Abstract
Carcinoma cuniculatum (CC) is a rare, distinct clinico-pathological variant of squamous cell carcinoma (SCC) that is defined histologically by the characteristic infiltrative pattern of a deep, broad, and complex proliferation of stratified squamous epithelium with keratin cores and keratin-filled crypts. Herein, we present a case report of CC of the oral tongue and discuss its diagnosis, management, and outcome, as well as briefly review the world literature. To our knowledge, this is the first documented case of CC of the tongue to be reported in the English literature. We draw attention to its clinico-pathological features and highlight that awareness of this entity as a distinct variant of SCC facilitates its correct management.
Keywords: Carcinoma cuniculatum, Verrucous carcinoma, Squamous cell carcinoma, Tongue
Introduction
Carcinoma cuniculatum (CC) is a rare, distinct clinico-pathological variant of squamous cell carcinoma (SCC) [1]. It is defined histologically by the characteristic infiltrative pattern of a deep, broad, and complex proliferation of stratified squamous epithelium with keratin cores and keratin-filled crypts. Notably, there is a consistent absence of any significant cytological atypia which, in the past, has led to failure in recognising this neoplasm as malignant. The tumour’s name, suggested by Aird et al. [2] in the first description in 1954 refers to its characteristic tendency to “burrow” into the underlying connective tissue. The original authors described this tumour as being restricted to the sole of the foot but since then, involvement of various other cutaneous sites has been reported. However, only 22 cases to date have been reported in sites within the upper aerodigestive tract (Table 1). Within the oral cavity, the majority of reported cases have presented in the alveolar mucosa or hard palate. We present a case of CC of the oral tongue, which, to our knowledge, is the first documented case in the English literature.
Table 1.
Summary of previously published reports of carcinoma cuniculatum arising in the upper aerodigestive tract
| Author, year | Age, sex | Site | Metastasis | Pre-operative diagnosis |
|---|---|---|---|---|
| Flieger and Owinski, 1977 [14] | 50, M | Maxillary molar region and sinus | N0 M0 | Osteomyelitis |
| 60, M | Maxillary molar region | N0 M1 | Tuberculosis | |
| 9, M | Maxillary premolar region | N0 M0 | Not stated | |
| 69, F | Hard palate | N0 M0 | Not stated | |
| Kahn et al., 1991 [4] | 62, M | Maxillary alveolus and sinus | N0 M0 | Cystic lesion |
| 49, M | Submandibular space | N0 M0 | Not stated | |
| 52, M | Anterior floor of mouth | N0 M0 | Not stated | |
| 54, F | Mandibular molar region | N0 M0 | Not stated | |
| Delahaye et al., 1994 [3] | 51, M | Retromolar trigone | N1 M0 | Squamous cell carcinoma |
| 55, M | Tonsil, floor of mouth | N0 M0 | Verrucous carcinoma | |
| 63, M | Subglottic larynx | N0 M0 | Not stated | |
| 31, M | Hard palate | N0 M0 | Not stated | |
| 52, M | Buccal mucosa | N0 M0 | Not stated | |
| Huault et al., 1998 [15] | 55, M | Mandibular alveolus | N1 M0 | Hyperkeratotic papilloma |
| Allon et al., 2002 [9] | 56, M | Maxillary gingiva | N0 M0 | Not stated |
| De Petris et al., 2005 [6] | 73, M | Oesophagus | N0 M0 | Verrucous carcinoma |
| 58, M | Oesophagus | N0 M0 | Verrucous carcinoma | |
| Puxeddu et al., 2008 [16] | 72, M | Larynx | N0 M0 | Pseudoepitheliomatous hyperplasia |
| Kruse and Graetz, 2009 [17] | 74, F | Maxillary alveolus | N0 M0 | Squamous cell carcinoma |
| Pons et al., 2010 [18] | 72, M | Mandibular molar region | N0 M0 | Inflammatory granuloma |
| 82, M | Mandibular molar region | N0 M0 | Not stated | |
| 43, M | Mandibular retromolar region | N0 M0 | Keratocyst |
Case Report
Clinical Presentation
A 61-year-old white male presented with a 1 year history of painless dysphagia and difficulty with articulation. On intra-oral examination, the tongue was notably swollen and firm with an ill-defined mass within the body of the tongue without overlying dorsal mucosal ulceration. There was a striking tethering effect causing marked restriction of tongue mobility. No palpable cervical lymphadenopathy was noted. Following examination under general anaesthesia and incisional biopsy, the Head & Neck Oncology Multidisciplinary Team treatment recommendation was for primary surgical ablative management.
Radiological Assessment
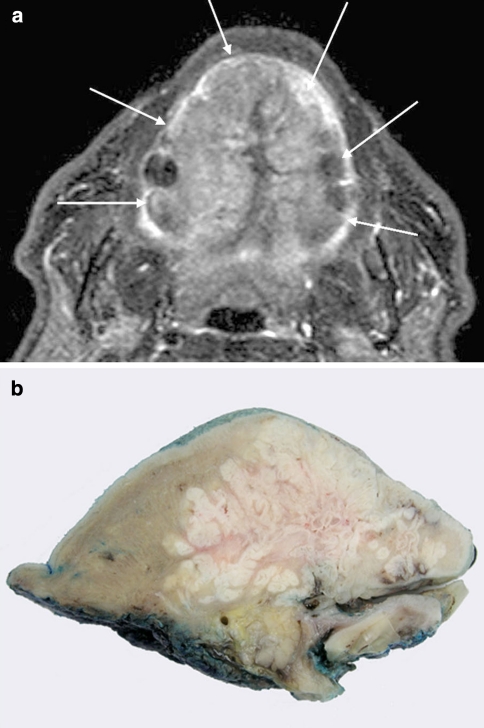
Magnetic resonance imaging was performed and demonstrated an extensive mass that replaced almost all of the oral tongue sparing the extrinsic muscles apart from the superior genioglossus (Fig. 1a). Despite its large size, there was no extension posteriorly into the tonsil and glosso-tonsillar sulcus or inferior extension to the floor of mouth. There was no radiographic evidence of cervical lymph node involvement.
Fig. 1.
Radiological and macroscopic appearances. a T1 STIR axial MRI through oral cavity and tongue demonstrating extensive tumour (white arrows) involving the whole dorsal surface of the tongue. b Macroscopic appearance of a sagittal slice of the resection specimen demonstrating a complex endophytic pattern of infiltration
Surgical Management
The ablative procedure included a total glossectomy, via a cervical visor approach, with bilateral selective neck dissection. A drop-down procedure facilitated the tongue and floor of mouth contents to be pulled through into the neck by osteotomy of the mandibular mental insertion of the digastric muscles. The tongue could then be removed in continuity with a bilateral selective neck dissection of levels I-III. The digastric muscle insertions were returned to their original position at the end of the procedure. The reconstructive procedure comprised an antero-lateral thigh microvascular flap anastomosed to the facial artery and internal jugular vein. This was domed in lateral and antero-posterior dimensions to facilitate the first stage of deglutition. A period of intensive post-operative swallowing therapy was instituted following decannulation of the temporary tracheostomy.
Histopathological Evaluation
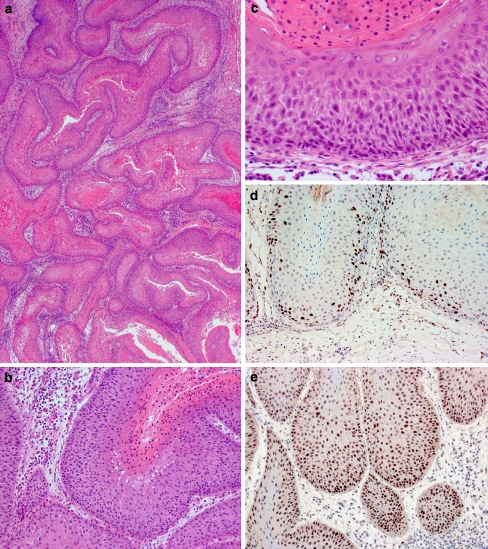
The subtotal glossectomy was received in continuity with a bilateral supra-omohyoid neck dissection. On sectioning, the firm, white, irregularly circumscribed tumour was seen to infiltrate muscle in an inverted endophytic pattern. The epicentre of the tumour appeared to be located in the ventral tongue, from which it infiltrated, superiorly, the full thickness of the intrinsic lingual musculature to abut the dorsal surface (Fig. 1b). Microscopically, this was composed of a complex network of stratified squamous epithelium with keratin-filled cores and crypts demonstrating a characteristic burrowing pattern (Fig. 2a, b). The crypts were lined by well-differentiated stratified squamous epithelium demonstrating no more than mild atypia in the form of basal cell crowding, anisonucleosis and nuclear pleomorphism (Fig. 2c). Many of the crypt lumina contained keratin-rich neutrophil microabscesses. Focally, there was fusion of the tumour with the ventral tongue mucosa, which showed no ulceration. However, in these areas, the surface epithelium demonstrated mild epithelial dysplasia. The tumour was excised by a clearance of 5 mm and there were no metastases in any of the 62 lymph nodes retrieved from the upper cervical neck dissection.
Fig. 2.
Pathological features of carcinoma cuniculatum of the tongue. a Low power photomicrograph demonstrating an anastomosing network of squamous epithelium (H&E, original magnification ×20); b Medium power photomicrograph demonstrating keratin-filled crypts (H&E, original magnification ×100); c High power photomicrograph demonstrating architectural and cytological atypia (H&E, original magnification ×200). d–e. Immunohistochemistry demonstrating Ki-67 expression in approximately 15% of basal and suprabasal cells (d) and strong p53 expression in the majority of basal and suprabasal cells, respectively (e, original magnification ×100)
Ancillary studies revealed that the tumour was negative for human papillomavirus (HPV) by immunohistochemistry using an antibody against a broad spectrum of subtypes (DAKO, M3528) and by in situ DNA hybridisation using probes against low- and high-risk subtypes (Ventana Medical Systems, INFORM HPV Family 6 and Family 16). Immunohistochemical staining for the proliferation marker Ki-67 (Novocastra,KI67-MM1-L-CE) highlighted approximately 15% of cells in cycle, all limited to the basal and suprabasal layers (Fig. 2d). Immunohistochemistry for p53 (Novocastra, P53-DO7-L-CE), which recognizes wild-type and mutant forms, demonstrated strong positivity in >90% of basal and suprabasal cells (Fig. 2e). Image-based ploidy analysis performed using a Fairfield analyser (Hamamatsu Photonics K.K., Japan, C4742-95) revealed the tumour to be diploid.
Follow-up
At review 24 months postoperatively, the patient remains clinically and radiologically disease-free with excellent recovery of his speech and swallowing function. He is on soft oral diet without need for tube-dependent nutritional support and video fluoroscopy revealed no aspiration during swallowing. He has resumed his professional activities as university lecturer. Long term surveillance will be continued with 6 month interval ultrasound scans and regular clinical follow-up.
Discussion
The World Health Organisation (WHO) recognises CC as a distinct variant of SCC [1]. CC can be distinguished from other forms of well-differentiated SCC by its characteristic burrowing inverted pattern of complex keratin-filled crypts with neutrophil microabscess formation. The tumour has previously been described under an array of synonyms including epithelioma cuniculatum, Buschke-Lowenstein tumour and inverted verrucous carcinoma (VC). The plethora of synonyms together with its rarity may have, at least in part, contributed to a general lack of awareness of the distinctiveness of CC from other variants of SCC. CC of the oral cavity occurs over a wide age range (9–87 years), with a mean of 50 years and a male preponderance (M: F = 3:1).
Of the cases occurring within the oral cavity, 78% presented on the alveolar gingiva or hard palate, where burrowing into underlying bone is a feature. Reports of cases arising at other oral sites, such as the tongue, floor of the mouth, and buccal mucosa have appeared as three cases in the French literature [3, 4] and one case in a German language publication [5]. However, these reports do not specify whether CC arose primarily within the tongue or whether there was direct spread form adjacent sites within the oral cavity.
CC may be confused clinically and histologically with VC. Although the WHO distinguishes between these two entities as separate variants of SCC [1], in the past, both these terms have been used interchangeably. It is possible that the failure to apply defining criteria to distinguish VC and CC may have resulted in the latter being underreported. Both are locally aggressive, well-differentiated forms of SCC that may have endophytic and exophytic components. In fact, CC has been erroneously diagnosed as VC at the time of incisional biopsy [3, 6]. However, despite these superficial similarities, there are sufficient clinico-pathological differences that warrant distinction between the two entities.
CC is characterised by the formation of keratin-filled crypts extending down into the supporting tissues, including skeletal muscle and bone. Neutrophil microabscesses are frequently found within these channels. By contrast, VC features a broad “pushing” infiltrative front that lacks the complex interconnecting network of channels characteristic of CC. In addition, mandibular invasion by CC may result in a large radiolucent cavity, whereas VC abutting the jawbone tends to cause surface resorption without invasion. Furthermore, foci of conventional SCC may be found in up to 20% of VC [7], but this phenomenon has not been reported in CC. Nevertheless, distinguishing between the two entities is important in order to avoid under-treatment, since CC is likely to be more locally aggressive compared to VC [8].
Alcohol and tobacco have been implicated as aetiological factors in the development of CC of the upper aerodigestive tract [9], but the rarity of the entity precludes any meaningful correlation. Whilst the presence of HPV DNA has been demonstrated in CC at cutaneous sites [10, 11], immunohistochemistry for HPV capsid protein, expressed in HPV types 6, 11, 16, 18, 31, 33, 42, 51, 52, 56 and 58, as well as in situ hybridisation using probes against high-risk (16, 18, 31,33, 35, 39, 45, 51, 52, 56, 58, and 66) and low-risk (6 and 11) HPV subtypes, were both negative in the current case, in keeping with other reports of CC in the upper aerodigestive tract [6, 9]. Therefore, the association of HPV and CC remains, at best, a site-specific observation.
A single previous attempt failed to demonstrate the expression of p53 in CC by immunohistochemistry [9]. By contrast, in the current case, p53 over-expression was detected in >90% of basal and suprabasal cells (Fig. 2e). This disparity, may in part, be explained by methodological differences (i.e, antigen retrieval), clonal variation, and/or variations in the degree of cytological atypia between the two cases, since p53 expression often correlates with the degree of dysplasia [12]. The over-expression of p53 in the current case, therefore, may be explained by the greater degree of atypia. However, it is not certain whether p53 is of wild-type or mutant form, and the significance of HPV-independent p53 accumulation in the aetiopathogenesis of CC remains to be elucidated. To date, there have been no reports of the DNA ploidy status of CC prior to the current report. The diploid nature of this tumour suggests a more favourable clinical outcome [13].
The treatment of choice for CC is complete surgical resection, which provides a highly favourable outcome. There was no record of recurrence in any of the 19 cases of CC of the upper aero-digestive tract within the follow-up period ranging from 7 to 96 months. Only one patient with regional nodal metastases has been described in CC of the upper aerodigestive tract [3], despite the long-standing nature and frequently advanced size of these tumours. In keeping with this phenomenon, there was no evidence of metastasis in the current case. A bilateral supra-omohyoid neck dissection was included in the management of the current case in order to facilitate surgical access for a sub-total glossectomy.
In conclusion, we report a case of CC of the tongue and draw attention to its distinct clinico-pathological features. CC needs to be distinguished from other variants of SCC since recognition of this entity and awareness of its clinical behaviour lead to the appropriate management. Furthermore, the clinico-pathological features of this case, in keeping with previous reports, suggests a favourable prognostic outcome of this entity despite advanced disease stage at presentation.
References
- 1.Johnson N, Franceschi S, Ferlay J, et al. Squamous cell carcinoma. In: Barnes EL, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics of head and neck tumours. Kleihues P, Sobin LH, series eds. World Health Organization Classification of Tumours. Lyon, France: IARC Press, 2005. p. 168–175.
- 2.Aird I, Johnson HD, Lennox B, et al. Epithelioma cuniculatum: a variety of squamous carcinoma peculiar to the foot. Br J Surg. 1954;42:245–250. doi: 10.1002/bjs.18004217304. [DOI] [PubMed] [Google Scholar]
- 3.Delahaye JF, Janser JC, Rodier JF, et al. Cuniculatum carcinoma. 6 cases and review of the literature. J Chir (Paris) 1994;131:73–78. [PubMed] [Google Scholar]
- 4.Kahn JL, Blez P, Gasser B, et al. Carcinoma cuniculatum. Apropos of 4 cases with orofacial involvement. Rev Stomatol Chir Maxillofac. 1991;92:27–33. [PubMed] [Google Scholar]
- 5.Gassler N, Helmke B, Schweigert HG, et al. Carcinoma cuniculatum of the oral cavity. A contribution to the differential diagnosis of potentially malignant papillary lesions of mouth mucosa. Pathologe. 2002;23:313–317. doi: 10.1007/s00292-002-0525-5. [DOI] [PubMed] [Google Scholar]
- 6.Petris G, Lewin M, Shoji T. Carcinoma cuniculatum of the esophagus. Ann Diagn Pathol. 2005;9:134–138. doi: 10.1016/j.anndiagpath.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Medina JE, Dichtel W, Luna MA. Verrucous-squamous carcinomas of the oral cavity. A clinicopathologic study of 104 cases. Arch Otolaryngol. 1984;110:437–440. doi: 10.1001/archotol.1984.00800330019003. [DOI] [PubMed] [Google Scholar]
- 8.Odell EW. Morgan PR.Biopsy pathology of the oral tissues. London: Chapman & Hall Medical; 1997. [Google Scholar]
- 9.Allon D, Kaplan I, Manor R, et al. Carcinoma cuniculatum of the jaw: a rare variant of oral carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:601–608. doi: 10.1067/moe.2002.126913. [DOI] [PubMed] [Google Scholar]
- 10.Wastiaux H, Dreno B. Recurrent cuniculatum squamous cell carcinoma of the fingers and virus. J Eur Acad Dermatol Venereol. 2008;22:627–628. doi: 10.1111/j.1468-3083.2007.02416.x. [DOI] [PubMed] [Google Scholar]
- 11.Knobler RM, Schneider S, Neumann RA, et al. DNA dot-blot hybridization implicates human papillomavirus type 11-DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33–37. doi: 10.1002/jmv.1890290107. [DOI] [PubMed] [Google Scholar]
- 12.Abbas NF, Labib El-Sharkawy S, Abbas EA, et al. Immunohistochemical study of p53 and angiogenesis in benign and preneoplastic oral lesions and oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:385–390. doi: 10.1016/j.tripleo.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Rubio Bueno P, Naval Gias L, Garcia Delgado R, et al. Tumor DNA content as a prognostic indicator in squamous cell carcinoma of the oral cavity and tongue base. Head Neck. 1998;20:232–239. doi: 10.1002/(SICI)1097-0347(199805)20:3<232::AID-HED8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Flieger S, Owinski T. Epithelioma cuniculatum an unusual form of mouth and jaw neoplasm. Czas Stomatol. 1977;30:395–401. [PubMed] [Google Scholar]
- 15.Huault M, Laroche C, Levy J, et al. Epithelioma cuniculatum. Apropos of a case in the anterior gingiva with involvement of the mandibular symphyseal bone and reconstruction using a fibular osteocutaneous flap and integrated implants. Rev Stomatol Chir Maxillofac. 1998;99:143–148. [PubMed] [Google Scholar]
- 16.Puxeddu R, Cocco D, Parodo G, et al. Carcinoma cuniculatum of the larynx: a rare clinicopathological entity. J Laryngol Otol. 2008;122:1118–1123. doi: 10.1017/S0022215107000163. [DOI] [PubMed] [Google Scholar]
- 17.Kruse AL, Graetz KW. Carcinoma cuniculatum: a rare entity in the oral cavity. J Craniofac Surg. 2009;20:1270–1272. doi: 10.1097/SCS.0b013e3181ace06b. [DOI] [PubMed] [Google Scholar]
- 18.Pons Y, Kerrary S, Cox A, et al. Mandibular cuniculatum carcinoma: Apropos of 3 cases and literature review. Head Neck. 2010. doi:10.1002/hed21493. [DOI] [PubMed]