery; 2005.
- 5.Lowe NJ, Maxwell CA, Lowe P, Duick MG, Shah K. Hyaluronic acid skin fillers: adverse reactions and skin testing. J Am Acad Dermatol. 2001;45:930–933. doi: 10.1067/mjd.2001.117381. [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann US, Clerici TJ. The histological aspects of fillers complications. Semin Cutan Med Surg. 2004;23:241–250. doi: 10.1016/j.sder.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Ghislanzoni M, Bianchi F, Barbareschi M, Alessi E. Cutaneous granulomatous reaction to injectable hyaluronic acid gel. Br J Dermatol. 2006;154:755–758. doi: 10.1111/j.1365-2133.2005.07074.x. [DOI] [PubMed] [Google Scholar]
- 8.Carruthers J, Carruthers A. A prospective, randomized, parallel group study analyzing the effect of BTX-A (Botox) and non animal sourced hyaluronic acid (NASHA, Restylane) in combination compared with NASHA (Restylane) alone in severe glabellar rhytides in adult female subjects: Treatment of severe glabellar rhytides with a hyaluronic acid derivative compared with the derivative and BTX-A. Dermatol Surg. 2003;29:802–809. doi: 10.1046/j.1524-4725.2003.29212.x. [DOI] [PubMed] [Google Scholar]
- 9.Andre P. Evaluation of the safety of a non-animal stabilized hyaluronic acid (NASH- Q-Medical, Sweden) in European countries: a retrospective study from 1997 to 2001. J Eur Acad Dermatol Venereol. 2004;18:422–425. doi: 10.1111/j.1468-3083.2004.00934.x. [DOI] [PubMed] [Google Scholar]
- 10.Sanchis-Bielasa JM, Bagan JV, Poveda R, Salvador I. Foreign body granulomatous reactions to cosmetic fillers: a clinical study of 15 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:237–241. doi: 10.1016/j.tripleo.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 11.Ferandez-Acenero MJ, Zamora E, Borbujo J. Granulomatous foreign body reaction against hyaluronic acid: report of a case after lip augmentation. Dermatol Surg. 2003;29:1225–1226. doi: 10.1111/j.1524-4725.2003.29392.x. [DOI] [PubMed] [Google Scholar]
- 12.Alijotas-Reig J, Garcia-Gimenez V. Delayed immune-mediated adverse effects related to hyaluronic acid and acrylic hydrogel dermal fillers: clinical findings, long-term follow-up and review of the literature. J Eur Acad Dermatol Venereol. 2008;22:150–161. doi: 10.1111/j.1468-3083.2007.02354.x. [DOI] [PubMed] [Google Scholar]
- 13.Weinberg MJ, Solish N. Complications of hyaluronic acid fillers. Facial Plast Surg. 2009;25:324–328. doi: 10.1055/s-0029-1243081. [DOI] [PubMed] [Google Scholar]
- 14.Requena L, Requena C, Christensen L, Zimmermann US, Kutzner H, Cerroni L. Adverse reactions to injectable soft tissue fillers. J Am Acad Dermatol. 2011;64:1–34. doi: 10.1016/j.jaad.2010.02.064. [DOI] [PubMed] [Google Scholar]
- 15.Wolfram D, Tzankov A, Piza-Katzer H. Surgery for foreign body reactions due to injectable fillers. Dermatology. 2006;213:300–304. doi: 10.1159/000096193. [DOI] [PubMed] [Google Scholar]
- 16.Bennett R, Taher M. Restylane persistent for 23 months found during Mohs micrographic surgery: a source of confusion with hyaluronic acid surrounding basal cell carcinoma. Dermatol Surg. 2005;31:1366–1369. doi: 10.1111/j.1524-4725.2005.31223. [DOI] [PubMed] [Google Scholar]
- 17.Anatelli F, Chapman MS, Brennick J. Amorphous basophilic deposit in the superficial dermis of the lip in an 80 year old. Am J Dermatopathol. 2010;32:306–309. doi: 10.1097/DAD.0b013e3181b9e5ab. [DOI] [PubMed] [Google Scholar]
- 18.American Society for Aesthetic Plastic Surgery. Cosmetic Surgery National Data bank: 2009 Statistics. www.surgery.org/sites/default/files/2009state.pdf. Accessed 22 July 2010. [DOI] [PubMed]
- 19.Romagnoli M, Belmontesi M. Hyaluronic acid-based fillers: theory and practice. Clin Dermatol. 2008;26:123–159. doi: 10.1016/j.clindermatol.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Ellis DA, Makdessian AS, Brown DJ. Survey of future injectables. Facial Plast Surg Clin North Am. 2001;9:405–411. [PubMed] [Google Scholar]
- 21.Waris E, Pakkanen M, Lassila K, Törmälä P, Konttinen YT, Suuronen R, et al. Alloplastic injectable biomaterials for soft tissue augmentation: a report on two cases with complications associated with a new material (DermaLive) and a review of the literature. Eur J Plast Surg. 2003;26:350–355. doi: 10.1007/s00238-003-0564-z. [DOI] [Google Scholar]
- 22.Fagien S. Facial soft-tissue augmentation with injectable autologous and allogenic human tissue collagen matrix (autologen and dermalogen) Plast Reconstr Surg. 2000;105:362–373. doi: 10.1097/00006534-200001000-00057. [DOI] [PubMed] [Google Scholar]
- 23.Laurent TC. Biochemistry of hyaluronan. Acta Otolaryngol Suppl. 1987;442:7–24. doi: 10.3109/00016488709102833. [DOI] [PubMed] [Google Scholar]
- 24.Monheit GD, Coleman KM. Hyaluronic acid fillers. Dermatol Ther. 2006;19:141–150. doi: 10.1111/j.1529-8019.2006.00068.x. [DOI] [PubMed] [Google Scholar]
- 25.Redbord KP, Busso M, Hanke CW. Soft tissue augmentation with hualuronic acid and calcium hydroxyl apatite fillers. Dermatol Ther. 2011;24:71–81. doi: 10.1111/j.1529-8019.2010.01380.x. [DOI] [PubMed] [Google Scholar]
- 26.Friedman PM, Mafong EA, Kauvar AN, Geronemus RG. Safety data of injectable nonanimal stabilized hyaluronic acid gel for soft tissue augmentation. Dermatol Surg. 2002;28:491–494. doi: 10.1046/j.1524-4725.2002.01251.x. [DOI] [PubMed] [Google Scholar]
- 27.Lowe NJ, Maxwell CA, Patnaik R. Adverse Reactions to dermal fillers: Review. Dermatol Surg. 2005;31:1616–1625. [PubMed] [Google Scholar]
- 28.Pollack SV. Some new injectable dermal filler materials: Hyaloform®, Restylane® and Artecoll®. J Cutan Med Surg. 1999;Suppl 4:S27–S35. [PubMed] [Google Scholar]
- 29.Shafir R, Amir A, Gur E. Long-term complications of facial injections with Restylane (injectable hyaluronic acid) Plast Reconstr Surg. 2000;106:1215–1216. doi: 10.1097/00006534-200010000-00048. [DOI] [PubMed] [Google Scholar]
- 30.Rongioletti F, Cattarini G, Sottofattori E, Rebora A. Granulomatous reaction after intradermal injections of hyaluronic acid gel. Arch Dermatol. 2003;139:815–816. doi: 10.1001/archderm.139.6.815. [DOI] [PubMed] [Google Scholar]
- 31.Dal Sacco D, Cozzani E, Parodi A, Rebora A. Scar Sarcoidosis after hyaluronic acid injection. Int J Dermatol. 2005;44:411–412. doi: 10.1111/j.1365-4632.2005.01930.x. [DOI] [PubMed] [Google Scholar]
- 32.Descamps V, Landry J, Frances C, Marinho E, Ratziu V, Chosidow O. Facial cosmetic filler injections as possible target for systemic Sarcoidosis in patients treated with interferon for chronic hepatitis: two cases. Dermatology. 2008;217:81–84. doi: 10.1159/000128281. [DOI] [PubMed] [Google Scholar]
- 33.Schanz S, Schippert W, Ulmer A, Rassner G, Fierlbeck G. Arterial embolization caused by injection of hyaluronic acid (Restylane) Br J Dermatol. 2002;146:928–929. doi: 10.1046/j.1365-2133.2002.04707.x. [DOI] [PubMed] [Google Scholar]
- 34.Lorenzi C, Weinberg M, Solish N, Swift A. Multicenter study of the efficacy and safety of subcutaneous non-animal-stabilized hyaluronic acid in aesthetic facial contouring: interim report. Dermatol Surg. 2006;32:205–211. doi: 10.1111/j.1524-4725.2006.32035.x. [DOI] [PubMed] [Google Scholar]
- 35.Lemperle G, Gauthier-Hazan N, Wolters M, Eiscmann-Klein M, Zimmermann U, Duffy DM. Foreign body granulomas after all injectable dermal fillers: part 1. Possible causes. Plast Reconstr Surg. 2009;123:1842–1863. doi: 10.1097/PRS.0b013e31818236d7. [DOI] [PubMed] [Google Scholar]
- 36.Lemperle G, Morhenn V, Charrier U. Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthetic Plast Surg. 2003;27:354–366. doi: 10.1007/s00266-003-3022-1. [DOI] [PubMed] [Google Scholar]