Abstract
Screening coagulation tests and assays for thrombosis and fibrinolysis were performed in 80 cases of malaria at presentation and during the course of the disease. Close correlation between the degree of thrombocytopenia (observed in >97% cases) and the presence hemorrhagic manifestations at presentation, and improvement in the platelet count in parallel with clinical recovery emphasised the role of platelets in the pathogenesis of coagulopathy in malaria. A potential selection bias resulting from inclusion of only patients admitted at a tertiary care hospital could explain the higher incidence (27.5%) of clinical bleeding observed in this study compared to that reported in the literature. Although a significant correlation between overt bleeding and abnormal PT/INR and APTT (observed in 20–37% cases) could not be demonstrated, a good correlation existed between normal screening coagulation tests and the absence of bleeding complications. Elevated D-Dimer and FDP levels in almost all cases (90%) of both types of malaria confirmed the high prevalence of disseminated intravascular coagulation and fibrinolysis. A correlation between rising D-Dimer levels and the incidence of bleeding was observed. Follow up studies in six cases with complications showed normalization of platelet counts and of screening coagulation assays with clinical recovery. D-Dimer and FDP levels however, remained elevated in most of these cases indicating the continuation of a smouldering coagulopathy even after full clinical recovery possibly due to the persistence of residual damage to the cells caused by the parasitic infection. Knowledge of this fact is important for avoiding unnecessary investigations and longer hospital stay in patients admitted with malaria.
Keywords: Malaria, Coagulopathies, DIC, Thrombosis, Fibrinolysis
Introduction
Cases of malaria are known to be associated with variable degrees of coagulopathy as evident from abnormalities of screening coagulation assays in a large number of patients during the course of the illness [1]. A direct interaction between the parasites and the endothelium of the microcirculation causes endothelial cell injury and sets up a series of reactions characterized by release of a large variety of cytokines and inflammatory mediators by the endothelium, the leucocytes and the other cells in the body. These in turn activate the coagulation pathway leading to widespread thrombin deposition in small arteries and arterioles (disseminated intravascular coagulation) and fibrinolysis [2]. This explains the abnormalities of a number of coagulation parameters, including the markers of thrombosis and fibrinolysis that have been reported in malaria [1–3]. However, attempts to use these laboratory parameters as indicators of the severity of the underlying coagulopathy and for monitoring disease progression have met with only a partial success [4]. Therefore, we wanted to revisit this subject in a prospective study involving a large number of malaria patients admitted at a tertiary care hospital. We found the platelet count to be a good indicator of the underlying vasculopathy and of the hemorrhagic tendency and that normal screening coagulation assays were associated with the absence of hemorrhagic complications. We also observed that D-Dimer and fibrin degradation products, markers of thrombosis and fibrinolysis respectively, remain elevated even at the time of discharge from the hospital suggesting persistence of cellular dysfunction caused by malaria even after full clinical recovery. These findings would help in devising rational approach for the management of malaria patients.
Patients and Methods
This study was carried out over a 2 year period. Patients were recruited in the study according to the following inclusion and exclusion criteria.
Inclusion criteria
Only smear-proven patients with malaria admitted in a teaching tertiary care hospital between April, 2007 and May, 2009 were included in this prospective study. Cases with positive “rapid malaria” tests (e.g. DIA MED “Optimal”) were not included to avoid inclusion of other febrile illnesses which can lead to a false positive result with this test.
Patients were enrolled in the study consecutively. Only patients admitted in the hospital were enrolled in the study.
Both male and female patients from the age of 13 to 70 years were included.
Exclusion criteria
Cases with mixed malarial infection were excluded from the study.
Cases of co-infection with malaria and dengue were excluded from the study.
Pregnant women were excluded.
Patients with acute hepatitis, leptospirosis, Salmonella and those with other bacterial and viral infections; immune-compromised individuals, e.g. AIDS and patients having other causes of coagulopathy were excluded.
The study was approved by the concerned hospital authorities.
Peripheral Blood Smear Examination
Smears were collected in all cases at the spike of fever with chills and rigor. Both thick and thin smears were examined for demonstration of malarial parasites. After staining with Leishman stain [5] the smears were examined under both 40 and 100X lenses for detection of the parasite and identification of the species. A minimum of 10,000 cells were counted to determine the parasite infestation rate. Multiple smears were examined by two observers in each case to increase the sensitivity of detection of the parasite.
Platelet Count
Platelet counts were performed as a part of complete blood count on an automated Hematology analyzer that was calibrated against standards with traceability. Two levels of controls were run on these analyzers every morning to ensure accuracy and reproducibility of the data. Normal platelet count ranged from 150.0 to 400.0 × 109/l [5].
Prothrombin Time (PT) and International Normalized Ratio (INR)
PT was measured on a semi-automated coagulometer using rabbit brain thromboplastin with a low ISI value by a clotting based assay [5]. INR of each result was calculated using the following formula,
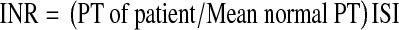 |
where ISI represents the international sensitivity index of the reagent. The mean normal PT (MNPT) was established from 20 normal subjects from the arithmetic mean of the individual values.
Activated Partial Thromboplastin Time (APTT)
APTT was measured using commercially available APTT reagent containing an activator [5]. Results were expressed against a normal range established locally from plasma of 20 normal adults. Values over 7 s of the normal upper limit were considered abnormal.
Fibrinogen Degradation Product (FDP)
FDP was measured using anti-FDP antibody coated latex particles wherein an agglutination of the latex particles by the patient’s plasma indicates the presence of FDP beyond a pre-defined value [5]. By using a serial dilution of the plasma the approximate level of FDP in plasma can be determined. The results were expressed in μg/ml.
D-Dimer
The principle of measurement of D-Dimer is similar to the one for FDP described above [5]. The anti-D-Dimer antibodies used in this test are monoclonal and don’t cross-react with FDP or with the other products of fibrinolysis. Here too the results are expressed in μg/ml FEU (fibrin equivalent unit).
Statistical Analysis
Statistical calculations were performed using SPSS (statistical package for social sciences) software. Chi-square test and in some cases Anova test were applied. P value of less than 0.05 was considered statistically significant.
Results
A total of 80 cases of malaria with only single parasite infection (40 each of P. falciparum and P. vivax) that fulfilled predefined inclusion and exclusion criteria mentioned above (Patients and Methods) were prospectively studied over a 2-year period. There was a high male preponderance in both types of malaria (overall male: female = 9:1) and most of the patients were in the early fourth decade of life (mean age 32 years).
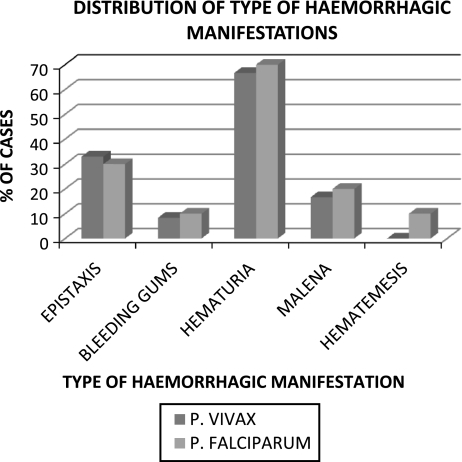
Hemorrhagic complications as evident from clinical bleeding were the commonest complications and were encountered in 27.5% (22/80) cases of malaria, followed by cerebral malaria (18.7%), acute renal failure (13.7%) and acute respiratory distress syndrome (8.7%). Bleeding complications ranged from purpura and ecchymoses to frank hematuria and epistaxis (Fig. 1). The high incidence of hemorrhagic complications seen in malaria cases in this study (P 0.017 and 0.002 for P. vivax and P. falciparum respectively) could reflect a selection bias on account of the fact that all cases included in this study required admission at a tertiary care hospital. Hematuria (macroscopic and microscopic) was the commonest form of bleeding in both types of malaria with an overall incidence of 63.5% (65% of cases of P. falciparum and 62% cases of P. vivax) followed by epistaxis and malena. The relative incidence of the various types of bleeding manifestations was similar in the two types of malaria.
Fig. 1.
Incidence of different types of hemorrhagic manifestations in malaria
Thrombocytopenia (platelets < 150 × 109/l) was observed almost universally in malaria (in 78/80 cases) and severe thrombocytopenia (platelets < 50 × 109/l) was seen in all cases (22/22; 100%) with clinical bleeding irrespective of the type of malaria. In contrast, 11/28 (39.3%) and 17/30 (56.7%) cases of P. vivax and P. falciparum respectively had severe thrombocytopenia in the absence of overt bleeding. The mean platelet count too was significantly lower (30.7 × 109/l) in patients with bleeding complications compared to those without hemorrhage (72.9 × 109/l) (P 0.001) (Table 1). This fact was further supported by a significant statistical correlation between the degree of thrombocytopenia and hemorrhagic manifestations in both P. vivax and P. falciparum with greater significance in the former (P 0.00 for P. vivax and 0.049 for P. falciparum). The nature and type of bleeding also pointed to abnormalities of primary hemostasis involving the microvasculature and platelets.
Table 1.
Mean platelet counts in malaria patients with and without hemorrhage
| Hemorrhage (number) | Mean platelet count (109/l) | Standard deviation |
|---|---|---|
| Yes (22) | 30.69 | 18.81 |
| No (58) | 72.90 | 37.22 |
| Total (80) | 52.32 | 36.36 |
Seven out of 12 (58.3%) patients with P. vivax with hemorrhagic manifestations had normal PT/INR and APTT while five patients (41.7%) had abnormal levels. This finding was of limited statistical significance (P 0.189 for PT/INR; 0.722 for APTT). Similarly, in patients with P. falciparum there was no correlation between hemorrhagic manifestation, PT/INR and APTT values (P 0.361 for PT/INR; 0.512 for APTT) (data not shown). However, there was a significant correlation between the absence of complications and normal PT and APTT (Tables 2, 3). This finding is of clinical significance since normal screening coagulation tests in a patient of malaria could indicate absence of bleeding complications.
Table 2.
Correlation of hemorrhagic manifestations with abnormality of PT/INR in P. vivax and P. falciparum
| Hemorrhage | P. vivax | P. falciparum | ||
|---|---|---|---|---|
| Normal PT | Abnormal PT | Normal PT | Abnormal PT | |
| Yes | 7/12 (58.3)a | 5/12 (41.7) | 7/10 (70) | 3/10 (30) |
| No | 22/28 (78.6) | 6/28 (21.4) | 25/30 (83.3) | 5/30 (16.7) |
| Total | 29/40 (72.5) | 11/40 (27.5) | 32/40 (80) | 8/40 (20) |
aFigures in the parenthesis indicate percentage incidence
Table 3.
Correlation of hemorrhagic manifestations with abnormality of APTT in P. vivax and P. falciparum
| Hemorrhage | P. vivax | P. falciparum | ||
|---|---|---|---|---|
| Normal APTT | Abnormal APTT | Normal APTT | Abnormal APTT | |
| Yes | 7/12 (58.3)a | 5/12 (41.7) | 7/10 (70) | 3/10 (30) |
| No | 18/28 (64.3) | 10/28 (35.7) | 24/30 (80) | 6/30 (20) |
| Total | 25/40 (62.5) | 15/40 (37.5) | 31/40 (77.5) | 9/40 (22.5) |
aFigures in the parenthesis indicate percentage incidence
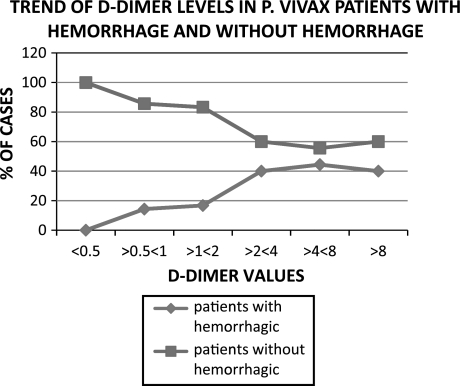
As shown in Table 4, all the 12 patients of P. vivax and all the 10 patients of P. falciparum presenting with hemorrhagic manifestations had high D-Dimer levels. However, this finding was of limited statistical value (P 0.238 for vivax and 0.168 for falciparum malaria) even though superficially the data appeared significant. This could possibly be due to the fact that a high percentage of patients (89% and 83% in P. vivax and in P. falciparum respectively) with no hemorrhagic manifestations also had high D-Dimer levels. It seems that as in the case of thrombocytopenia, elevation of D-Dimer levels is a common occurrence in malaria. When we looked at the association between D-Dimer values above 0.5 μg/ml and hemorrhagic manifestations (Table 5) in the two types of malaria, in P. vivax infection all the 12 patients with clinical hemorrhage had values above 5 μg/ml. However, this was not statistically significant (P 0.517). Interestingly though, one can observe a rising trend in the incidence of hemorrhage with rising D-Dimer values (Fig. 2). In contrast, in patients without hemorrhage the D-Dimer values were somewhat evenly distributed. All the 10 patients of P. falciparum with hemorrhagic manifestation had relatively higher D-Dimer levels (>2 μg/ml) compared to P. vivax cases. This association was statistically highly significant (P 0.018).
Table 4.
Correlation of hemorrhagic manifestations with high and normal D-Dimer levels in P. vivax and P. falciparum
| Hemorrhage | P. vivax | P. falciparum | ||
|---|---|---|---|---|
| Normal D-Dimer | Abnormal D-Dimer | Normal D-Dimer | Abnormal D-Dimer | |
| Yes | 0/12 (0)a | 12/12 (100) | 0/10 (0) | 10/10 (100) |
| No | 3/28 (10.7) | 25/28 (89.3) | 5/30 (16.7) | 25/30 (83.3) |
| Total | 3/40 (7.5) | 37/40 (92.5) | 5/40 (12.5) | 35/40 (87.5) |
aFigures in the parenthesis indicate percentage incidence
Table 5.
Correlation of D-Dimer levels with hemorrhage in patients with malaria
| D-Dimer levels (μg/ml) | P. vivax | P. falciparum | ||
|---|---|---|---|---|
| Hemorrhagic (%) | Non-hemorrhagic (%) | Hemorrhagic (%) | Non-hemorrhagic (%) | |
| <0.5 | 0/3 (0)a | 3/3 (100) | 0/5 (0) | 5/5 (100) |
| > 0.5 < 1 | 1/7 (14.3) | 4/7 (85.7) | 0/6 (0) | 6/6 (100) |
| > 1 <2 | 1/6 (16.7) | 5/6 (83.3) | 0/2 (0) | 2/2 (100) |
| > 2 < 4 | 4/10 (40.0) | 6/10 (60.0) | 4/6 (66.7) | 2/6 (33.3) |
| > 4 < 8 | 4/9 (44.4) | 6/9 (55.6) | 6/15 (40.0) | 9/15 (60) |
| >8 | 2/5 (40.0) | 3/5 (60.0) | 0/6 (0) | 6/6 (100) |
aFigures in the parenthesis indicate percentage incidence
Fig. 2.
Correlation between D-Dimer levels and clinical bleeding in vivax malaria. Similar trend was also observed in falciparum malaria
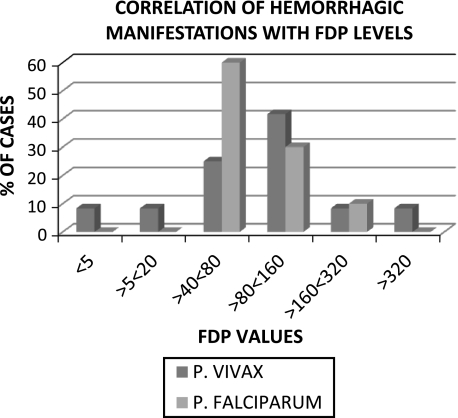
Table 6 shows that elevated FDP was observed in 95% (P 0.527) and 92.5% (P 0.298) of cases of P. vivax and P. falciparum respectively indicating high prevalence of elevated FDP in malaria irrespective of the type of parasite involved. High FDP therefore, seems to be a universal phenomenon in malaria. On further examination of the data, FDP > 40 μg/ml appears to represent a serious derangement in hemostasis since all 10 patients of P. falciparum and 10/12 patients of P. vivax with bleeding manifestations had FDP values above this level. However, the fact that 16 patients of P. vivax and 21 patients of P. falciparum did not have clinical bleeding in spite of having FDP > 40 μg weakens the clinical significance of high FDP vis-à-vis increased risk of bleeding in malaria and is reflected in the absence of statistical significance of this finding (P 0.081 for P. vivax; 0.333 for P. falciparum).
Table 6.
Correlation between FDP levels and bleeding manifestation in P. vivax and P. falciparum
| Incidence of hemorrhage | ||||
|---|---|---|---|---|
| FDP levels (μg/ml) | P. vivax | P. falciparum | ||
| Number/Total | % | Number/Total | % | |
| <5 | 1/2 | 50 | 0/3 | 0 |
| > 5 < 20 | 1/12 | 8.33 | 0/6 | 0 |
| > 20 < 40 | 0 | 0 | 0 | 0 |
| > 40 < 80 | 3/12 | 25 | 6/16 | 37.5 |
| > 80 < 160 | 5/8 | 62.5 | 3/8 | 37.5 |
| > 160 < 320 | 1/5 | 20 | 1/5 | 20.0 |
| >320 | 1/1 | 50 | 0/2 | 0 |
There was no correlation between liver dysfunction, as indicated by elevated levels of hepatic enzyme ALT and abnormalities of coagulation tests. In any case, all patients, except one, had ALT levels less than 150 units/dl indicating only a mild liver injury in most of the cases. This practically ruled out the possible role of decreased synthesis of coagulation factors as the cause of abnormal screening coagulation assays if one considers ALT as a surrogate marker of hepatic injury.
In six patients with complicated malaria (three each suffering from P. vivax and P. falciparum malaria) admitted in ICU setting, coagulation parameters were checked on admission, during treatment as well as following clinical recovery and prior to discharge. Several interesting observations could be made from comparison of the coagulation and other laboratory parameters on admission with those prior to discharge in these cases (Table 7).
All patients showed significant improvement in the platelet counts and the counts were either normal or near normal at the time of discharge.
In patients with abnormal results in screening coagulation assays (PT/INR and APTT) the values became normal with clinical recovery.
There was a significant decline in D-Dimer levels compared to those at presentation. However, the values remained mildly to moderately elevated even prior to discharge in all cases, i.e. >0.5 to <1 μg/ml in two patients, >2 to <4 μg/ml in three cases and >4 to <8 μg/ml in one case.
The decline in FDP levels was less impressive on discharge compared to the D-Dimer levels. Interestingly, although in all but one patient the FDP levels declined on discharge compared to those at presentation, they remained above 40 μg/ml.
There was a lack of correlation in individual patients between the degree of drop in D-Dimer and that in FDP levels at the time of discharge.
Table 7.
Coagulation parameters on admission and at the time of discharge in six patients of malaria with complications
| SN | Hospital stay (day) | Malaria species | Platelets (109/l) | PT/INR | APTT | D-Dimer (μg/ml) | FDP (μg/ml) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adm | Disch | Adm | Disch | Adm | Disch | Adm | Disch | Adm | Disch | |||
| 1. | 13 | Pv | 10 | 170 | Abn | Norm | Abn | Norm | > 4 < 8 | > 0.5 < 1 | > 80 < 160 | > 40 < 80 |
| 2. | 11 | Pv | 20 | 155 | Abn | Norm | Abn | Norm | > 4 < 8 | > 2 < 4 | > 80 < 160 | > 80 < 160 |
| 3. | 8 | Pv | 40 | 134 | Norm | Norm | Norm | Norm | >8 | > 4 < 8 | > 80 < 160 | > 40 < 80 |
| 4. | 10 | Pf | 15 | 180 | Norm | Norm | Abn | Norm | > 4 < 8 | > 0.5 < 1 | > 80 < 160 | > 40 < 80 |
| 5. | 9 | Pf | 70 | 190 | Norm | Norm | Norm | Norm | >8 | > 2 < 4 | > 80 < 160 | > 5 < 20 |
| 6. | 14 | Pf | 11 | 140 | Abn | Norm | Abn | Norm | > 4 < 8 | > 2 < 4 | > 80 < 160 | > 80 < 160 |
SN serial number, d days, PvPlasmodium vivax, PfPlasmodium falciparum, Adm on admission, Disch at discharge Abn abnormal, Norm normal
These findings point to a residual prothrombotic and an associated fibrinolytic process in malaria patients with complications even after a significant clinical recovery and clearance of parasites from the circulation.
Discussion
The higher incidence of malaria in males in early fourth decade observed in this study could be related to multiple socio-economic factors including employment-related movement of populations, poor living conditions, the amount of outdoor activities, travel undertaken by the patients and the fact that a state of immunological balance with malaria is achieved late in adulthood. Differences in the clothing habits of males and females in India and also the feeding habits of Anopheles vectors in the evening and outdoors would have contributed to this difference in sex incidence.
Thrombocytopenia (platelet count <150,000/mm3) was present in almost all (97.5%) patients (78/80; P. vivax 39/40 and P. falciparum 39/40) in this study in accordance with similar high incidence reported by earlier studies [4, 6]. In order to find correlation between thrombocytopenia and the presence or absence of hemorrhage, the platelet counts were categorized into five subgroups representing different degrees of thrombocytopenia (Table 8). A statistically significant correlation was observed between these two parameters in that all the 22 patients with clinical bleeding had platelet counts below 50,000/mm3 (P 0.000 for P. vivax and 0.049 for P. falciparum). This finding supports the critical role of platelets in the pathogenesis of microvascular lesion in malaria irrespective of the species involved and of thrombocytopenia in clinical bleeding in malaria.
Table 8.
Relative incidence of hemorrhagic manifestation at different platelet counts in patients with P. vivax and P. falciparum
| Platelet count (× 10/9l) | P. vivax | P. falciparum | ||||
|---|---|---|---|---|---|---|
| Hemorrhage (n = 12) | No hemorrhage (n = 28) | Total (n = 40) | Hemorrhage (n = 10) | No hemorrhage (n = 30) | Total (n = 40) | |
| ≤10 | 1(8.3)a | 0(0) | 1(2.5) | 0(0) | 0(0) | 0(0) |
| 10–20 | 6(50) | 1(3.6) | 7(17.5) | 5(50) | 5(16.7) | 10(25) |
| 20–50 | 5(41.7) | 10(35.7) | 15(37.5) | 5(50) | 12(40) | 17(42.5) |
| 50–100 | 0(0) | 13(46.4) | 13(32.5) | 0(0) | 9(30) | 9(22.5) |
| ≥100 | 0(0) | 4(14.3) | 4(10) | 0(0) | 4(13.3) | 4(10) |
aFigures in the parenthesis indicate percentage incidence
Some other workers have noted that although mild to moderate thrombocytopenia is a common finding in malaria, hemorrhagic manifestations and clinical evidence of disseminated intravascular coagulation (DIC) are rare (~5 and 5–10% respectively) [2]. In contrast to these reports, hemorrhagic manifestations were observed in 25% cases of P. falciparum and 30% cases of P. vivax in this study and there was a clear-cut correlation between thrombocytopenia and incidence hemorrhage in both P. vivax and P. falciparum (Table 1) [8]. The higher incidence of hemorrhagic manifestations encountered by us could have partly been contributed by the fact that all our patients were admitted to a tertiary care hospital with malaria and ~49% of them had various types of complications including hemorrhage. Wiwanitkit [3] has recently highlighted the magnitude of hemorrhagic complications in a large study involving Thai patients with malaria and has reported a high incidence of clinical bleeding in these patients.
The pathogenesis of thrombocytopenia in malaria is multifactorial and involves (a) the consumption of platelets in the thrombotic process triggered by the infection, (b) immune-mediated destruction, (c) adhesion of platelets to the activated/injured endothelial surface, (d) clearing by the reticulo-endothelial system of platelets that have phagocytised parasites, (e) hypersplenism, (f) apoptosis of platelets – all of which are also intimately related to the pathophysiology of malaria. Role of circulating immune complexes and inflammatory mediators (e.g. TNF-α, IL-6 and Interferon) has also been postulated [4, 6]. It is now well established that patients with thrombocytopenia due to peripheral destruction of normally functioning platelets and normal bone marrow (e.g. immune thrombocytopenic purpura) usually do not bleed above a platelet count of 20 × 109/l [7]. This is ascribed to the presence of “macroplatelets” representing young platelets which are larger in size and are functionally more active than platelets found normally in the circulation. However, this logic does not hold true when there are additional in vivo factors that cause platelet dysfunction, e.g. presence of high levels of circulating FDP and other products of fibrinolysis, anti-platelet drugs such as analgesics and antipyretics, low fibrinogen level and over-activation of platelets, one or more of which are known to co-exist in patients of malaria [8] and could be responsible for the overt bleeding encountered in this study in patients with platelet counts ranging from 20 to 50,000/mm3. This finding is of clinical significance both in assessing the risk of bleeding in malaria patients and in monitoring disease progression and/or recovery because improvement in the platelet count would reflect arrest and even reversal of the complex processes that lead to thrombocytopenia in malaria in the first place. Improvement in platelet count has been directly correlated with clinical recovery in malaria [1, 2]. In this study too, we followed up the laboratory parameters, including the platelet count regularly in all these cases during their hospital stay (data not shared here) and noticed improvement in the platelet counts as the patients responded to the antimalarial treatment. As shown in Table 7 wherein follow up results of laboratory tests in six representative cases of malaria with complication are listed, platelet counts in all cases became normal or near normal prior to discharge.
We examined the possibility of associated liver dysfunction influencing the PT/INR values in these cases since in 31% cases of P. vivax and in 44% cases of P. falciparum there was evidence of liver dysfunction as indicated by mildly elevated levels of ALT. However, there was no correlation between raised ALT levels and high PT/INR in either type of malaria. Therefore, it can be concluded that the reduction in coagulation factors leading to prolongation of PT/INR in these cases was not due to liver failure.
The clinical significance of prolonged APTT observed in this study is questionable as it did not seem to result in an increased incidence of bleeding in either type of malaria for there was no correlation between hemorrhagic manifestations and prolongation of APTT in these cases. On the other hand, however, there was a close correlation between normal PT/INR and APTT results and the absence of clinical bleeding. So this finding could have a negative predictive value as far as the risk of bleeding in malaria is concerned.
Plasma level of D-Dimer is considered to be a good measure of an underlying active thrombotic process and that of FDP as an indicator of the fibrinolytic pathway [9]. D-Dimer levels were high in most of the patients in both types of malaria at presentation (in 92% cases of P. vivax and in 87.5% cases of P. falciparum) suggesting the presence of an underlying smouldering and subclinical thrombosis (DIC) in the majority of patients in line with the observations made above. On further analysis, a linear relationship was observed between rising titres of D-Dimer and the incidence of bleeding in P. vivax and less so, in P. falciparum cases (Fig. 2; Tables 4, 5).
The incidence of elevated FDP in this study was universally high in that this was seen in over 90% cases of both types of malaria (Table 6). Other studies have also reported similar findings. However, there was no correlation between high FDP levels and (a) presence or absence of complications; (b) presence or absence of bleeding and (c) presence or absence of cerebral malaria in either type of malaria or in the total patient population. This finding reflects the now well established fact that activation of the fibrinolytic pathway is integral to malarial infection and could be a sequel to the widespread activation of the thrombotic pathway, and that it does not necessarily increase the risk of bleeding in these cases. Nevertheless, both D-Dimer and FDP are useful indicators of the underlying coagulopathy and could be useful in management of patients if used judiciously (Fig. 3).
Fig. 3.
Correlation between FDP levels and clinical bleeding in malaria. Please note that most number of patients with bleeding had FDP >40 μg/ml
Follow up platelet counts and the other coagulation parameters in three cases each of P. vivax and P. falciparum with complications are listed in Table 7 as representatives of the general trend observed in all cases who recovered. The hospital stay in patients with complications was longer as expected, ranging from 8 to 14 days. From the data presented in this table it can also be concluded that recovery in these patients was heralded by improvement in platelet counts. When one considers the pathophysiology and the high prevalence of thrombocytopenia in malaria, it is not surprising that changes in platelet count closely reflect the course of the disease. Therefore, monitoring of platelet count in patients with malaria can be a reliable way of predicting the course of illness and would facilitate timely modulation of therapy. The fact that we found a close association between thrombocytopenia and major complications in malaria, namely cerebral malaria and incidence of bleeding also adds to the diagnostic and prognostic significance to platelet count in monitoring disease progression in all patients of both types of malaria.
Along with normalization of platelet counts in most of the patients prior to discharge, the screening coagulation tests too became normal in all cases in which these were prolonged at presentation (Table 7). This reflects recovery of the coagulation factors that were reduced as a result of the consumptive coagulopathy caused by malarial infection. However, since these tests don’t detect mildly reduced levels of coagulation factors, normalization of PT/INR and APTT does not necessarily mean total reversal of the thrombosis-fibrinolysis cascade that was set into motion by the infection. That this indeed is the case is suggested by continued generation of small amounts of D-Dimer even when the screening coagulation tests became normal at the time of discharge of our patients. Similarly, persistence of high levels of FDP at discharge could point to the smouldering activation of the fibrinolytic pathway originally initiated by the factors that led to increased thrombin generation in these patients. Awareness of these facts should (a) decrease the tendency for ordering unnecessary and often expensive investigations during the recovery phase of malarial infection and (b) reduce hospital stay of patients with associated benefits on both counts.
References
- 1.Miller LH, Baruch DI, Marsh K, et al. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 2.Clark IA, Cowden WB. The pathophysiology of falciparum malaria. Pharmacol Ther. 2003;99:221–260. doi: 10.1016/S0163-7258(03)00060-3. [DOI] [PubMed] [Google Scholar]
- 3.Wiwanitkit V. Overt bleeding in malaria patients: experience & review. Blood Coagul Fibrinolysis. 2008;19:1–4. doi: 10.1097/MBC.0b013e3282f185b9. [DOI] [PubMed] [Google Scholar]
- 4.Moxon CA, Heyderman RS, Wassmer SC. Dysregulation of coagulation in cerebral malaria. Mol Biochem Parasitol. 2009;166:99–108. doi: 10.1016/j.molbiopara.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laffan M, Manning R. Investigations of hemostasis. In: Lewis SM, Bain BJ, Bates I, editors. Dacie and Lewis practical hematology. Amsterdam: Elsevier; 2006. pp. 379–440. [Google Scholar]
- 6.Francischetti IMB. Does activation of the blood coagulation cascade play a role in malaria pathogenesis? Trends Parasitol. 2008;24:258–263. doi: 10.1016/j.pt.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slichter SJ. Relationship between platelet count and bleeding risk in thrombocytopenic patients. Transfusion Med Reviews. 2004;18:153–167. doi: 10.1016/j.tmrv.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Shen Y-MP, Frenkel EP. Acquired platelet dysfunction. Hematol Oncol Clin North Am. 2007;21:647–661. doi: 10.1016/j.hoc.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Sato N, Takahashi H, Shibata S. Fibrinogen/fibrin products and D-dimer in clinical practice: Interpretation of discrepant results. Am J Hematol. 1995;48:168–174. doi: 10.1002/ajh.2830480306. [DOI] [PubMed] [Google Scholar]