Abstract
G protein-coupled receptors (GPCRs) play critical roles in cellular signal transduction and are important targets for therapeutics. Although these receptors have been intensely studied for quite some time, our understanding about their mechanism of action is still incomplete. GPCR activity has traditionally been viewed within the context of two-state models where the receptor is in equilibrium between a single inactive state and a single active state. This framework is too simple and restrictive to accommodate more recent observations made on these receptors, which instead point to a situation where the receptor can adopt several different active conformational substates with distinct functional effects. Structural and functional evidence for this emerging view is presented in this review. Implications of this emerging view in rationalizing diseased states and in drug discovery are also discussed.
Keywords: Conformational substates, drug action, functional selectivity, receptor activation, receptor pharmacology, membrane protein, signal transduction, two-state model
INTRODUCTION
Membrane proteins serve an important role in biology by transmitting information between the external environment and the inside of the cell. Membrane proteins form the largest class of therapeutic targets and therefore represent the largest portion of the “druggable genome” [1]. The largest family of membrane proteins, and also one of the largest groups of therapeutic targets, is the G protein-coupled receptor (GPCR) superfamily [1-3]. Despite the central role that GPCRs play in biology and drug discovery, our understanding about their mechanism of action is still incomplete.
GPCRs span biological membranes via seven transmembrane alpha helices. In the classical view of GPCR signaling, agonist binding, or a photon of light in the case of the visual pigment rhodopsin, activates the receptor to initiate the signaling cascade. The activated receptor can then promote the activation of a heterotrimeric G protein coupled at the intracellular surface, which then goes on to regulate downstream effectors to generate the cellular response. Signaling is terminated by a competing set of events that deactivate the receptor. These events include phosphorylation of the receptor by GPCR kinase (GRK), binding of arrestin to the cytoplasmic surface of the receptor, and, in most systems, internalization of the receptor. The current view of GPCR signaling has become considerably more complex than that of the classical view [4]. Contributing to this complexity are issues directly related to the activation mechanism of the receptor. In this review, an overview will be provided for an emerging view that updates the classical view of GPCR activation; namely, the notion that GPCRs function via multiple active conformational substates rather than a single active state.
TWO-STATE MODEL OF GPCR ACTIVITY
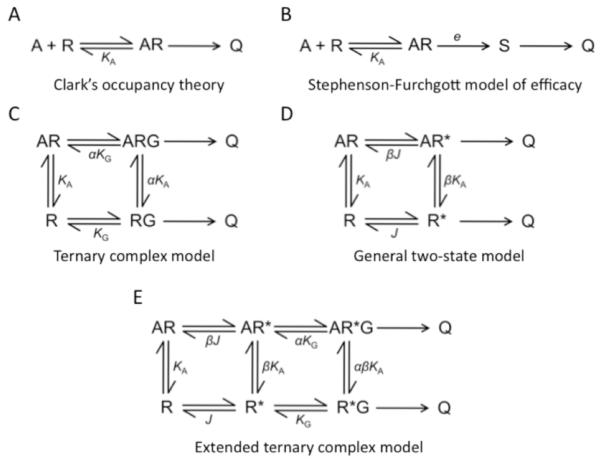
Efforts to gain mechanistic insight about GPCR activity began even before the molecular identity of these receptors was known through the formulation of mathematical models describing dose-response relationships [5-7]. Common among these early models was the idea that the receptor must bind and form a complex with the agonist to generate a cellular response (Figs. 1A and 1B) [8-10]. Besides this idea, these types of schemes provided no mechanistic details about events occurring at the level of the receptor; that is, the models are ambiguous as to what the agonist is doing to the receptor molecule to generate the cellular response.
Fig. (1). Models describing GPCR action.
(A), The earliest model describing the action of GPCRs was Clark’s occupancy theory [8]. In this model, the agonist (A) binds receptor (R) to from a complex (AR), which promotes the cellular response (Q). (B), Clark’s model was later updated to introduce a dimensionless quantity called the stimulus (S) and the concept of efficacy (e) [9, 10]. (C), The ternary complex model introduced the idea of a third component involved in GPCR signaling, the heterotrimeric G protein (G) [11]. The receptor must be in complex with the G protein to generate a cellular response. (D), The general two-state model introduced the idea that the receptor exists in two states, an inactive state (R) and an active state (R*) [15]. R* is the state that is capable of generating a cellular response. (E), The extended ternary complex model is a combination of the ternary complex model and general two-state model [13].
The availability of radioligands and molecular biology would provide access to additional insights resulting in updates to these earlier models. The complex patterns observed in ligand binding curves obtained at graded concentrations of agonist and the sensitivity of those patterns to guanyl nucleotides led to the formulation of the ternary complex model (Fig. 1C) [11]. This model accommodated the allosteric effect of G proteins on the receptor and pointed to an agonist-promoted complexing of the receptor to the G protein required for the generation of the cellular response. Even with the inclusion of effects of the G protein, the ternary complex model was still ambiguous as to what is occurring at the level of the receptor. The emergence of the notion of constitutive activity and inverse agonism would result in a more explicit description of the action of agonists on the receptor in the form of two-state models (Fig. 1D) [12-16]. This description included the idea that the receptor exists in two states, an inactive state (R) and an active state (R*), that are intrinsic properties of the receptor [17]. The general two-state model was first considered in pharmacology to describe the open and closed states of ligand-gated ion channels [18]. This general two-state model was combined with the ternary complex model to include effects of the G protein, which resulted in the extended ternary complex model and the cubic ternary complex model (Fig. 1E) [13, 16]. These latter models are the most updated mechanistic schemes that are commonly used to rationalize the activity of GPCRs.
Common in these updated two-state model descriptions of GPCR activity is the notion that ligands act by shifting the equilibrium between R and R* and that the activated R* state leads to the cellular response by coupling to the G protein. Ligands binding at the orthosteric binding site have traditionally been classified as agonists, partial agonists, inverse agonists, and antagonists [19, 20]. Within the context of two-state models, inverse agonists shift the equilibrium in favor of the R state while agonists shift the equilibrium in favor of the R* state. The efficacy of the agonist is determined by how effectively it can shift the equilibrium towards the R* state. Antagonists are neutral and do not shift the equilibrium in either direction.
The two-state models of GPCR activity led to the expectation that conformational changes serve as an “on-off” switch allowing the receptor to switch between an inactive state and an active state. As biochemical and biophysical approaches emerged allowing for indirect structural insights about GPCRs, such as spin labeling, fluorescence quenching, sulfhydryl reactivity, and disulfide cross-linking methods, information about these expected conformational changes began to surface [21-24]. These earlier structural studies predicted an activation mechanism occurring via conformational changes within the transmembrane region of the receptor that include rigid body movements and rotation of transmembrane helices. Most recently, some of the challenges preventing the generation of crystal structures have begun to be overcome allowing for the determination of high-resolution structures for several GPCRs [25]. Some of the crystal structures of the activated state of GPCRs that are beginning to emerge display some of the predicted conformational changes expected from previous indirect biochemical and biophysical studies [26].
Within the context of two-state models, the activity of the receptor is defined by the equilibrium between R and R*. Thus, the level of receptor accumulated in either the inactive or active state will dictate the various facets of GPCR activity such as efficacy and differential modulation of signaling pathways. It is becoming apparent that this simple model is too restrictive to fully describe the increasing complexity observed for GPCR activity. Emerging evidence is pointing to a more complex scenario for receptor activation that includes multiple active conformational substates rather than a single active state as predicted in traditional two-state models.
MULTIPLE ACTIVE CONFORMATIONAL SUBSTATES
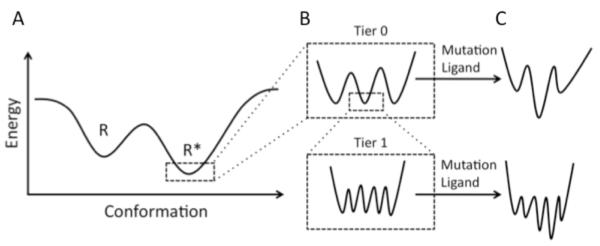
Energy landscapes have previously been suggested as a useful tool to understand GPCR activation [27, 28]. A similar approach is used here to describe the idea of conformational substates, which allows a better discussion of how the dynamic nature of GPCRs contributes to activation. The hierarchical classification of conformational substates has been adopted from a description of myoglobin function [29], and similar terminology and conventions are used here. Fig. (2A) depicts an idealized energy landscape with smooth hills and valleys that are consistent with two-state models. Each valley represents a minima corresponding to R and R* states of the receptor. The positions and the shape of these valleys can be modulated by factors affecting receptor function such as ligands, G protein, mutation, or other environmental perturbations. This energy landscape depiction, however, is too simplistic and the energy landscape that more closely describes GPCR function will be more complex. The bottom of each valley likely is not smooth but rather rugged (Fig. 2B), because of the presence of multiple conformational substates. The conformational substates can be separated into multiple tiers. Only the first two tiers are discussed here, however, more tiers may exist as has been suggested in the case of myoglobin [29].
Fig. (2). Conformational substates.
(A), An idealized energy landscape is shown for the inactive (R) and active (R*) states. In classical two-state models, the valleys are smooth with only a single minima corresponding to either the R or R* state. (B), The valleys in (A) are likely rugged with multiple minima corresponding to conformational substates. There are likely multiple tiers of conformational substates represented in each of these valleys. Only two tiers are represented here. (C), Mutations, ligands, and other factors can remodel the energy landscape so that one or more of the conformational substates becomes more energetically favorable. The figure and terminology are adapted from [29].
Conformational substates in tier 0 represent discrete structures. Thus, the mean structure of each substate in this tier will be different from others and therefore each mean structure can represent a distinct class. Substates with similar minima, and therefore isoenergetic, may be equally populated while those with higher minima are less populated. Perturbations, such as mutations or ligand binding, may reshape each minima causing one substate to be favored over others (Fig. 2C). Tier 0 conformational substates will be detectable in different crystal structures that are obtained at different experimental conditions that preferentially favors one of the substates over the others.
In contrast to tier 0 conformational substates, substates in tier 1 cannot be individually classified and require a statistical approach to be studied [29]. The substates in this tier represent those arising from thermal fluctuations, which alter the position of atoms around their position in the mean structures of tier 0 substates. The conformational substates in tier 1 have not been considered as much as those in tier 0. However, their occurrence is readily observable in NMR and molecular dynamics simulations and they play an important role in the function of proteins [30-33].
Several lines of investigation from both structural and functional perspectives point to a scenario where GPCRs form multiple active conformational substates. In the next section, evidence for active conformational substates of GPCRs in each of the two tiers will be overviewed. The two-dimensional energy landscapes used in the present discussion provide a good starting point to define the concept of conformational substates and will be useful to advance our understanding of receptor activation. However, in reality, energy landscapes describing GPCR function are three dimensional and will be even more complex (e.g, [34]). The trajectory leading to the bottom of each well likely is not smooth but instead rugged, resulting in inactive intermediates forming sequentially prior to achieving the active conformational substates (e.g., [27]). Moreover, GPCRs can exist as oligomers and form complexes with other signaling molecules [35, 36], which can provide an additional layer of complexity to energy landscapes describing GPCR activation. Insights into the energy landscape describing GPCR activation and the remodelling of the energy landscape upon ligand binding are beginning to emerge from computational studies [37, 38]. The focus of the discussion here is on the conformational substates of the active state of the receptor, however, conformational substates of the inactive state also likely are present as well.
EVIDENCE FOR ACTIVE CONFORMATIONAL SUBSTATES OF GPCRS
Functional Selectivity
The repertoire of signaling activities by GPCRs is considerably more complex than envisioned in the early periods of research that led to the formulation of classical models (Fig. 1). GPCR signaling can no longer be viewed as a single pathway consisting of a linear sequence of events. A single GPCR can affect several different biochemical pathways through different mechanisms. In classical view of GPCR signaling, each receptor couples to and signals via a single class of heterotrimeric G proteins to promote a specific cellular response [39]. Heterotrimeric G proteins can be broadly categorized into four major classes based on the identity of the β subunit: Gs, Gi/o, Gq/11, and G12/13 [40]. It is now clear that G proteins exhibit some promiscuity and a single GPCR can couple to and signal via G proteins from multiple classes [41], which results in the propagation of signals through multiple bioch emical pathways to achieve different cellular responses.
Also in the classical view, GPCRs signal exclusively via heterotrimeric G proteins to generate the cellular response. It is now apparent that G protein-independent signaling can occur within a cell via arrestin molecules to increase the diversity of cellular responses a single GPCR can generate. Arrestins in the classical view serve as proteins that bind phosphorylated GPCRs deactivate and, in most instances, internalize the receptor [42, 43]. The role arrestins play in the cell has now expanded to also include signaling functions. GPCRs can signal independently of G proteins via arrestins to mitogen-activated protein (MAP) kinases, which can regulate chemotaxis, apoptosis, cancer metastasis, and protein translation [44, 45]. Because of the capability of receptors to signal independently of the G protein, GPCRs are now sometimes referred to as seven-transmembrane receptors [46].
How do GPCRs navigate through this complexity to achieve specific and robust signaling? This complexity can partially be simplified in cells by excluding signaling molecules from interacting with GPCRs either at the level of protein synthesis or by restricting access through the formation of microdomains in the membrane such as caveolae or lipid rafts [47, 48]. Even with exclusion mechanisms in place, access to multiple signaling proteins in any given environment is still likely present and therefore GPCRs will still face choices of which signaling proteins to partner with in a particular cell or microdomain.
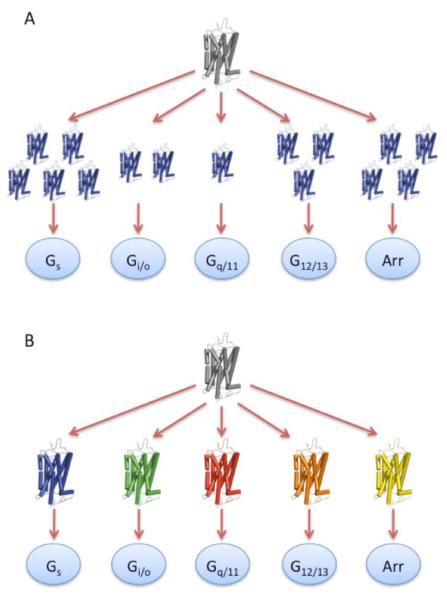
The classic two-state framework, where the receptor can adopt only a single active state, is restrictive in how GPCRs can achieve a diverse array of signaling properties within a single cell. This diversity can only be achieved by the strength of the signal promoted by agonists [49], which is dependent on how many receptors achieve the active state. Thus, the decision of which G protein to couple to or whether to signal via arrestin must be a direct result of the number of receptors present in the active state (Fig. 3A). The strength of the signal may account for some of the diversity observed in the signals generated by GPCRs [49]. However, observations from several functional studies cannot be accommodated within a two-state framework, but instead point to multiple active conformational substates that selectively couple the receptor to different biochemical pathways (Fig. 3B) (reviewed in [50-56]).
Fig. (3). Signaling diversity of GPCRs: strength of signal versus conformational substates.
(A), In a two-state view of GPCR activity, where the receptor displays only a single active state, the decision of which G protein to couple to or whether to choose a G protein-independent pathway via arrestin binding must be determined by the number of receptor molecules that achieve the active state (i.e., strength of signal). (B), In a conformational substate view, since a single receptor can adopt several different active conformational substates (depicted by different colored receptors), the decision of which G protein to couple to or whether to choose a G protein-independent pathway via arrestin binding can be determined by which conformational substate is attained.
One of the first observations from functional assays that indicated GPCRs select between different biochemical pathways by attaining specific conformational substates came from studies on the type-1 pituitary adenyly cyclase-activating polypeptide (PACAP) receptor [57]. Type-1 PACAP receptors can signal by coupling to the Gs class of G proteins to activate adenylate cyclase and generate cyclic AMP or to the Gq/11 class of G proteins to activate phospholipase C and generate inositol phosphate [40]. The relative potencies of the agonists PACAP-27 and PACAP-38 are reversed depending on whether signaling is viewed from the vantage point of cyclic AMP production or inositol phosphate production. This reversal of potencies cannot be accounted for by either exclusion mechanisms or strength of signals, but instead points to a situation where the different agonists promote distinct conformational substates that differentially couple with and signal to the Gs and Gq/11 class of G proteins [49].
The notion that different active conformational substates underlie the ability of GPCRs to achieve a diverse array of signaling properties is often referred to as functional selectivity. The concept of functional selectivity has been around for quite some time and is also referred to by several alternate names including “stimulus (or agonist) trafficking,” “biased agonism,” and “collateral efficacy” [49, 55]. Diversity within the context of functional selectivity arises because ligands promote distinct conformational substates, which in turn dictates the class of G protein to couple to or whether a G protein-independent pathway will be chosen (Fig. 3B). The result of functional selectivity is that the pharmacological properties of a ligand will depend upon the biochemical pathway that is being investigated. Thus, the relative potencies and efficacies of a set of ligands will be different depending on the biochemical pathway and the traditional classification of ligands may no longer hold true in some instances since antagonists or inverse agonists for one biochemical pathway may serve as an agonist for another pathway [20].
Biophysical Studies
Several biophysical studies on adrenergic receptors have provided structural evidence for multiple active conformational substates [58-64]. Single-molecule fluorescence spectroscopy of purified β2 adrenergic receptor labeled with fluorescein revealed multiple conformational substates in both the ligand-free and agonist bound forms [58]. These substates were not observed by traditional fluorescence spectroscopy [65]. At least three predominant active conformational substates were detected by these single-molecule studies, which likely represent tier 0 conformational substates. Conformational substates detected in the presence of agonist are absent or rarely populated in the ligand-free form of the receptor, which likely occurs by a change in the shape of the energy landscape upon agonist binding resulting in differently populated conformational substates.
Fluorescence lifetime studies on the same fluorescein-labeled purified β2 adrenergic receptors provided additional insights into conformational substates of the receptor [66]. Agonists displayed two major populations of lifetimes in contrast to only a single lifetime detected for the ligand-free receptor. The position of the distribution of the shorter lifetimes is different between full and partial agonists, thereby suggesting that full agonists stabilize different tier 0 conformational substates compared to partial agonists. Moreover, the width of the distribution of the shorter lifetimes was different between the two types of agonists, which may reflect different numbers of tier 1 conformational substates for different agonists. These data are inconsistent with two-state models, where the effect of full agonists and partial agonists is determined by their ability to shift the equilibrium between R and R*. Rather, they support the notion that agonists stabilize discrete conformational substates and that full agonists and partial agonists remodel the energy landscape differently to preferentially stabilize different subsets of conformational substates.
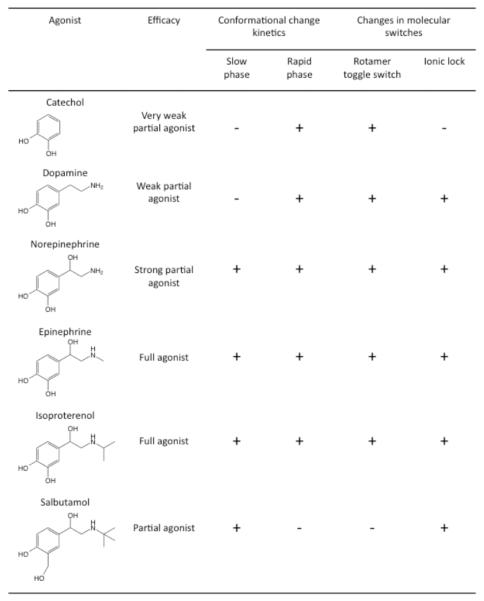
Two kinetic components, a slow and fast phase, were detected when monitoring agonist-promoted changes in fluorescence of tetramethylrhodamine-labeled purified β2 adrenergic receptors [59, 60]. The detection of the two kinetic components and their rate constants were dependent on the efficacy of the agonist and the presence of a catecholamine group in the agonist structure (Fig. 4). The kinetically distinguishable species were interpreted as intermediate states that form sequentially [59], however, it is equally possible that they represent distinct tier 0 conformational substates. Consistent with the previous examples, these studies point to two or more distinct active conformational substates. At the structural level, fluorescence spectroscopy has revealed that these conformational substates can differ at two molecular switch regions that are important for receptor activation (Fig. 4) [62]. Mass spectrometry has revealed additional structural differences between active conformational substates of β2 adrenergic receptors [64]. At the functional lev el, these conformational substates can differentially affect functional properties such as G protein activation and internalization mediated by GRK and arrestin [59], thereby providing structural correlates of how functional selectivity can be achieved.
Fig. (4). Different conformations of the β2 adrenergic receptor promoted by different agonists.
A table summarizing results from refs. [59, 60, 62] is shown. The structures of β2 adrenergic receptor agonists are shown along with the traditional efficacy classification for those agonists. Agonist promoted conformational changes display two kinetic species, a slow phase and a rapid phase. The ability of each agonist to generate these two kinetic species is indicated. Changes are observed in two molecular switch regions in the receptor structure upon receptor activation. The ability of each agonist to generate a change in these molecular switch regions is indicated.
Similar observations were made in Förster resonance energy transfer (FRET) studies on the β2A adrenergic receptor in HEK293 cells [61, 63]. The kinetics of the change in FRET, which was used as a reporter of conformational changes, differed depending on the efficacy of agonists tested. These kinetic differences are indicative of differential stabilization of tier 0 conformational substates. Similarly to conformational substates of β2 adrenergic receptors, the conformational substates of β2A adrenergic receptors can affect function by altering the speed of G protein activation [63].
These biophysical studies on the β2 adrenergic receptor and β2A adrenergic receptor demonstrate that different agonists can promote distinct active conformational substates to achieve functional selectivity. Studies on the β1 adrenergic receptor and β opioid receptor extend these findings to demonstrate that distinct conformational substates of the receptor can affect the interaction of the receptor with the G protein to achieve functional selectivity [67, 68]. The functional selectivity displayed in the differential ability of isoproterenol, bucindolol, and propranolol to activate the β1 adrenergic receptor and engage Gi-mediated MAP kinase signaling was correlated with differences in coupling between the G protein and receptor as assessed by bioluminescence resonance energy transfer (BRET) between tagged forms of the two proteins [68]. It must be noted that isoproterenol, bucindolol, propranol are traditionally classified as an agonist, partial agonist, and inverse angonist, respectively, based on pharmacological effects on Gs-mediated adenylate cyclase signaling. However, all three ligands can serve as agonists for the Gi-mediated MAP kinase signaling pathway. BRET studies on the β opioid receptor suggest that functional selectivity may be achieved not only by whether a conformational substate can couple to a particular G protein or not, but also through the ability of conformational substates to promote a particular conformation of the receptor-G protein complex [67].
Rhodopsin is assumed to be less plastic and dynamic compared to other GPCRs (e.g., [28]). However, even with rhodopsin there appears to be multiple active conformational substates. For rhodopsin, activation has been proposed to occur according to a two-state scheme where the receptor shifts from the inactive dark state, maintained by the chromophore 11-cis retinal, to the active MII state. Both species have a unique spectral fingerprint [69]. The spectrally distinct inactive intermediates that form sequentially preceding MII state formation are not considered here to simplify the discussion [70]. Several lines of evidence are suggestive of multiple conformational active substates for rhodopsin. Several different isochromic MII-like states have been distinguished kinetically under various experimental conditions [71-74]. Some of these isochromic MII-like states appear to form in a sequential manner, such as MIIa and MIIb, while others appear to originate from the same inactive species [72, 75]. Transducin peptide studies and partial agonist studies on rhodopsin indicate that different active states can be conformationally distinct [76, 77], thereby supporting the notion of tier 0 conformational substates. Lastly, NMR has revealed an ensemble of active rhodopsin states, which may represent tier 1 conformational substates [78].
Crystal Structures
High-resolution structures for GPCRs have not been available until relatively recently. We are now in a period where the emergence of crystal structures for GPCRs is appearing at an increased rate. The first crystal structure of a GPCR appeared in the year 2000 for the inactive dark-state of rhodopsin [79]. The next structure for a GPCR other than rhodopsin would come 7 years later for that of the β2 adrenergic receptor [80, 81]. There are now crystal structures of the inactive state for several different GPCRs. Active state crystal structures of GPCRs are now beginning to appear as well, thereby providing a structural perspective of conformational changes occurring upon receptor activation. The first active state structure for rhodopsin appeared in 2006 [82], and several other structures have appeared subsequently [83-86]. Most recently, several active state crystal structures of GPCRs other than rhodopsin have become available, including an active state crystal structure of the β2 adrenergic receptor in complex with a heterotrimeric G protein [87-92].
Efforts to understand GPCR activation at the structural level prior to the availability of crystal structures led to the expectation of significant conformational changes occurring in the transmembrane region in the form of rigid body movements and rotation of helices [21-24]. Some of the predicted conformational changes are indeed present in some of the crystal structures of the active state of GPCRs that are beginning to emerge. For instance, some of the active state crystal structures of rhodopsin/opsin, β2 adrenergic receptor, and A2A adenosine receptor display tilting and rotation of the intracellular portion of transmembrane helix VI, which was predicted to occur previously [83-85, 87, 88, 91]. Other significant conformational changes observed in all three receptors involve transmembrane helices III, V, and VII.
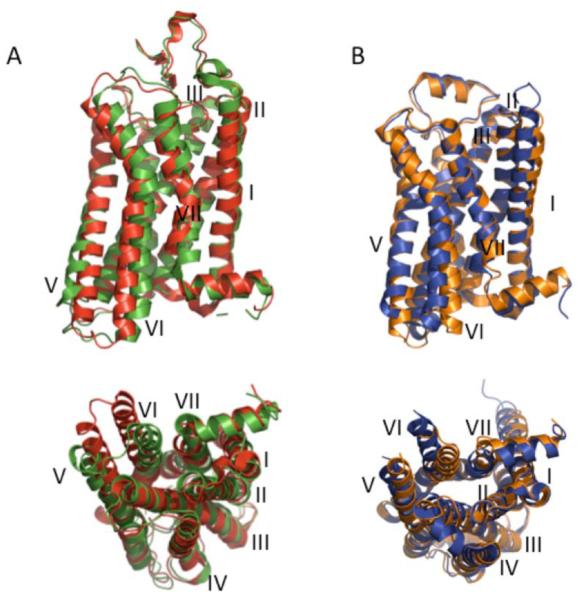
The sampling of active state crystal structures is currently small, so it is not yet possible to form a comprehensive structural perspective of GPCR activation. However, with the crystal structures currently available some issues are becoming apparent. There does seem to be some common structural changes that occur across most GPCRs as predicted previously and now illustrated with the available crystal structures [87]. However, there are also several differences that each GPCR has adopted for their specific functions. Moreover, the different active state crystal structures for the same receptor can display significantly different conformations. The available active state crystal structures for rhodopsin and the β2 adrenergic receptor display differences (Fig. 5). The first crystal structure of activated rhodopsin surprisingly displayed minimal differences within the transmembrane helices of the receptor compared to the structure of the inactive dark-state rhodopsin [82]. This is in contrast to the significant conformational changes that have been detected within the transmembrane helices in the crystal structures of opsin [83, 84], which exhibits constitutive activity, and reconstituted or mutant active rhodopsins [85, 86] (Fig. 5A).
Fig. (5). Active state crystal structures of rhodopsin and β2 adrenergic receptor.
(A), Two crystal structures for the active state of rhodopsin were aligned with PyMOL and are shown from the side view (top) and cytoplasmic view (bottom). The first active state crystal structure solved for rhodopsin (green, PDB: 2I35) [82], which displays minimal differences with the inactive state rhodopsin structure, has significant differences with an active state crystal structure derived from reconstituted rhodopsin (red, PDB: 3PXO) [85], which does display significant differences with the inactive state rhodopsin structure. (B), Two crystal structures for the active state of the β2 adrenergic receptor were aligned with PyMOL and are shown from the side view (top) and cytoplasmic view (bottom). The crystal structure derived from the receptor irreversibly bound to the agonist (orange, PDB: 3PDS) [90], which displays minimal differences with the inactive state β2 adrenergic receptor crystal structure, has significant differences with a crystal structure derived from a nanobody-stabilized active state receptor (blue, PDB: 3P0G) [91], which does display significant differences with the inactive state β2 adrenergic receptor crystal structure. Transmembrane helices are numbered I-VII.
Likewise, there is a similar discrepancy in the two crystal structures of the active state of the β2 adrenergic receptor (Fig. 5B). The active state structure for the β2 adrenergic receptor covalently bound to an agonist did not display significant differences within the transmembrane helices compared to the inactive structure [90]. Only minimal changes were observed in the ligand-binding pocket facilitated by hydrogen bonds formed between the agonist and serine residues in transmembrane helix V. These minimal changes are similar to those observed in the crystal structure for the active state of the β1 adrenergic receptor [89]. In this structure no significant conformational changes are detected within the transmembrane helices and only a 1Å contraction is observed in the ligand-binding pocket of the receptor. Interestingly, full agonists formed an additional hydrogen bond with a serine reside in transmembrane helix V compared to partial agonists, which may form the basis of the difference in efficacy of these different agonists. In contrast to these active state structures, the crystal structure of an active state of the β2 adrenergic receptor stabilized by a camelid antibody fragment displayed significant conformational changes within the transmembrane helices (Fig. 5B) [91]. This active state structure is largely similar to the active state crystal structure of the β2 adrenergic receptor in complex with a G protein except for small differences mainly in the cytoplasmic ends of transmembrane helices V and VI promoted by interactions with the G protein [92].
The two crystal structures for the active state of the A2A adenosine receptor are very similar to each other with only a 0.6 Å root mean square deviation within the transmembrane regions of the two structures [87, 88]. Though both structures display detectable conformational changes in transmembrane helices similar to those observed in some of the active state structures of rhodopsin/opsin and the β2 adrenergic receptor, the magnitude of the motion of helix VI is smaller compared to those other receptors. This difference was attributed by one group to a distinct conformational substate promoted by the conformationally selective agonist used [87], while the other group attributed the difference to an intermediate state between R and R* [88].
It is clear from the available active state GPCR crystal structures that there are significant differences between crystal structures of the same receptor and between crystal structures of different GPCRs. In each case, the difference may be attributed to the formation of an intermediate state that precedes the formation of a fully active state. For instance, in the β2 adrenergic receptor, the small contraction observed within the ligand-binding site facilitated by hydrogen bonding may precede the larger conformational changes occuring within the transmembrane helices [90]. Alternatively, the differences may represent one or more of the many tier 0 active conformational substates of the receptor. Experimental conditions and genetic engineering of receptors may preferentially stabilize one conformational substate over another, thereby resulting in th e crystallization of that particular conformational substate. It should be noted that some conformational substates formed by the receptor may avoid being detected altogether by crystallography since some conformational substates may not be suitably stabilized to form crystals even when present in appreciable quantities. The current observations from crystal structures cannot distinguish between intermediate states and distinct tier 0 conformational substates.
CONFORMATIONAL SUBSTATES IN DISEASE AND DRUG DISCOVERY
Multiple active conformational substates and functional selectivity provide an added level of complexity to GPCR-mediated signaling. While this complexity provides explanations for observations inconsistent with the classical view and enriches our mechanistic understanding of these systems, it is a double-edged sword in relation to understanding dysfunctional states leading to disease and in drug discovery efforts. On one hand, it provides much needed details about diseased states and opens new therapeutic opportunities to be exploited. On the other hand, interpretation of observations becomes more complex and extra caution must be applied since the entire repertoire of receptor activity must now be considered when assessing diseased states or in the development of therapeutics.
Mutations in GPCRs can cause diseased states through several different mechanisms. Rhodopsin demonstrates some of the diversity found in the types of defects that can arise from mutations in GPCRs. More than 120 point mutations have been discovered in rhodopsin that lead to the retinal degenerative disorder retinitis pigmentosa or congenital stationary night blindness [93]. Mutations in rhodopsin can cause several different types of defects in the protein molecule including misfolding or instability, mistrafficking, altered post-translational modifications, altered coupling and signaling to transducin, and constitutive activity. Multiple active conformational substates and functional selectivity allow for a more complex outcome as a result of these types of changes. Since GPCRs can signal via several different biochemical pathways, mutations affecting the function of GPCRs need not impact all pathways equally since the mutation may only affect a subset of conformational substates. Moreover, some active conformational substates may be more prevalent in pathological conditions compared to those present under normal conditions, thereby altering the signaling properties of the receptor.
Mutations causing constitutive activity present an example of the impact multiple active conformational substates can have in the disease phenotype. Similar to rhodopsin, mutations have been detected in other GPCRs that result in a constitutively active receptor and cause disease [94, 95]. Typically, all constitutive activity has been treated similarly and interpreted within the context of two-state models, where an appreciable population of receptors in a single active state is presumed to be present in the absence of agonist. However, in the situation where a GPCR can adopt numerous active conformational substates, not all constitutive activity may be equivalent and the resulting cellular effects may be quite different from those produced by agonist-promoted activity. An example of this is found with a G90D mutation in rhodopsin that causes congenital stationary night blindness. This mutation causes rhodopsin to be constitutively active, however, the active state promoted by the mutation generates a response with distinct physiological properties that are different from that promoted by light-activated rhodopsin [96]. Thus, much like functional selectivity is observed in the action of ligands, mutations causing constitutive activity may also exhibit a form of functional selectivity.
The concepts of active conformational substates and functional selectivity will have significant impact on drug discovery efforts [51, 52, 56, 97, 98]. Since ligands can have different efficacies and promote different physiological effects depending on the signaling pathway chosen, the effect of drugs being tested will depend on the readout of the assay. Thus, current strategies and testing of drugs can be misleading [99]. These same properties, however, open up new avenues for the development of targeted and specific drugs. New strategies can be employed to develop functionally selective drugs that stabilize a specific conformational substate, or a subset of conformational substates, to affect a particular aspect of signaling without affecting others. In some instances, stereoisomers of the same ligand can be developed as functionally selective drugs since even the subtle structural differences exhibited by stereoisomers can promote functional selectivity [100, 101]. Functional selectivity is not restricted to drugs binding the orthosteric ligand-binding site, but also extends to drugs acting through allosteric binding sites as well [102].
CONCLUDING REMARKS
Studies on GPCR systems span over a century of work and have played an important role in defining the field of pharmacology. Trying to understand the mechanism of action of GPCRs has been a goal since the beginning of research into these receptors. There is a rich history of the steps taken and milestones achieved to arrive at the current level of mechanistic understanding of GPCR action. The notion that GPCRs act via multiple active conformational substates provides an important update to the classical view of signal transduction. Structural and functional details about conformational substates are only beginning to emerge, however, and much work is still required to fully utilize these concepts to rationalize GPCR activity and to develop targeted therapeutics with reduced side effects. Advances in technology have provided access to missing details of our mechanistic understanding of these systems and must continue to advance to propel us even further. Advances in single-molecule technologies may particularly be useful in quantitatively assessing conformational substates of GPCRs to define mechanism just as they have done in mechanistic studies on ligand-gated ion channels [103].
ACKNOWLEDGEMENTS
I would like to thank Dr. Alejandro Colozo for comments on the manuscript and help with the generation of Fig. (5). This work was supported by grants from the National Institutes of Health (R00EY018085 and R01EY021731), Research to Prevent Blindness (Unrestricted Grant and Career Development Award), and the Ohio Lions Eye Research Foundation.
REFERENCES
- [1].Strachan RT, Ferrara G, Roth BL. Screening the receptorome: an efficient approach for drug discovery and target validation. Drug Discov. Today. 2006;11:708–716. doi: 10.1016/j.drudis.2006.06.012. [DOI] [PubMed] [Google Scholar]
- [2].Schlyer S, Horuk R. I want a new drug: G-protein-coupled receptors in drug development. Drug Discov. Today. 2006;11:481–493. doi: 10.1016/j.drudis.2006.04.008. [DOI] [PubMed] [Google Scholar]
- [3].Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat. Rev. Drug Discov. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- [4].Limbird LE. The receptor concept: a continuing evolution. Mol. Interv. 2004;4:326–336. doi: 10.1124/mi.4.6.6. [DOI] [PubMed] [Google Scholar]
- [5].Maehle AH, Prull CR, Halliwell RF. The emergence of the drug receptor theory. Nat. Rev. Drug. Discov. 2002;1:637–641. doi: 10.1038/nrd875. [DOI] [PubMed] [Google Scholar]
- [6].Maehle AH. The quantification and differentiation of the drug receptor theory, c. 1910-1960. Ann. Sci. 2005;62:479–500. doi: 10.1080/00033790412331312666. [DOI] [PubMed] [Google Scholar]
- [7].Rang HP. The receptor concept: pharmacology’s big idea. Br. J. Pharmacol. 2006;147:S9–S16. doi: 10.1038/sj.bjp.0706457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Clark AJ. The mode of action of drugs on cells. Edward Arnold & Co.; London: 1933. [Google Scholar]
- [9].Stephenson RP. A modification of receptor theory. Br. J. Pharmacol. 1956;11:379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Furchgott RF. The use of β-haloalkylamines in the differentiation of receptors and in the determination of dissociation constants of receptor-agonist complexes. Adv. Drug Res. 1966;3:21–55. [Google Scholar]
- [11].De Lean A, Stadel JM, Lefkowitz RJ. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled β-adrenergic receptor. J. Biol. Chem. 1980;255:7108–7117. [PubMed] [Google Scholar]
- [12].Costa T, Cotecchia S. Historical review: Negative efficacy and the constitutive activity of G-protein-coupled receptors. Trends Pharmacol. Sci. 2005;26:618–624. doi: 10.1016/j.tips.2005.10.009. [DOI] [PubMed] [Google Scholar]
- [13].Samama P, Cotecchia S, Costa T, Lefkowitz RJ. A mutation-induced activated state of the β2-adrenergic receptor. Extending the ternary complex model. J. Biol. Chem. 1993;268:4625–4636. [PubMed] [Google Scholar]
- [14].Chidiac P, Hebert TE, Valiquette M, Dennis M, Bouvier M. Inverse agonist activity of β-adrenergic antagonists. Mol. Pharmacol. 1994;45:490–499. [PubMed] [Google Scholar]
- [15].Leff P. The two-state model of receptor activation. Trends Pharmacol. Sci. 1995;16:89–97. doi: 10.1016/s0165-6147(00)88989-0. [DOI] [PubMed] [Google Scholar]
- [16].Weiss JM, Morgan PH, Lutz MW, Lydic R, Kenakin TP. The cubic ternary complex receptor-occupancy model I. Model description. J. Theor. Biol. 1996;178:151–167. doi: 10.1006/jtbi.1996.0139. [DOI] [PubMed] [Google Scholar]
- [17].Sum CS, Park PS-H, Wells JW. Effects of N-ethylmaleimide on conformational equilibria in purified cardiac muscarinic receptors. J. Biol. Chem. 2002;277:36188–36203. doi: 10.1074/jbc.M201731200. [DOI] [PubMed] [Google Scholar]
- [18].Del Castillo J, Katz B. Interaction at end-plate receptors between different choline derivatives. Proc. R. Soc. Lond. B Biol. Sci. 1957;146:369–381. doi: 10.1098/rspb.1957.0018. [DOI] [PubMed] [Google Scholar]
- [19].Neubig RR, Spedding M, Kenakin T, Christopoulos A. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on terms and symbols in quantitative pharmacology. Pharmacol. Rev. 2003;55:597–606. doi: 10.1124/pr.55.4.4. [DOI] [PubMed] [Google Scholar]
- [20].Kenakin TP. Pharmacological onomastics: what’s in a name? Br. J. Pharmacol. 2008;153:432–438. doi: 10.1038/sj.bjp.0707407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kobilka BK. Agonist-induced conformational changes in the β2 adrenergic receptor. J. Pept. Res. 2002;60:317–321. doi: 10.1034/j.1399-3011.2002.21062.x. [DOI] [PubMed] [Google Scholar]
- [22].Hubbell WL, Altenbach C, Hubbell CM, Khorana HG. Rhodopsin structure, dynamics, and activation: a perspective from crystallography, site-directed spin labeling, sulfhydryl reactivity, and disulfide cross-linking. Adv. Protein Chem. 2003;63:243–290. doi: 10.1016/s0065-3233(03)63010-x. [DOI] [PubMed] [Google Scholar]
- [23].Meng EC, Bourne HR. Receptor activation: what does the rhodopsin structure tell us? Trends Pharmacol. Sci. 2001;22:587–593. doi: 10.1016/s0165-6147(00)01825-3. [DOI] [PubMed] [Google Scholar]
- [24].Wess J, Han SJ, Kim SK, Jacobson KA, Li JH. Conformational changes involved in G-protein-coupled-receptor activation. Trends Pharmacol. Sci. 2008;29:616–625. doi: 10.1016/j.tips.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kobilka B, Schertler GF. New G-protein-coupled receptor crystal structures: insights and limitations. Trends Pharmacol. Sci. 2008;29:79–83. doi: 10.1016/j.tips.2007.11.009. [DOI] [PubMed] [Google Scholar]
- [26].Hofmann KP, Scheerer P, Hildebrand PW, Choe HW, Park JH, Heck M, Ernst OP. A G protein-coupled receptor at work: the rhodopsin model. Trends Biochem. Sci. 2009;34:540–552. doi: 10.1016/j.tibs.2009.07.005. [DOI] [PubMed] [Google Scholar]
- [27].Deupi X, Kobilka BK. Energy landscapes as a tool to integrate GPCR structure, dynamics, and function. Physiology (Bethesda) 2010;25:293–303. doi: 10.1152/physiol.00002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kobilka BK, Deupi X. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol. Sci. 2007;28:397–406. doi: 10.1016/j.tips.2007.06.003. [DOI] [PubMed] [Google Scholar]
- [29].Frauenfelder H, Sligar SG, Wolynes PG. The energy landscapes and motions of proteins. Science. 1991;254:1598–1603. doi: 10.1126/science.1749933. [DOI] [PubMed] [Google Scholar]
- [30].Cooper A, Dryden DT. Allostery without conformational change. A plausible model. Eur. Biophys. J. 1984;11:103–109. doi: 10.1007/BF00276625. [DOI] [PubMed] [Google Scholar]
- [31].Wand AJ. Dynamic activation of protein function: a view emerging from NMR spectroscopy. Nat. Struct. Biol. 2001;8:926–931. doi: 10.1038/nsb1101-926. [DOI] [PubMed] [Google Scholar]
- [32].Mittermaier A, Kay LE. New tools provide new insights in NMR studies of protein dynamics. Science. 2006;312:224–228. doi: 10.1126/science.1124964. [DOI] [PubMed] [Google Scholar]
- [33].Vendruscolo M, Dobson CM. Structural biology. Dynamic visions of enzymatic reactions. Science. 2006;313:1586–1587. doi: 10.1126/science.1132851. [DOI] [PubMed] [Google Scholar]
- [34].Dill KA, Chan HS. From Levinthal to pathways to funnels. Nat. Struct. Biol. 1997;4:10–19. doi: 10.1038/nsb0197-10. [DOI] [PubMed] [Google Scholar]
- [35].Park PS-H, Filipek S, Wells JW, Palczewski K. Oligomerization of G protein-coupled receptors: past, present, and future. Biochemistry. 2004;43:15643–15656. doi: 10.1021/bi047907k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bockaert J, Fagni L, Dumuis A, Marin P. GPCR interacting proteins (GIP) Pharmacol. Ther. 2004;103:203–221. doi: 10.1016/j.pharmthera.2004.06.004. [DOI] [PubMed] [Google Scholar]
- [37].Bhattacharya S, Vaidehi N. Computational mapping of the conformational transitions in agonist selective pathways of a G-protein coupled receptor. J. Am. Chem. Soc. 2010;132:5205–5214. doi: 10.1021/ja910700y. [DOI] [PubMed] [Google Scholar]
- [38].Provasi D, Artacho MC, Negri A, Mobarec JC, Filizola M. Ligand-induced modulation of the free-energy landscape of G protein-coupled receptors explored by adaptive biasing techniques. PLoS Comput. Biol. 2011;7:e1002193. doi: 10.1371/journal.pcbi.1002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gilman AG. G proteins: transducers of receptor-generated signals. Annu. Rev. Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- [40].Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol. Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- [41].Hermans E. Biochemical and pharmacological control of the multiplicity of coupling at G-protein-coupled receptors. Pharmacol. Ther. 2003;99:25–44. doi: 10.1016/s0163-7258(03)00051-2. [DOI] [PubMed] [Google Scholar]
- [42].Pierce KL, Lefkowitz RJ. Classical and new roles of β-arrestins in the regulation of G-protein-coupled receptors. Nat. Rev. Neurosci. 2001;2:727–733. doi: 10.1038/35094577. [DOI] [PubMed] [Google Scholar]
- [43].Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- [44].DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. β-arrestins and cell signaling. Annu. Rev. Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- [45].Shenoy SK, Lefkowitz RJ. β-arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol. Sci. 2011 doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- [47].Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu. Rev. Pharmacol. Toxicol. 2008;48:359–391. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Neubig RR. Membrane organization in G-protein mechanisms. FASEB J. 1994;8:939–946. doi: 10.1096/fasebj.8.12.8088459. [DOI] [PubMed] [Google Scholar]
- [49].Kenakin T. Agonist-receptor efficacy. II. Agonist trafficking of receptor signals. Trends Pharmacol. Sci. 1995;16:232–238. doi: 10.1016/s0165-6147(00)89032-x. [DOI] [PubMed] [Google Scholar]
- [50].Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional selectivity and classical concepts of quantitative pharmacology. J. Pharmacol. Exp. Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- [51].Perez DM, Karnik SS. Multiple signaling states of G-protein-coupled receptors. Pharmacol. Rev. 2005;57:147–161. doi: 10.1124/pr.57.2.2. [DOI] [PubMed] [Google Scholar]
- [52].Galandrin S, Oligny-Longpre G, Bouvier M. The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol. Sci. 2007;28:423–430. doi: 10.1016/j.tips.2007.06.005. [DOI] [PubMed] [Google Scholar]
- [53].Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat. Rev. Drug. Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kenakin T, Miller LJ. Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol. Rev. 2010;62:265–304. doi: 10.1124/pr.108.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kenakin T. Collateral efficacy in drug discovery: taking advantage of the good (allosteric) nature of 7TM receptors. Trends Pharmacol. Sci. 2007;28:407–415. doi: 10.1016/j.tips.2007.06.009. [DOI] [PubMed] [Google Scholar]
- [56].Aplin M, Christensen GL, Hansen JL. Pharmacologic perspectives of functional selectivity by the angiotensin II type 1 receptor. Trends Cardiovasc. Med. 2008;18:305–312. doi: 10.1016/j.tcm.2009.01.003. [DOI] [PubMed] [Google Scholar]
- [57].Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- [58].Peleg G, Ghanouni P, Kobilka BK, Zare RN. Single-molecule spectroscopy of the β2 adrenergic receptor: observation of conformational substates in a membrane protein. Proc. Natl. Acad. Sci. U. S. A. 2001;98:8469–8474. doi: 10.1073/pnas.151239698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Swaminath G, Xiang Y, Lee TW, Steenhuis J, Parnot C, Kobilka BK. Sequential binding of agonists to the β2 adrenoceptor. Kinetic evidence for intermediate conformational states. J. Biol. Chem. 2004;279:686–691. doi: 10.1074/jbc.M310888200. [DOI] [PubMed] [Google Scholar]
- [60].Swaminath G, Deupi X, Lee TW, Zhu W, Thian FS, Kobilka TS, Kobilka B. Probing the β2 adrenoceptor binding site with catechol reveals differences in binding and activation by agonists and partial agonists. J. Biol. Chem. 2005;280:22165–22171. doi: 10.1074/jbc.M502352200. [DOI] [PubMed] [Google Scholar]
- [61].Vilardaga JP, Steinmeyer R, Harms GS, Lohse MJ. Molecular basis of inverse agonism in a G protein-coupled receptor. Nat. Chem. Biol. 2005;1:25–28. doi: 10.1038/nchembio705. [DOI] [PubMed] [Google Scholar]
- [62].Yao X, Parnot C, Deupi X, Ratnala VR, Swaminath G, Farrens D, Kobilka B. Coupling ligand structure to specific conformational switches in the β2-adrenoceptor. Nat. Chem. Biol. 2006;2:417–422. doi: 10.1038/nchembio801. [DOI] [PubMed] [Google Scholar]
- [63].Nikolaev VO, Hoffmann C, Bunemann M, Lohse MJ, Vilardaga JP. Molecular basis of partial agonism at the neurotransmitter β2A-adrenergic receptor and Gi-protein heterotrimer. J. Biol. Chem. 2006;281:24506–24511. doi: 10.1074/jbc.M603266200. [DOI] [PubMed] [Google Scholar]
- [64].Kahsai AW, Xiao K, Rajagopal S, Ahn S, Shukla AK, Sun J, Oas TG, Lefkowitz RJ. Multiple ligand-specific conformations of the β2-adrenergic receptor. Nat. Chem. Biol. 2011;7:692–700. doi: 10.1038/nchembio.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ghanouni P, Steenhuis JJ, Farrens DL, Kobilka BK. Agonist-induced conformational changes in the G-protein-coupling domain of the β2 adrenergic receptor. Proc. Natl. Acad. Sci. U. S. A. 2001;98:5997–6002. doi: 10.1073/pnas.101126198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ghanouni P, Gryczynski Z, Steenhuis JJ, Lee TW, Farrens DL, Lakowicz JR, Kobilka BK. Functionally different agonists induce distinct conformations in the G protein coupling domain of the β2 adrenergic receptor. J. Biol. Chem. 2001;276:24433–24436. doi: 10.1074/jbc.C100162200. [DOI] [PubMed] [Google Scholar]
- [67].Audet N, Gales C, Archer-Lahlou E, Vallieres M, Schiller PW, Bouvier M, Pineyro G. Bioluminescence resonance energy transfer assays reveal ligand-specific conformational changes within preformed signaling complexes containing β-opioid receptors and heterotrimeric G proteins. J. Biol. Chem. 2008;283:15078–15088. doi: 10.1074/jbc.M707941200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Galandrin S, Oligny-Longpre G, Bonin H, Ogawa K, Gales C, Bouvier M. Conformational rearrangements and signaling cascades involved in ligand-biased mitogen-activated protein kinase signaling through the β1-adrenergic receptor. Mol. Pharmacol. 2008;74:162–172. doi: 10.1124/mol.107.043893. [DOI] [PubMed] [Google Scholar]
- [69].Park PS, Lodowski DT, Palczewski K. Activation of G protein-coupled receptors: beyond two-state models and tertiary conformational changes. Annu. Rev. Pharmacol. Toxicol. 2008;48:107–141. doi: 10.1146/annurev.pharmtox.48.113006.094630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Shichida Y, Imai H. Visual pigment: G-protein-coupled receptor for light signals. Cell. Mol. Life Sci. 1998;54:1299–1315. doi: 10.1007/s000180050256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hoffmann W, Siebert F, Hofmann KP, Kreutz W. Two distinct rhodopsin molecules within the disc membrane of vertebrate rod outer segments. Biochim. Biophys. Acta. 1978;503:450–461. doi: 10.1016/0005-2728(78)90144-5. [DOI] [PubMed] [Google Scholar]
- [72].Straume M, Mitchell DC, Miller JL, Litman BJ. Interconversion of metarhodopsins I and II: a branched photointermediate decay model. Biochemistry. 1990;29:9135–9142. doi: 10.1021/bi00491a006. [DOI] [PubMed] [Google Scholar]
- [73].Jager S, Szundi I, Lewis JW, Mah TL, Kliger DS. Effects of pH on rhodopsin photointermediates from lumirhodopsin to metarhodopsin II. Biochemistry. 1998;37:6998–7005. doi: 10.1021/bi9728194. [DOI] [PubMed] [Google Scholar]
- [74].Arnis S, Hofmann KP. Two different forms of metarhodopsin II: Schiff base deprotonation precedes proton uptake and signaling state. Proc. Natl. Acad. Sci. U. S. A. 1993;90:7849–7853. doi: 10.1073/pnas.90.16.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Knierim B, Hofmann KP, Ernst OP, Hubbell WL. Sequence of late molecular events in the activation of rhodopsin. Proc. Natl. Acad. Sci. U. S. A. 2007;104:20290–20295. doi: 10.1073/pnas.0710393104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Downs MA, Arimoto R, Marshall GR, Kisselev OG. G-protein alpha and beta-gamma subunits interact with conformationally distinct signaling states of rhodopsin. Vision Res. 2006;46:4442–4448. doi: 10.1016/j.visres.2006.07.021. [DOI] [PubMed] [Google Scholar]
- [77].Knierim B, Hofmann KP, Gartner W, Hubbell WL, Ernst OP. Rhodopsin and 9-demethyl-retinal analog: effect of a partial agonist on displacement of transmembrane helix 6 in class A G protein-coupled receptors. J. Biol. Chem. 2008;283:4967–4974. doi: 10.1074/jbc.M703059200. [DOI] [PubMed] [Google Scholar]
- [78].Struts AV, Salgado GF, Martinez-Mayorga K, Brown MF. Retinal dynamics underlie its switch from inverse agonist to agonist during rhodopsin activation. Nat. Struct. Mol. Biol. 2011;18:392–394. doi: 10.1038/nsmb.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le T,I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- [80].Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK. Crystal structure of the human β2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- [82].Salom D, Lodowski DT, Stenkamp RE, Le Trong I, Golczak M, Jastrzebska B, Harris T, Ballesteros JA, Palczewski K. Crystal structure of a photoactivated deprotonated intermediate of rhodopsin. Proc. Natl. Acad. Sci. U. S. A. 2006;103:16123–16128. doi: 10.1073/pnas.0608022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Scheerer P, Park JH, Hildebrand PW, Kim YJ, Krauss N, Choe HW, Hofmann KP, Ernst OP. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008;455:497–502. doi: 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- [84].Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature. 2008;454:183–187. doi: 10.1038/nature07063. [DOI] [PubMed] [Google Scholar]
- [85].Choe HW, Kim YJ, Park JH, Morizumi T, Pai EF, Krauss N, Hofmann KP, Scheerer P, Ernst OP. Crystal structure of metarhodopsin II. Nature. 2011;471:651–655. doi: 10.1038/nature09789. [DOI] [PubMed] [Google Scholar]
- [86].Standfuss J, Edwards PC, D’Antona A, Fransen M, Xie G, Oprian DD, Schertler GF. The structural basis of agonist-induced activation in constitutively active rhodopsin. Nature. 2011;471:656–660. doi: 10.1038/nature09795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Xu F, Wu H, Katritch V, Han GW, Jacobson KA, Gao ZG, Cherezov V, Stevens RC. Structure of an agonist-bound human A2A adenosine receptor. Science. 2011;332:322–327. doi: 10.1126/science.1202793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lebon G, Warne T, Edwards PC, Bennett K, Langmead CJ, Leslie AG, Tate CG. Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation. Nature. 2011 doi: 10.1038/nature10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Warne T, Moukhametzianov R, Baker JG, Nehme R, Edwards PC, Leslie AG, Schertler GF, Tate CG. The structural basis for agonist and partial agonist action on a β1-adrenergic receptor. Nature. 2011;469:241–244. doi: 10.1038/nature09746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Rosenbaum DM, Zhang C, Lyons JA, Holl R, Aragao D, Arlow DH, Rasmussen SG, Choi HJ, Devree BT, Sunahara RK, Chae PS, Gellman SH, Dror RO, Shaw DE, Weis WI, Caffrey M, Gmeiner P, Kobilka BK. Structure and function of an irreversible agonist-β2 adrenoceptor complex. Nature. 2011;469:236–240. doi: 10.1038/nature09665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Rasmussen SG, Choi HJ, Fung JJ, Pardon E, Casarosa P, Chae PS, Devree BT, Rosenbaum DM, Thian FS, Kobilka TS, Schnapp A, Konetzki I, Sunahara RK, Gellman SH, Pautsch A, Steyaert J, Weis WI, Kobilka BK. Structure of a nanobody-stabilized active state of the β2 adrenoceptor. Nature. 2011;469:175–180. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, Mathiesen JM, Shah ST, Lyons JA, Caffrey M, Gellman SH, Steyaert J, Skiniotis G, Weis WI, Sunahara RK, Kobilka BK. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Mendes HF, van der Spuy J, Chapple JP, Cheetham ME. Mechanisms of cell death in rhodopsin retinitis pigmentosa: implications for therapy. Trends Mol. Med. 2005;11:177–185. doi: 10.1016/j.molmed.2005.02.007. [DOI] [PubMed] [Google Scholar]
- [94].Smit MJ, Vischer HF, Bakker RA, Jongejan A, Timmerman H, Pardo L, Leurs R. Pharmacogenomic and structural analysis of constitutive G protein-coupled receptor activity. Annu. Rev. Pharmacol. Toxicol. 2007;47:53–87. doi: 10.1146/annurev.pharmtox.47.120505.105126. [DOI] [PubMed] [Google Scholar]
- [95].Seifert R, Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2002;366:381–416. doi: 10.1007/s00210-002-0588-0. [DOI] [PubMed] [Google Scholar]
- [96].Dizhoor AM, Woodruff ML, Olshevskaya EV, Cilluffo MC, Cornwall MC, Sieving PA, Fain GL. Night blindness and the mechanism of constitutive signaling of mutant G90D rhodopsin. J. Neurosci. 2008;28:11662–11672. doi: 10.1523/JNEUROSCI.4006-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kenakin T. Functional selectivity and biased receptor signaling. J. Pharmacol. Exp. Ther. 2011;336:296–302. doi: 10.1124/jpet.110.173948. [DOI] [PubMed] [Google Scholar]
- [98].Mailman RB. GPCR functional selectivity has therapeutic impact. Trends Pharmacol. Sci. 2007;28:390–396. doi: 10.1016/j.tips.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Kenakin T. Ligand-selective receptor conformations revisited: the promise and the problem. Trends Pharmacol. Sci. 2003;24:346–354. doi: 10.1016/S0165-6147(03)00167-6. [DOI] [PubMed] [Google Scholar]
- [100].Seifert R, Dove S. Functional selectivity of GPCR ligand stereoisomers: new pharmacological opportunities. Mol. Pharmacol. 2009;75:13–18. doi: 10.1124/mol.108.052944. [DOI] [PubMed] [Google Scholar]
- [101].Woo AY, Wang TB, Zeng X, Zhu W, Abernethy DR, Wainer IW, Xiao RP. Stereochemistry of an agonist determines coupling preference of β2-adrenoceptor to different G proteins in cardiomyocytes. Mol. Pharmacol. 2009;75:158–165. doi: 10.1124/mol.108.051078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Leach K, Sexton PM, Christopoulos A. Allosteric GPCR modulators: taking advantage of permissive receptor pharmacology. Trends Pharmacol. Sci. 2007;28:382–389. doi: 10.1016/j.tips.2007.06.004. [DOI] [PubMed] [Google Scholar]
- [103].Colquhoun D. The quantitative analysis of drug-receptor interactions: a short history. Trends Pharmacol. Sci. 2006;27:149–157. doi: 10.1016/j.tips.2006.01.008. [DOI] [PubMed] [Google Scholar]