Abstract
Understanding the early immunologic events accompanying reactivated tuberculosis (TB) in HIV-infected individuals may yield insight into causes of reactivation and improve treatment modalities. We used the cynomolgus macaque (Macaca fascicularis) model of HIV–Mycobacterium tuberculosis coinfection to investigate the dynamics of multifunctional T cell responses and granuloma T cell phenotypes in reactivated TB. CD4+ and CD8+ T cells expressing Th1 cytokines (IFN-γ, IL-2, TNF) and Th2 cytokines (IL-4 and IL-10) were followed from latent M. tuberculosis infection to reactivation after coinfection with a pathogenic SIV. Coinfected animals experienced increased Th1 cytokine responses to M. tuberculosis Ags above the latent-response baseline 3–5 wk post-SIV infection that corresponded with peak plasma viremia. Th2 cytokine expression was not Ag specific, but strong, transient IL-4 expression was noted 4–7 wk post-SIV infection. Animals reactivating <17 wk post-SIV infection had significantly more multifunctional CD4+ T cells 3–5 wk post-SIV infection and more Th2-polarized and fewer Th0-, Th1-polarized CD8+ T cells during weeks 1–10 post-SIV infection than animals reactivating >26 wk post-SIV infection. Granuloma T cells included Th0-, Th1-, and Th2-polarized phenotypes but were particularly rich in cytolytic (CD107+) T cells. When combined with the changes in peripheral blood T cells, these factors indicate that events during acute HIV infection are likely to include distortions in proinflammatory and anti-inflammatory T cell responses within the granuloma that have significant effects on reactivation of latent TB. Moreover, it appears that mycobacteria-specific multifunctional T cells are better correlates of Ag load (i.e., disease status) than of protection.
Nearly a third of the world’s population is estimated to be infected with Mycobacterium tuberculosis, a pathogenic bacterium that causes tuberculosis (TB). Individuals with normal immune systems can maintain subclinical infections for decades in an asymptomatic state known as latent TB (1, 2). Infection with HIV strongly predisposes individuals with latent TB to experience reactivated disease (3). Although HIV-infected individuals with low CD4+ T cell numbers are at severe risk of developing TB (4, 5), individuals with better preserved CD4+ T cell numbers are also at increased risk of primary or reactivated TB well before the onset of AIDS (6). The combined effects of HIV and M. tuberculosis coinfection have an unfortunate and deadly synergy and make TB the leading cause of death among HIV-infected individuals (6).
Cytokine-mediated communication between innate and adaptive immune systems in the granuloma is required to mount protective anti-mycobacterial responses. The cytokines most commonly associated with protection are IFN-γ (7) and tumor necrosis factor-α (TNF) (8), both of which are released by M. tuberculosis-specific T cells and cause macrophage activation and bactericidal activity (9, 10). IL-2 acts in conjunction with IFN-γ and TNF (Th1 cytokines) by promoting T cell survival and proliferation (11). The roles of Th2 cytokines such as IL-4 and IL-10 are less understood and more controversial but correlated with diminished protection against TB and poor prognosis (12–18). IL-4 polarizes naive T cells toward Th2 responses (12) possibly causing decreased IFN-γ and macrophage activation (13), and abundant IL-4+CD4+ bronchoalveolar lavage (BAL) T cells are present in cavitary TB (14). The relationship between IL-10 and TB is also unclear, but IL-10–expressing T cells are associated with anergy (15) and delayed responses to drug therapy (16). Moreover, IL-10 may downregulate monocyte and macrophage costimulatory molecules, thereby impairing CD4+ and CD8+ T cell-mediated cytotoxicity in TB patients (19). The importance of anti-inflammatory factors, such as IL-10 and regulatory T cells (Tregs), in the granuloma as a means of limiting pathology also must be considered. We previously demonstrated that Tregs rapidly migrated from blood to lungs after M. tuberculosis infection and that higher levels of peripheral Tregs prior to infection correlated with better outcome of infection (20). However, other groups have demonstrated in mice that substantial reduction in Treg CD4+ T cells results in higher bacterial burden (21). The available data support that the balance of proinflammatory and anti-inflammatory factors in the granuloma may play a major role in control of infection.
The push to develop T cell-based vaccines has led to questions on what defines a protective T cell response, how T cell responses change after infection, and how to drive responses in directions favoring protection. Multifunctional T cells, which express combinations of multiple cytokines (22–25), are of particular interest in vaccine development. Multifunctional T cells expressing Th1 cytokines have been described as immune correlates for protection against intracellular pathogens including HIV (26), SIV (27), and leishmania (11). In contrast to these responses, however, multifunctional CD4+ T cell responses in humans with TB have been correlated with active disease or higher bacterial burden rather than protection (28–30). The divergence in multifunctional responses between HIV and M. tuberculosis and the interest in development of T cell-based vaccines necessitates a better understanding of T cell responses to coinfection, yet the number of studies examining multifunctional CD4+ and CD8+ T cell responses in HIV–M. tuberculosis coinfection remain limited (29, 31, 32). Day et al. (32) found that the majority of CD4+ T cells in the blood of HIV-positive individuals with latent TB are multifunctional, that the frequency of IL-2–expressing cells inversely correlates with viral load, and that these cells are among the first populations depleted by HIV. Moreover, responses in CD4+ BAL T cells from HIV-positive individuals are less multifunctional than T cells from HIV-negative individuals with TB (33). Although controversial, multifunctional T cells expressing both IFN-γ and Th2 cytokines, referred to as the Th0 phenotype, are associated with active TB (34, 35). Initiation of anti-tuberculous drug treatment has been associated with decreased frequencies of Th0-polarized T cells and shifts toward protective Th1-polarized T cells, suggesting a link between Th2 cytokine expression and active disease (35–38).
Despite the consequences of HIV–M. tuberculosis coinfection, much of the basic biology underlying interactions between these pathogens and the host remain poorly understood. Studying human coinfections is inherently difficult and complicated by numerous uncontrollable variables. Non-human primates offer alternative systems for studying human coinfections under experimental conditions. We recently used cynomolgus macaques, which accurately represent latent and active TB (39), to model HIV coinfection of individuals with latent M. tuberculosis infection (40). In the current study, we examined the effects of SIV infection on T cell cytokine responses in cynomolgus macaques from latent M. tuberculosis infection, acute SIV infection, and through reactivated TB. We found that SIV infection was followed by increased anti-mycobacterial Th1 cytokine responses during acute viremia. A previously undocumented peak in IL-4–expressing T cells occurring postinfection was temporally associated with diminished IFN-γ and IL-2 production. The animals reactivating shortly after SIV infection had fewer Th0- and Th1-polarized T cells and more Th2-polarized CD8+ T cells than animals reactivating later, suggesting that in addition to depleting T cells, SIV influences the T cell phenotypes providing protective anti-mycobacterial responses. Granuloma T cell responses were considerably different from peripheral blood T cell responses and were dominated by cytolytic T cells distinct from the cytokine-producing T cells found in peripheral blood. These findings suggest that changes in T cell function during HIV infection, as early as the acute phase of virus infection, become critical factors in reactivated TB long before the onset of AIDS.
Materials and Methods
Experimental animals
Eighteen M. tuberculosis-, SIV-, simian HIV-, or simian retrovirus D-free adult (>4 y of age) cynomolgus macaques (Macaca fascicularis) were used for these studies (Covance, Alice, TX; USA Valley Biosystems, West Sacramento, CA). The experimental design used in these studies is presented in Supplemental Fig. 1. The SIV-only macaques (11507, 11807, 19506, 19706) and coinfected (SIV–M. tuberculosis) macaques (1207, 1407, 1807, 1907, 2407, 3007, 10405) are the same as those previously reported in Diedrich et al. (40). SIV-infected animals were housed under BSL-2 conditions, and M. tuberculosis-infected animals were housed under BSL-3 conditions. BAL, gastric aspirate, and other procedures were performed as previously described (16, 41). Blood from monkeys 3708, 3809, 4908, 5008, 5508, 5608, and 19408 were used as SIV-negative controls for active TB. Similarly, granulomas from monkeys 3809 and 19408 were controls for SIV-negative monkeys with active TB. These studies followed all animal experimentation guidelines, and all experimental manipulations and protocols were approved by the University of Pittsburgh’s Institutional Animal Care and Use Committee.
M. tuberculosis and SIV infection
Cynomolgus macaques were infected with ~25 CFU Erdman strain M. tuberculosis via intrabronchial instillation as previously described (41). Monkeys 3708, 4908, 5008, 5508, and 5608, referred to as high-dose (hd) M. tuberculosis monkeys, were similarly infected with ~200 CFU Erdman strain M. tuberculosis and developed active TB. Infection was confirmed by conversion of negative to positive tuberculin skin test and PBMC responses elevated from baseline in lymphocyte proliferation and ELISPOT assays (39). Animals were classified as latent or active 8–10 mo postinfection, and seven animals with latent infections were selected for SIV infection (36). Latent monkeys are defined as TST positive without signs of clinical disease (23, 41). All animals were infected with SIVmac251 (provided by Dr. Keith Reimann, Beth Israel Deaconess Medical Center, Harvard University). Concentrated SIV stock was diluted in RPMI 1640 medium, and 106 to 107 tissue culture-infective dose (TCID)50 units were injected intravenously. Two groups of animals were infected: four M. tuberculosis-uninfected macaques (SIV-only control group) and seven macaques with latent TB (coinfected group). The criteria for determining reactivated TB included weight loss, increased ESR, M. tuberculosis-positive gastric aspirates or BAL cultures, and/or abnormal chest radiograph and was confirmed at necropsy as higher bacterial burdens and gross pathology scores (40).
PBMC isolation and necropsy procedures
Blood was drawn from hd M. tuberculosis monkeys at the time of infection and subsequently 9 wk post-M. tuberculosis infection, at which point these animals were transferred to other studies. Blood was sampled from SIV-only and coinfected animals every week for the first 8 wk postinfection. Thereafter, sampling from M. tuberculosis–SIV coinfected animals was done every other week and from SIV-only animals monthly. PBMCs were isolated via Percoll gradient centrifugation as previously described (42). Quantitative RT-PCR was performed on plasma as described (40), and results were previously reported by Diedrich et al. (40). Animals were humanely euthanized and necropsies performed as previously described (41) when reactivated TB occurred. Blood and tissues were processed as described (39), with tissue mechanically homogenized into single-cell suspensions for immunologic analysis with Medimachines (BD Biosciences, San Jose, CA).
Intracellular cytokine staining and flow cytometry
Freshly isolated PBMCs were resuspended in RPMI 1640 media supplemented with 10% human AB serum (Gemini Bio-Products, West Sacramento, CA), 1% l-glutamine (Sigma-Aldrich, St. Louis, MO), and 1% HEPES (Sigma) at a density of 5 × 105 to 1 × 106 cells/ml and transferred to FACS tubes for stimulation with mycobacterial or SIV Ags. Cells were stimulated with M. tuberculosis culture filtrate protein (CFP; 10 µg/ml; Tuberculosis Vaccine Testing and Research Materials Contract [National Institutes of Health, National Institute of Allergy and Infectious Diseases NO1-AI-40091], Colorado State University, Fort Collins, CO) or SIV-mac239 gag and pol peptide pools (10 µg/ml each; AIDS Research and Reference Reagent Program, National Institutes of Health, Germantown, MD). SIV Gag and Pol stimulators were 15mer peptides with 11 amino acid overlaps between sequential peptides. Phorbol 12,13-dibutyrate and ionomycin (50 nM and 10 µM final concentration, respectively; Sigma) were used as positive controls. Sham-stimulated (media-only) cells were used as negative (unstimulated) controls. Cells were stimulated for 1 h at 37°C/5% CO2 prior to addition of brefeldin A (GolgiPlug; BD Biosciences) and incubated for another 4.5–5 h at 37°C/5% CO2 prior to being stained. Surface CD107 was used as an indicator of degranulation and cytotoxicity (43). Tissue cells and PBMCs were stained with Abs against CD107a (clone eBioH4A; eBioscience, San Diego, CA) that were conjugated with QDot 565 (Invitrogen, Carlsbad, CA), or a similarly conjugated isotype Ab (eBioscience), in the presence of monensin (BD Bioscience) and brefeldin A while being stimulated 4.5–5 h at 37°C/5% CO2. PBMCs and tissue cells were stained for viability with Blue Viability Dye (Invitrogen) and then stained for CD3 (clone SP34-2; BD Biosciences), CD4 (clone L200; BD Biosciences), CD8 (clone DK25 [Dako, Carpinteria, CA] or clone OKT8 [eBioscience]) and CD20 (clone 2H7; eBioscience). Cells were fixed and permeabilized with Fix-Perm reagents (BD) and stained intracellularly for IFN-γ (clone B27; BD Bioscience), IL-2 (clone MQ1-17H12; eBioscience), and TNF (clone mab11; eBioscience) (Th1 tube) or IFN-γ, IL-10 (clone JES3-9D7; eBioscience), and IL-4 (clone 8D4-8; eBioscience) (Th0/2 tube). Cell phenotypes were read with an LSR II flow cytometer (BD Biosciences) with the objective of acquiring 30,000 viable CD3+ events per tube. Only data from freshly isolated cells are presented because of inconsistencies in cytokine production by cryopreserved cells.
Data analysis
Flow cytometry data were analyzed with the FlowJo software package (Tree Star, Ashland, OR). Cytokine-positive gates were based on unstimulated (media-only) controls set so the frequency of events in the cytokine-positive gate was generally <0.05%. Pie charts depicting multifunctional responses were plotted with SPICE (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD). Data were analyzed using Prism (GraphPad Software, San Diego, CA). Statistical comparisons were done using nonparametric statistics to account for data that were not normally distributed. Pairwise comparisons were performed using Mann–Whitney U rank sum test and the Kruskal–Wallis one-way ANOVA where multiple groups were to be compared with p < 0.05 considered statistically significant.
Results
M. tuberculosis and SIV infection
Seven cynomolgus macaques with latent M. tuberculosis infection were infected with SIVmac251 as described by Diedrich et al. (40). All animals experienced peak viremia with ~107 virus copies/ml plasma between 3 and 5 wk postinfection, a time frame coinciding with the most severe depletion of peripheral CD4+ T cells (40). After the acute phase of virus infection (1–8 wk post-SIV infection, a period consistent with 103 to 107 virus copies/ml plasma), viral loads decreased substantially, and T cell populations rebounded to numbers within normal ranges for these monkeys (pre-SIV infection range for coinfected animals on the date of SIV infection: 208–1170 CD4+ T cells/µl, 367–1357 CD8+ T cells/µl). Indications of reactivated TB were observed in three animals (1207, 1407, 2407) 4–6 wk post-SIV infection, and necropsies were performed on these monkeys 12–17 wk post-SIV infection (40). Four other monkeys (1807, 1907, 3007, 10405) showed indications of reactivation 16–47 wk post-SIV infection and were euthanized 26–48 wk post-SIV infection (40). Based on this, the monkeys are separated into two groups according to their time to necropsy, with animals undergoing necropsy <17 wk post-SIV infection considered early reactivators and animals undergoing necropsy >26 wk post-SIV infection considered late reactivators. The monkeys displayed a spectrum of disease ranging from a few lung granulomas in late reactivators to widely disseminated pathology in early reactivators (40).
M. tuberculosis-specific Th1 cytokine responses are perturbed during acute SIV infection
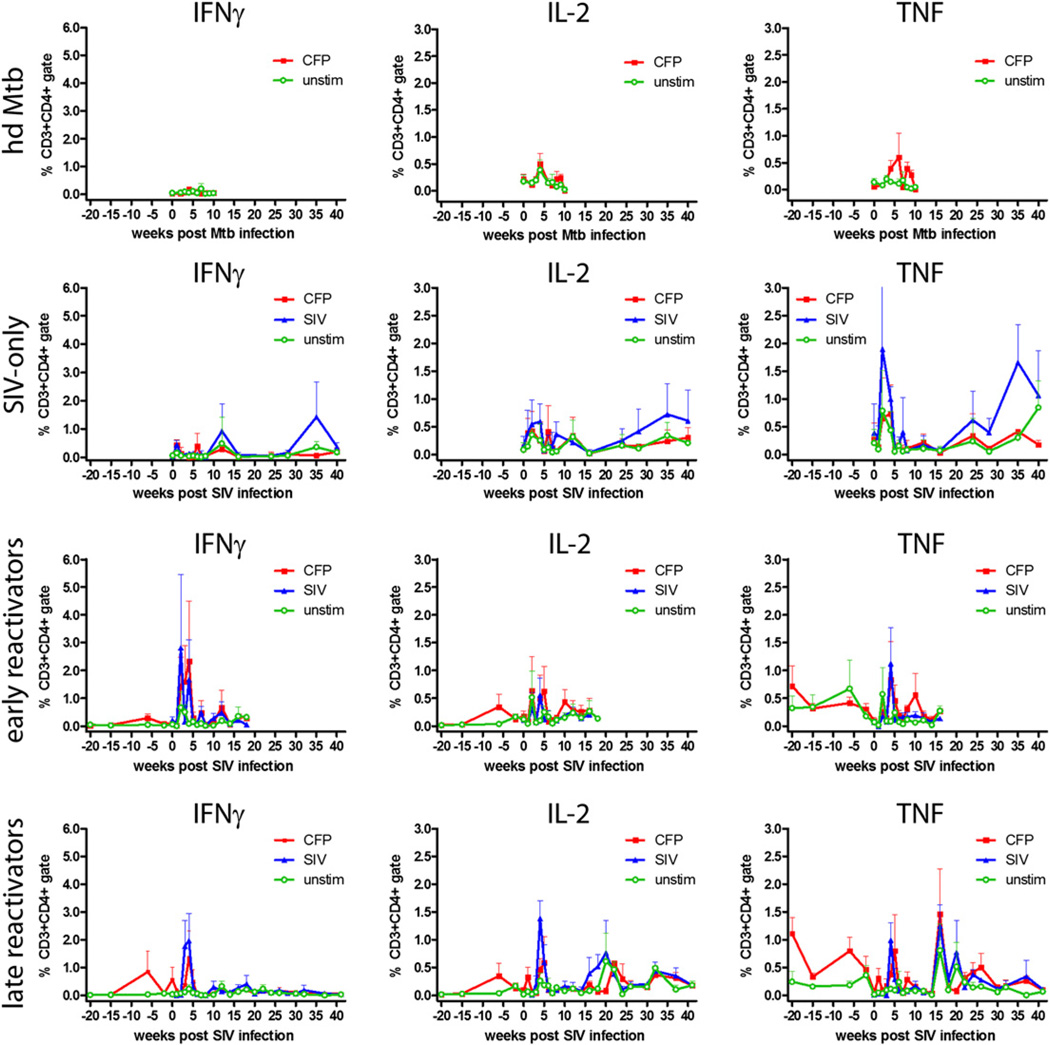
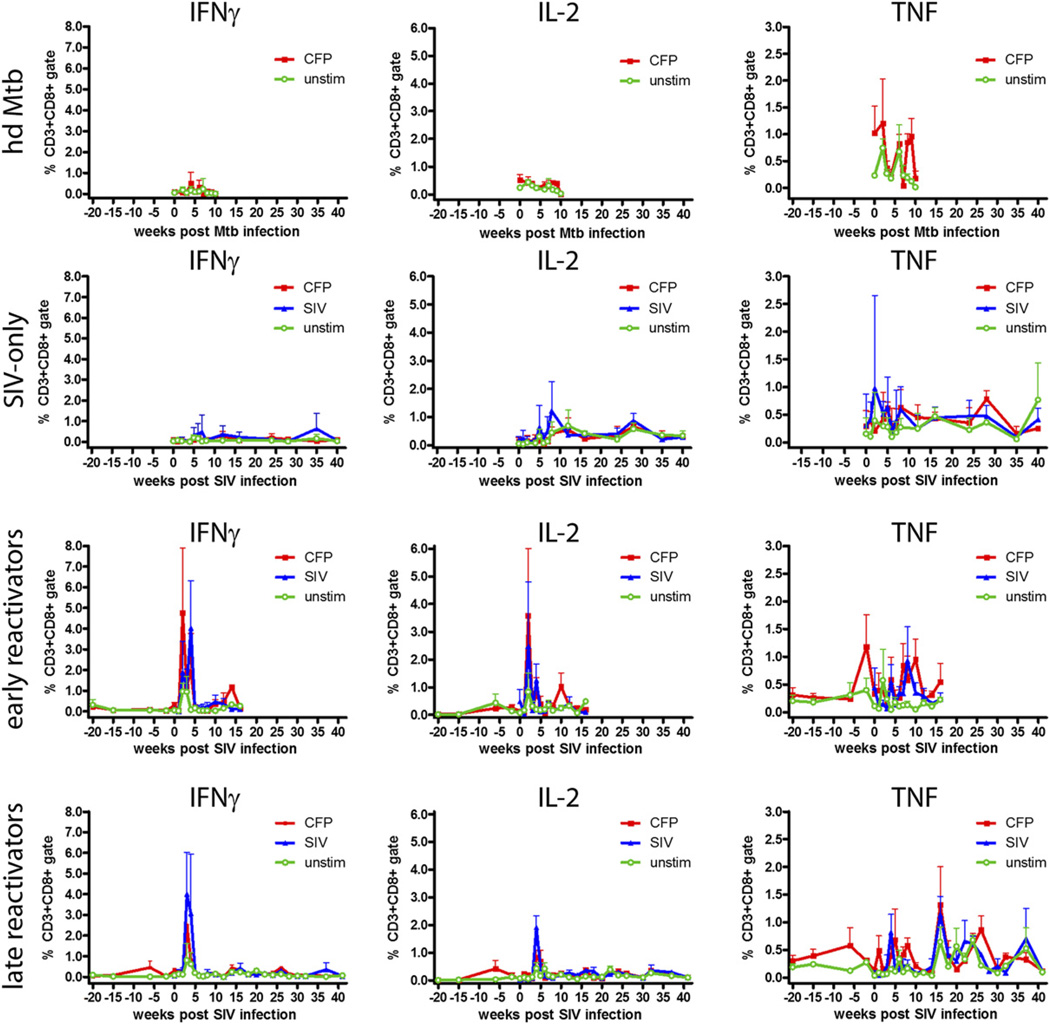
Th1 (IFN-γ, IL-2, TNF) and Th2 (IL-4, IL-10) responses in CD4+ and CD8+ T cells were measured to determine how SIV and M. tuberculosis–SIV coinfection modified Ag-specific responses (Supplemental Fig. 2). IL-10 is a cytokine with pleomorphic effects, and for the purposes of this study IL-10 was considered a Th2 cytokine because it has the capacity to downregulate Th1 responses (44). M. tuberculosis-specific T cell responses varied widely among animals and within the same animal. The hd M. tuberculosis animals were used to monitor responses to acute M. tuberculosis infection in M. tuberculosis- and SIV-naive animals and showed small numbers of IFN-γ– and IL-2–producing T cells in the periphery after CFP stimulation (Figs. 1, 2). SIV-only animals demonstrated SIV-specific responses but negligible M. tuberculosis-specific responses (Figs. 1, 2). Animals with latent infection, which were subsequently infected with SIV, showed low M. tuberculosis-specific responses that were similar to responses observed in the hd M. tuberculosis monkeys. SIV infection of these animals led to substantially increased M. tuberculosis-specific responses. These animals experienced significant increases in the frequency of M. tuberculosis-specific IFN-γ– and IL-2– expressing T cells 2–5 wk post-SIV infection that were above pre-SIV expression levels (Figs. 1, 2). After the initial spike in Ag-specific T cells, the frequencies of IFN-γ– and IL-2–expressing T cells decreased to low levels. The initial spike in responses was not observed in the hd M. tuberculosis animals, suggesting the large peak in peripheral responses was unique to monkeys with previously primed anti-mycobacterial responses. The frequency of TNF+ T cells in the coinfected period was variable and similar to the pre-SIV infection period of latent disease (Figs. 1, 2).
FIGURE 1.
Pathogen-specific CD4+ T cell responses increase during the acute phase of infection. Data represent the mean frequency of cytokine-positive CD4+ T cells ± SEM; only upper error bars have been shown for clarity. Early reactivators are animals reactivating <17 wk post-SIV infection, and late reactivators are animals reactivating >26 wk post-SIV infection. Pre-SIV infection data from monkey 10405 were not available. hdMtb, high-dose M. tuberculosis.
FIGURE 2.
Pathogen-specific CD8+ T cell responses increase during the acute phase of infection. Data represent the mean frequency of cytokine-positive CD8+ T cells ± SEM; only upper error bars have been shown for clarity. Early reactivators are animals reactivating <17 wk post-SIV infection, and late reactivators are animals reactivating >26 wk post-SIV infection. Pre-SIV infection data from monkey 10405 were not available. hdMtb, high-dose M. tuberculosis.
Significant numbers of IL-4–expressing T cells are present for a short period postinfection
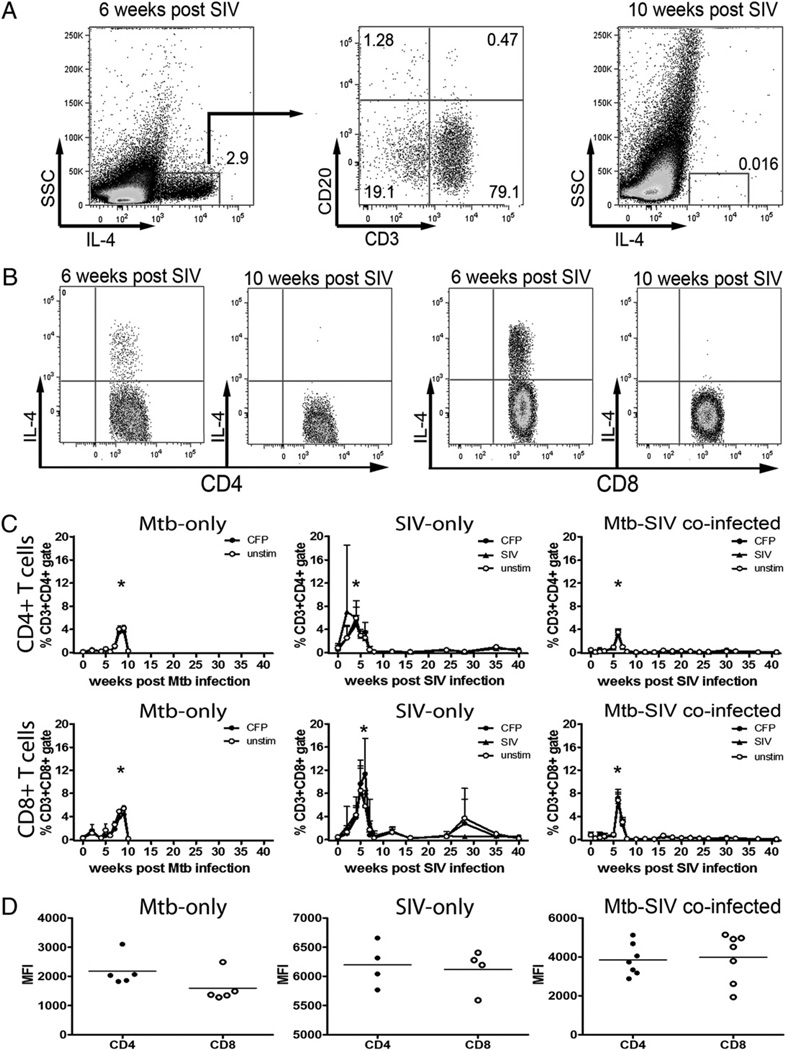
Th2 cytokines have been linked to diminished resistance to mycobacterial infection (12–18, 45) so we assessed the effect of SIV infection on IL-4 and IL-10 expression in the macaques. IL-10+ T cells were present at low frequencies on a constitutive basis over the course of infection, but their frequency did not increase above baseline levels in response to stimulation with CFP or Gag/Pol Ags (data not shown). However, the macaques experienced a transient pulse of IL-4–expressing T cells that was significantly above baseline IL-4 expression (p < 0.05; Kruskal–Wallis test) after infection with M. tuberculosis or SIV (SIV-only or coinfected monkeys) (Fig. 3). IL-4 expression was largely restricted to CD3+ T cells with only a small fraction of CD20+ B cells expressing IL-4 (Fig. 3A). This pulse of IL-4 expression included more CD8+ T cells than CD4+ T cells (Fig. 3B) and was not repeated over the course of study in either SIV-only or coinfected macaques (Fig. 3B, 3C). The quantity of IL-4 on a per cell basis, as measured by mean fluorescence units, was similar between CD4+ and CD8+ T cells (Fig. 3D). The cause of this sudden and short-lived burst of IL-4+ cells remains to be determined, but it temporally correlated with a period of reduced IFN-γ and IL-2 expression (Figs. 1, 2) in coinfected animals, suggesting that it downregulated proinflammatory cytokine expression.
FIGURE 3.
Transient IL-4 expression occurs in macaques after infection. All animals experienced a pulse of IL-4+ T cells after SIV or M. tuberculosis infection. FACS plots showing IL-4 expression are from monkey 3007, a coinfected animal. A, IL-4 expression is largely restricted to CD3+ T cells. B and C, Peak periods of IL-4 expression included a significant proportion of CD4+ and CD8+ T cells and were not repeated. Greater frequencies of CD8+ T cells expressed IL-4 compared with frequencies of CD4+ T cells that expressed IL-4. Pre-SIV infection IL-4 data for coinfected animals were not available. Data represent the mean frequency of IL-4+ T cells ± SEM; only upper error bars are shown for clarity. Asterisks indicate statistically significant increases at week 6 in coinfected animals and at weeks 2–6 in SIV-only animals compared with postpeak IL-4 time points (p < 0.05, Kruskal–Wallis one-way ANOVA) and at weeks 8–9 in M. tuberculosis-only animals compared with other time points (p < 0.001, Kruskal–Wallis one-way ANOVA). D, IL-4 expression on a per-cell basis was not significantly different between CD4+ or CD8+ T cell populations. Data are the mean values from each monkey of the unstimulated and CFP-stimulated (coinfected animals) or SIV Gag/Pol-stimulated (SIV-only group) T cells. IL-4 expression in unstimulated and stimulated T cells was not significantly different. Data were acquired on different dates with different FACS compensation settings, thus were not directly comparable. Mtb, M. tuberculosis.
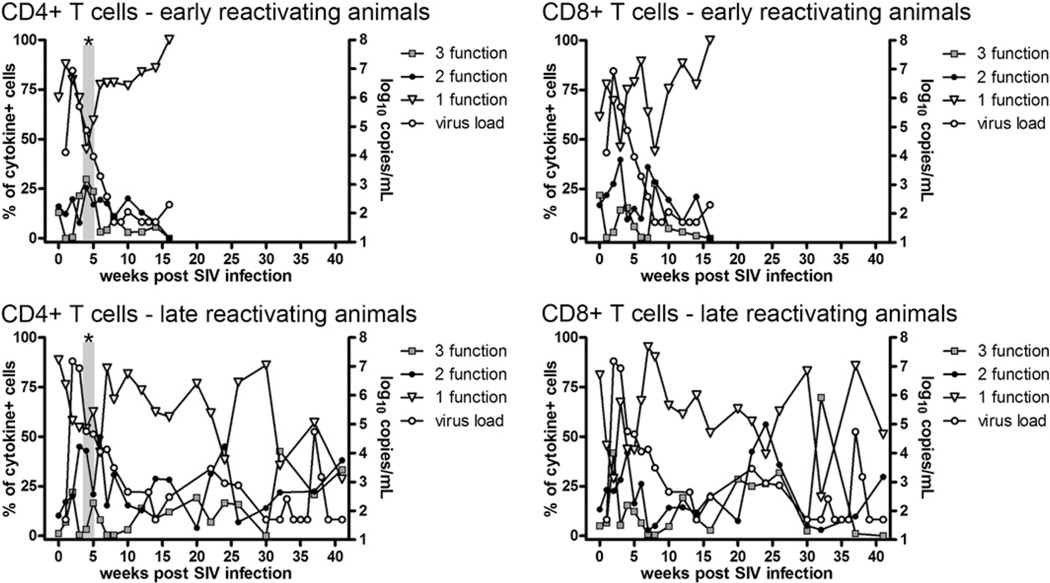
Multifunctional Th1 cytokine responses increase with acute SIV infection
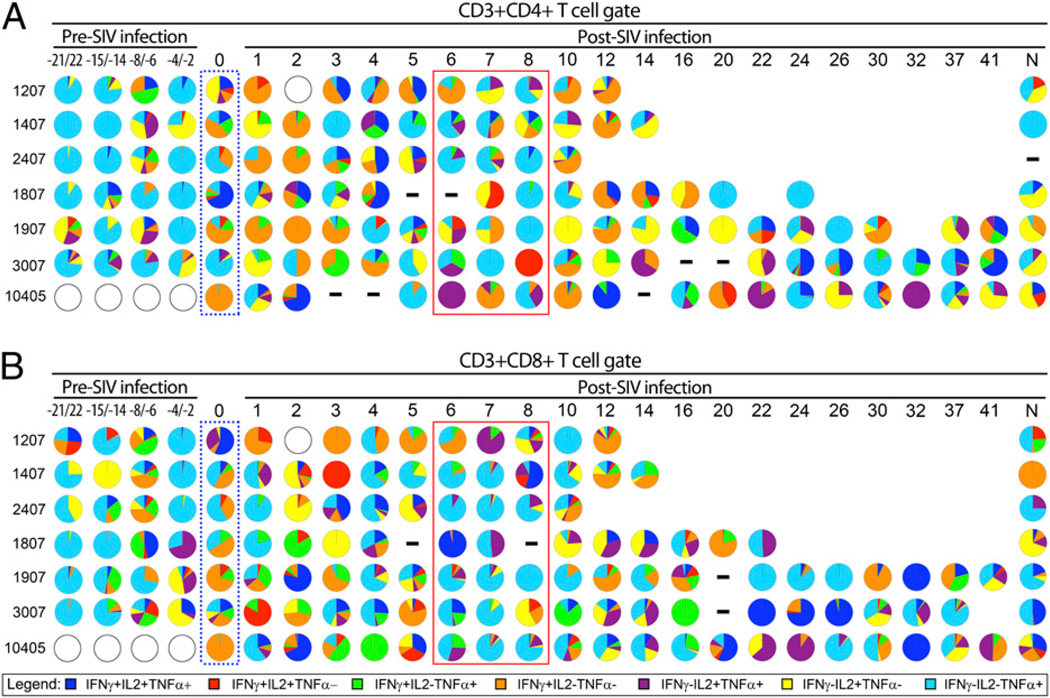
Multifunctional T cells are postulated to be immune correlates of protection (11). Given this, we measured the multifunctionality of mycobacteria-specific T cell responses to determine whether higher frequencies of multifunctional T cells correlated with protection against reactivated TB. Most cytokine-positive cells were positive for only a single cytokine with a period of abundant multifunctional CD4+ T cells temporally consistent with peak plasma viremia (Fig. 4). There was a drop in single-positive cells concurrent with increased frequencies of multifunctional cells, suggesting these multifunctional cells differentiated from single-function cells as proposed by Seder et al. (46). Significant differences in two- and three-function CD8+ T cell frequencies were not observed. Early reactivating monkeys had significantly more IFN-γ+IL-2+TNF+CD4+ T cells over weeks 3–5, when aggregated together, than late reactivating monkeys (p = 0.021, Mann–Whitney U) over the same time period (Fig. 4). The early reactivating animals had greater bacterial burdens and gross pathology at necropsy (40), and the abundance of Ag-specific IFN-γ+IL-2+TNF+CD4+ T cells during acute SIV infection suggests multifunctional T cell proliferation may relate to increased levels of mycobacterial Ags. Separating the multifunctional analysis into discrete functional units (e.g., IFN-γ+IL-2+ compared with IFN-γ+TNF+) demonstrated the remarkably diverse response elicited by CFP stimulation (Fig. 5). CD4+ T cell responses during weeks 1–3 post-SIV infection included increased frequencies of IFN-γ+CD4+ T cells (Fig. 5A, orange pie slices) relative to the pre-SIV latent period. CD8+ T cell responses appeared more diverse than CD4+ T cell responses, especially regarding the IFN-γ+TNF+CD8+T cells (Fig. 5B, green pie slices) in late reactivating monkeys. Notably, weeks 6–8 were characterized by an unusual lack of CD8+ T cell multifunctionality where all monkeys demonstrated an abundance of TNF-only responses (Fig. 5B, royal blue slices, red box). This trend was less evident in CFP-stimulated CD4+ T cells (Fig. 5A).
FIGURE 4.
Frequency of cytokine-positive T cells positive for one, two, or three cytokines in coinfected macaques correlated with plasma viral loads. Data from cytokine-positive CFP-stimulated T cells were Boolean gated and grouped into three categories: IFN-γ+IL-2+TNF+ 3-function cells (open squares), 2-function cells (filled circles) expressing two cytokines, and 1-function cells expressing only IFN-γ, IL-2, or TNF (open triangles). Plasma viral loads (open circles) were previously reported by Diedrich et al. (40). Data represent the mean frequency of cytokine-positive cells for each functional group with error bars omitted for clarity. Asterisks indicates a statistically significant difference (p = 0.021, Mann–Whitney U) in the mean frequency of 3-function CD4+ T cells between early (n = 3) and late (n = 4) reactivating animals for the pooled data from weeks 3, 4, 5 post-SIV infection (shaded box).
FIGURE 5.
CFP stimulation induces multifunctional phenotypes in CD4+ and CD8+ T cells from coinfected macaques. Boolean gated data are presented as pie charts with each slice representing a percentage of the total number of cytokine-positive cells. Time points where data were not available are represented by open circles, and points where responses in CFP-stimulated tubes were less than or equal to the unstimulated tube are indicated by a horizontal dash. The dotted blue box indicates T cell responses on the day of SIV infection. The solid red line indicates a 3 wk period rich in CD8+ T cells only producing TNF. Monkeys 1207, 1407, and 2407 are early reactivating animals, whereas 1807, 1907, 3007, and 10405 are late reactivating animals. The time pre- and post-SIV infection is indicated in weeks; monkeys 1207, 1407, and 1807 were sampled −21, −14, −6, and −2 wk pre-SIV infection, and monkeys 1907, 2407, and 3007 were sampled at −20, −15, −8, and −4 wk pre-SIV infection. N, T cell responses at the time of necropsy. A, CD4+ T cell responses. B, CD8+ T cell responses.
CD8+ T cell polarization during acute SIV infection correlates with reactivation
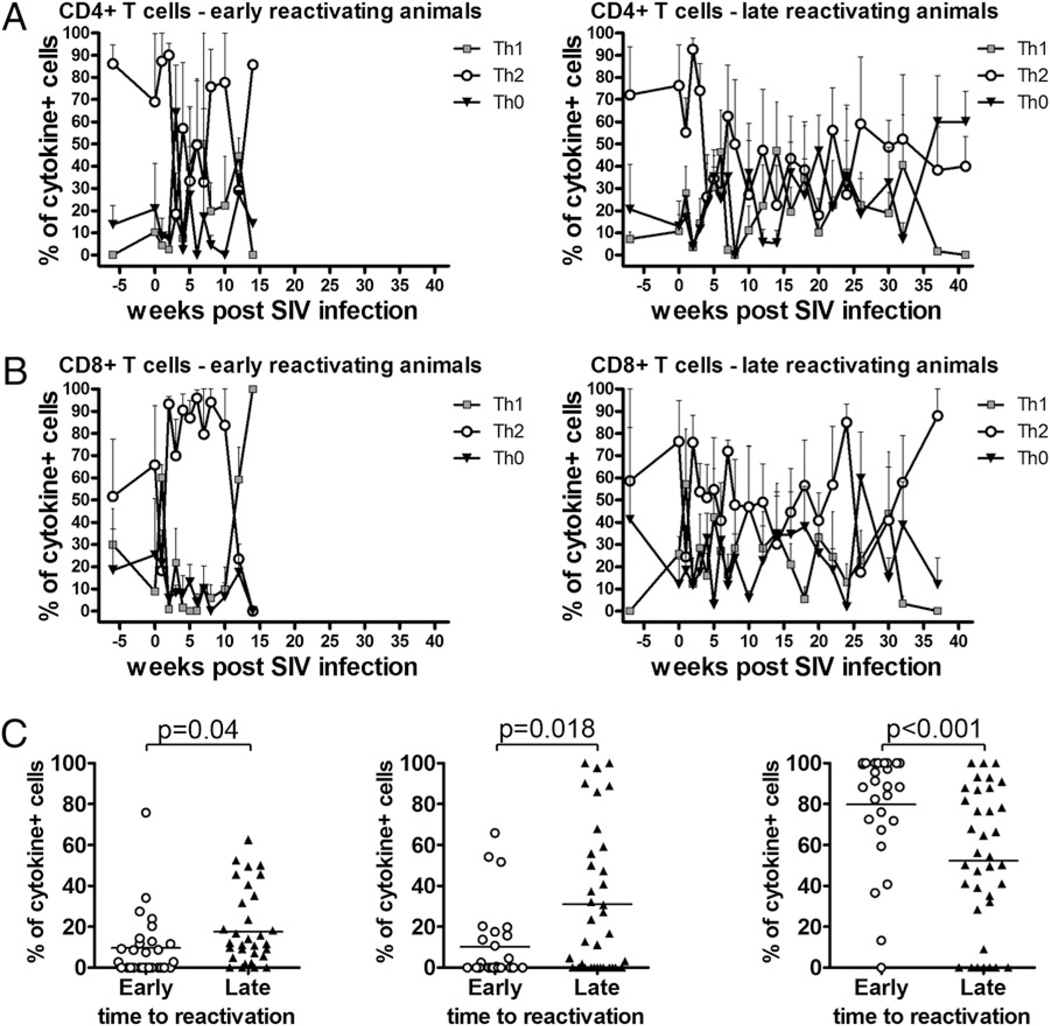
There is some evidence that individuals with less severe TB have more robust peripheral Th1-cytokine responses than those of individuals with more severe disease who have T cells biased toward Th2-cytokine production (19, 47). Similarly, untreated TB may be accompanied by mixed proinflammatory and anti-inflammatory responses mediated by Th0-polarized T cells that shift toward proinflammatory Th1 cytokine production after successful drug therapy (35–37). To investigate the contributions of Th0- and Th2-polarized cells to SIV-induced reactivated TB, we evaluated the phenotypes of cells stained for IFN-γ, IL-4, and IL-10 to compare frequencies of Th0- (IFN-γ+IL-4+IL-10+, IFN-γ+IL-4+, IFN-γ+IL-10+), Th1- (IFN-γ+), and Th2- (IL-4+IL-10+, IL-4+, IL-10+) polarized T cells in early and late reactivating monkeys. CD4+ T cell polarization was not significantly different between early and late reactivating monkeys (Fig. 6A). In contrast to CD4+ T cell responses, we found late reactivating monkeys had fewer Th2-polarized CD8+ T cells and more Th0- and Th1-polarized T cells (Fig. 6B). When the responses from the weeks 1–10 post-SIV infection were aggregated and compared, the early reactivating animals had more Th2-polarized (p = 0.023, Mann–Whitney U), fewer Th0-polarized (p = 0.043, Mann–Whitney U), and fewer Th1-polarized (p < 0.001, Mann–Whitney U) CD8+ T cells than late reactivating animals (Fig. 6C). In contrast, polarization states of CD4+ T cells from early and late reactivating monkeys were not significantly different (data not shown). These data suggest a predominance of purely Th2-polarized CD8+ T cells coupled with a lack of Th1 cytokine production had detrimental effects on the maintenance of latency.
FIGURE 6.
Early and late reactivating animals show differences in frequencies of Th0-, Th1-, and Th2-polarized CD8+ T cells. Responses of CFP-stimulated cells were combined to reflect populations of Th1-polarized (IFN-γ), Th2-polarized (IL-4+IL-10+, IL-4+, or IL-10+), or Th0-polarized (IFN-γ+IL-4+IL-10+, IFN-γ+IL-4+, or IFN-γ+IL-10+) T cells. A, CD4+ T cell responses. B, CD8+ T cell responses. Data represent the mean frequency of cytokine-expressing cells ± SEM; lower error bars have been omitted for clarity. C, CD8+ T cell phenotypes were determined as previously described and the response for weeks 1–10 obtained by pooling the responses for early and late reactivating monkeys. Pre-SIV phenotypes were not significantly different between groups of animals (data not shown). Early reactivating animals had significantly fewer Th0- and Th1-polarized CD8+ T cells (p = 0.004 and p = 0.018, respectively; Mann–Whitney U) and more Th2-polarized CD8+ T cells (p < 0.001; Mann–Whitney U).
Mycobacteria-specific responses in the granuloma differ from those of the PBMCs
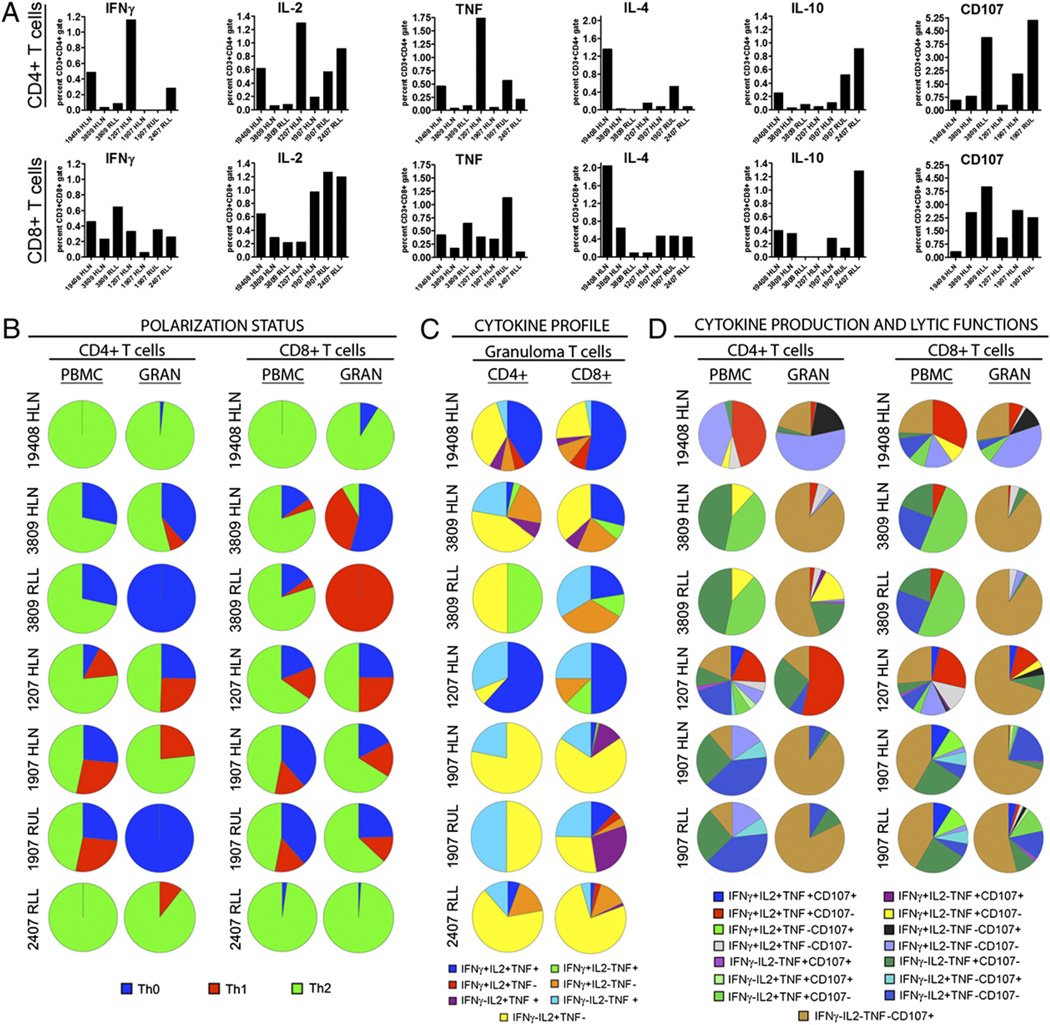
Granuloma-containing tissues from M. tuberculosis-only and coinfected monkeys were homogenized into single-cell suspensions and the T cell responses assessed by flow cytometry. Each granuloma is unique, in terms of bacterial numbers and even cellular composition (39), and we found the T cell cytokine responses among granulomas were highly variable and different from those of the peripheral blood. Granuloma T cells tended to have high frequencies of M. tuberculosis-specific Th1-polarized T cells (Fig. 7A). IL-4– and IL-10–expressing cells were observed (Fig. 7A), but similar to responses in the peripheral blood, expression of these cytokines was not upregulated by CFP stimulation. CD107+ cells were observed and generally more abundant than the cytokine-positive cells (Fig. 7A). In a multifunctional analysis examining the frequency of Th0- (IFN-γ+IL-4+IL-10+, IFN-γ+IL-4+, or IFN-γ+IL-10+), Th1- (IFN-γ+), and Th2- (IL-4+, IL-10+, or IL-4+IL-10+) polarized T cells, the majority of granuloma T cell responses were found to be Th0- and Th2-polarized (Fig. 7B). Separating the cytokine-positive cells into distinct multifunctional populations demonstrated the heterogeneity in responses between monkeys and between PBMC and tissue cells within a monkey (Supplemental Table I). Th1 cytokines were upregulated after stimulation, and expression of these cytokines was compared against upregulated responses from the peripheral blood. Single positive IL-2–producing T cells were the dominant phenotype, with single positive cells expressing TNF being the other major cytokine-secreting phenotype (Fig. 7C). Inclusion of surface CD107 expression, an indicator of T cell cytolytic activity (43), indicated significant numbers of T cells in the granuloma were positive for only CD107 (Fig. 7D, brown pie slides), with cytokine-producing cells making up smaller fractions of the total response (Fig. 7D). These data suggest that T cell responses in the granuloma are balanced between Th1 and Th2 responses and substantially different from those of peripheral blood T cells, particularly with respect to the abundance of cytolytic T cells.
FIGURE 7.
Profiles of T cells isolated from granulomas differ from peripheral blood T cells. T cells from homogenized granulomas were assayed for cytokine production and surface CD107 expression after CFP stimulation and compared against similarly treated peripheral blood T cells obtained at necropsy. Monkeys 19408 and 3809 are SIV-negative control animals with active TB. HLN, hilar lymph node; RLL, right lower lung granuloma. A, Cytokine responses of granuloma T cells as a percentage of gated cells. Bar order is (left to right on the x-axes): 19408 HLN, 3809 HLN, 3809 RLL, 1207 HLN, 1907 HLN, 1907 RLL, 2407 RLL. CD107 data were unavailable for monkey 2407. B, Th0-, Th1-, and Th2-polarized T cells in the granuloma and peripheral blood. T cell polarization states were determined as indicated in the figure. The separate phenotypes of cytokine-positive cells, expressed as percentages of the total cytokine-positive population, used to derive these data are listed in Supplemental Table I. C, Cytokine profiles of CD4+ and CD8+ T cells in the granuloma. Peripheral blood T cell data for coinfected animals is included in Fig. 5, last column (N). D, Characterization of T cells for effector function, including cytokine production and cytolytic function. CD107 data were not available for monkey 2407.
Discussion
Immune events associated with HIV coinfection of individuals with latent TB are challenging to investigate through clinical studies, and the lack of a biologically relevant coinfection model has limited the research that can be performed. We recently developed and characterized an animal model of HIV-induced reactivation of latent TB using cynomolgus macaques to address this need. Although there was an initial peak in viral loads, the cynomolgus macaques did not recapitulate high plasma virus set-point observed in human HIV infections (40, 48), but even at low peripheral viral loads, these animals were sufficiently immunosuppressed by SIV that they all developed reactivated TB (40). Previously, we identified significant correlations between CD4+ T cell numbers and time to reactivation (40) but did not focus on the changes in cytokine expression (outside of ELISPOT-assessed IFN-γ production) after SIV infection that may have influenced reactivation. Considering this, we sought to address this question by measuring CD4+ and CD8+ T cell cytokine responses from latent TB through the early events after coinfection with SIV and development of reactivated TB.
Our data demonstrate that SIV infection causes remarkable immunologic flux in cynomolgus macaques with latent TB. Increased cytokine responses reminiscent of amplified cytokine responses observed during acute HIV infection (49) included significantly elevated frequencies of M. tuberculosis-specific IFN-γ–, IL-2–, and TNF-expressing T cells. We have postulated that SIV depletes T cells coordinating anti-mycobacterial responses in stable granulomas during acute infection, releasing immune pressures that normally limit bacterial replication, and leading to an increased abundance of mycobacterial Ags (40). The data presented in this study suggest that the increased Ag load stimulates proliferation of M. tuberculosis-specific T cells, which are detected as Th1 cytokine-positive cells in the peripheral blood. Thus, the abundance of M. tuberculosis-specific multifunctional T cells during acute SIV infection may represent higher Ag loads, and we note that the earliest reactivating animals had both the highest frequencies of IFN-γ+IL-2+TNF+CD4+ T cells during acute infection and the highest bacterial burden at necropsy (40). Moreover, later reactivating animals showed increasing frequencies of three-function CD4+ T cells toward the end of the study period. In humans, multifunctional IFN-γ+IL-2+TNF+CD4+ T cells have been associated with active TB (29), and it is noted that frequencies of IFN-γ+IL-2+TNF+CD4+ T cell frequencies decrease after antituberculous drug therapy (28). Considering these data, it appears that multifunctional T cells in TB are peripheral indicators of higher Ag or bacterial loads rather than of protection. Whether the presence of multifunctional cells present prior to infection, for example from a vaccination protocol, could limit mycobacterial infection, as suggested by Darrah et al. (11), remains to be seen. The fate of the large number of cytokine-producing cells, as shown by their disappearance after acute infection, is unknown. It has been demonstrated that HIV selectively depletes M. tuberculosis-specific T cells prior to the loss of T cells specific for opportunistic pathogens including Candida albicans and CMV (50), and these cells may be eliminated by virus infection. Alternatively, T cell exhaustion is prominent in HIV infection (51), and the decreased frequencies of cytokine-producing cells may indicate exhaustion. MHC tetramers are not available for cynomolgus macaques, and staining for PD-1, a cell-surface receptor associated with exhaustion in HIV patients (52), has been inconclusive (data not shown), thus the processes leading to diminished Th1 cytokine expression remain unclear. Finally, it is possible that these cells migrate from the periphery to the site of infection and therefore are no longer available for serial sampling.
Peripheral blood T cell responses can be sampled with relative ease, but understanding T cell function in the granuloma is necessary to determine how infection is controlled at the local level. Moreover, data on factors constituting appropriate and protective granuloma T cell responses will be important for designing T cell-based vaccines against M. tuberculosis. Although protective correlates in the granuloma are largely unknown, the greatest protection will likely be achieved by balancing responses that activate macrophages (IFN-γ and TNF), stimulate proliferation (IL-2), reduce inflammation, and induce apoptosis in M. tuberculosis-infected cells. Our data indicate that M. tuberculosis-specific T cells in the granuloma have lytic functions or produce IL-2 and are less likely than peripheral blood T cells to express IFN-γ or TNF. We reported a similar distinction in the CD8+ T cell subset in mice infected with M. tuberculosis, where CD8+ T cells were either cytotoxic or IFN-γ producing, but rarely both (53). This suggests that the T cell response in the granuloma during active TB is predominately focused on lysis of infected cells, with a minority of T cells having roles activating macrophages or stimulating T cell proliferation.
Excessive inflammation may negatively affect protection by damaging lung tissue peripheral to the granuloma; consequently, balancing proinflammatory Th1 cytokine expression with appropriate expression of Th2 cytokines or anti-inflammatory factors may also be protective. In concordance with this, we found granulomas from SIV-negative and coinfected animals contained both Th1- and Th2-polarized cells as well as Th0-polarized T cells. Peripheral blood CD8+ T cells in early reactivating monkeys had responses skewed toward IL-4 and IL-10 expression, whereas late reactivating monkeys had lower frequencies of Th2-polarized CD8+ T cells and higher frequencies of IFN-γ–expressing CD8+ T cells during acute SIV infection, indicating a balance between Th1 and Th2 responses may be critical in maintenance of latency. Moreover, appropriately timed expression of Th2 cytokines may also be important and function to dampen tissue-damaging inflammation after acute responses to pathogens. The brief pulse of IL-4–expressing T cells in M. tuberculosis-only, SIV-only, and M. tuberculosis–SIV coinfected macaques may reflect a systemic effort to downregulate inflammatory processes, but as of yet, mechanisms regulating this pulse of IL-4 and its function remain unclear. We found that the Th1 and Th2 cytokine profiles of granuloma T cells from SIV-negative and coinfected animals were highly variable, even between granulomas from the same animal. In all cases, these responses were measured during active disease and it is possible that after reactivation has occurred, granuloma T cell responses from SIV-infected monkeys may be similar to responses from SIV-negative monkeys with active TB. Consequently, it may be difficult retrospectively to link reactivated TB to cytokine expression of a single cell subset after reactivation has already occurred. Instead, latent granulomas, or granulomas in the early stages of SIV coinfection, should be studied in the future to address these questions.
Our results indicate that immunologic events during the acute phase of HIV infection are critically important in determining susceptibility to early reactivation of latent TB. These data indicate that the immune dysregulation occurring after HIV infection, even in the absence of severe CD4+ T cell depletion and high viral loads, correlates with reactivated TB. Based on our data from these SIV-infected cynomolgus macaques, we conjecture that HIV-induced distortions in T cell populations mediating proinflammatory and anti-inflammatory cytokine responses at the granuloma level may alter the equilibrium between bacterial growth and immune pressure in favor of reactivation. Furthermore, our data highlight functional differences between the peripheral blood and granuloma T cell responses that may be relevant to development of T cell-based vaccines against M. tuberculosis. We expect that studies using models of HIV–M. tuberculosis coinfection will enable a better understanding of the basic biology of host–pathogen interaction and allow development of novel therapies and vaccines fostering protective responses that diminish the likelihood of reactivated TB in immunocompromised individuals.
Supplementary Material
Acknowledgments
We thank Dr. Edwin Klein and Dr. Chris Janssen for performing necropsies, Mark Rodgers, Catherine Cochran, and Kelly Wyatt for superb technical assistance, and Jennifer Kerr, Melanie O’Malley, Jamie Tomko, Dan Fillmore, and Paul Johnston for outstanding animal care and procedures.
This work was supported by National Institutes of Health Grants RO1 HL074845-04 (to J.L.F.), T32 AI060525-05 (to J.L.F., supporting J.T.M.), and F32 AI077183-01A1 (to J.T.M.) and by the Heiser Program for Research in Tuberculosis and Leprosy (to J.T.M.).
Abbreviations used in this article
- BAL
bronchoalveolar lavage
- CFP
culture filtrate protein
- hd
high-dose
- TB
tuberculosis
- TNF
tumor necrosis factor-α
- Treg
regulatory T cell
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Selwyn PA, Alcabes P, Hartel D, Buono D, Schoenbaum EE, Klein RS, Davenny K, Friedland GH. Clinical manifestations and predictors of disease progression in drug users with human immunodeficiency virus infection. N. Engl. J. Med. 1992;327:1697–1703. doi: 10.1056/NEJM199212103272401. [DOI] [PubMed] [Google Scholar]
- 2.Cardona PJ, Ruiz-Manzano J. On the nature of Mycobacterium tuberculosis-latent bacilli. Eur. Respir. J. 2004;24:1044–1051. doi: 10.1183/09031936.04.00072604. [DOI] [PubMed] [Google Scholar]
- 3.Selwyn PA, Hartel D, Lewis VA, Schoenbaum EE, Vermund SH, Klein RS, Walker AT, Friedland GH. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N. Engl. J. Med. 1989;320:545–550. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 4.Wolday D, Hailu B, Girma M, Hailu E, Sanders E, Fontanet AL. Low CD4+ T-cell count and high HIV viral load precede the development of tuberculosis disease in a cohort of HIV-positive Ethiopians. Ethiop. Med. J. 2003;41 Suppl 1:67–73. [PubMed] [Google Scholar]
- 5.Djoba Siawaya JF, Ruhwald M, Eugen-Olsen J, Walzl G. Correlates for disease progression and prognosis during concurrent HIV/TB infection. Int. J. Infect. Dis. 2007;11:289–299. doi: 10.1016/j.ijid.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Lawn SD, Churchyard G. Epidemiology of HIV-associated tuberculosis. Curr. Opin. HIV AIDS. 2009;4:325–333. doi: 10.1097/COH.0b013e32832c7d61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, Bloom BR. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 9.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 10.Newport MJ, Huxley CM, Huston S, Hawrylowicz CM, Oostra BA, Williamson R, Levin M. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N. Engl. J. Med. 1996;335:1941–1949. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 11.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 12.Li-Weber M, Krammer PH. Regulation of IL4 gene expression by T cells and therapeutic perspectives. Nat. Rev. Immunol. 2003;3:534–543. doi: 10.1038/nri1128. [DOI] [PubMed] [Google Scholar]
- 13.Bogdan C, Vodovotz Y, Paik J, Xie QW, Nathan C. Mechanism of suppression of nitric oxide synthase expression by interleukin-4 in primary mouse macrophages. J. Leukoc. Biol. 1994;55:227–233. doi: 10.1002/jlb.55.2.227. [DOI] [PubMed] [Google Scholar]
- 14.Mazzarella G, Bianco A, Perna F, D’Auria D, Grella E, Moscariello E, Sanduzzi A. T lymphocyte phenotypic profile in lung segments affected by cavitary and non-cavitary tuberculosis. Clin. Exp. Immunol. 2003;132:283–288. doi: 10.1046/j.1365-2249.2003.02121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boussiotis VA, Tsai EY, Yunis EJ, Thim S, Delgado JC, Dascher CC, Berezovskaya A, Rousset D, Reynes JM, Goldfeld AE. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J. Clin. Invest. 2000;105:1317–1325. doi: 10.1172/JCI9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Djoba Siawaya JF, Beyers N, van Helden P, Walzl G. Differential cytokine secretion and early treatment response in patients with pulmonary tuberculosis. Clin. Exp. Immunol. 2009;156:69–77. doi: 10.1111/j.1365-2249.2009.03875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manca C, Reed MB, Freeman S, Mathema B, Kreiswirth B, Barry CE, III, Kaplan G. Differential monocyte activation underlies strain-specific Mycobacterium tuberculosis pathogenesis. Infect. Immun. 2004;72:5511–5514. doi: 10.1128/IAI.72.9.5511-5514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ordway DJ, Costa L, Martins M, Silveira H, Amaral L, Arroz MJ, Ventura FA, Dockrell HM. Increased Interleukin-4 production by CD8 and gammadelta T cells in health-care workers is associated with the subsequent development of active tuberculosis. J. Infect. Dis. 2004;190:756–766. doi: 10.1086/422532. [DOI] [PubMed] [Google Scholar]
- 19.de la Barrera S, Aleman M, Musella R, Schierloh P, Pasquinelli V, Garcia V, Abbate E, Sasiain Mdel. C. IL-10 down-regulates costimulatory molecules on Mycobacterium tuberculosi-pulsed macrophages and impairs the lytic activity of CD4 and CD8 CTL in tuberculosis patients. Clin. Exp. Immunol. 2004;138:128–138. doi: 10.1111/j.1365-2249.2004.02577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green AM, Mattila JT, Bigbee C, Bongers KS, Lin PL, Flynn JL. CD4(+) regulatory T cells in a cynomolgus macaque model of Mycobacterium tuberculosis Infection. J. Infect. Dis. 2010;202:533–541. doi: 10.1086/654896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott-Browne JP, Shafiani S, Tucker-Heard G, Ishida-Tsubota K, Fontenot JD, Rudensky AY, Bevan MJ, Urdahl KB. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J. Exp. Med. 2007;204:2159–2169. doi: 10.1084/jem.20062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makedonas G, Betts MR. Polyfunctional analysis of human t cell responses: importance in vaccine immunogenicity and natural infection. Springer Semin. Immunopathol. 2006;28:209–219. doi: 10.1007/s00281-006-0025-4. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed N, Gottschalk S. How to design effective vaccines: lessons from an old success story. Expert Rev. Vaccines. 2009;8:543–546. doi: 10.1586/erv.09.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sáez-Cirión A, Pancino G, Sinet M, Venet A, Lambotte O ANRS EP36 HIV Controllers study group. HIV controllers: how do they tame the virus? Trends Immunol. 2007;28:532–540. doi: 10.1016/j.it.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Bolton DL, Roederer M. Flow cytometry and the future of vaccine development. Expert Rev. Vaccines. 2009;8:779–789. doi: 10.1586/erv.09.41. [DOI] [PubMed] [Google Scholar]
- 26.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcondes MC, Penedo MC, Lanigan C, Hall D, Watry DD, Zandonatti M, Fox HS. Simian immunodeficiency virus-induced CD4+ T cell deficits in cytokine secretion profile are dependent on monkey origin. Viral Immunol. 2006;19:679–689. doi: 10.1089/vim.2006.19.679. [DOI] [PubMed] [Google Scholar]
- 28.Caccamo N, Guggino G, Joosten SA, Gelsomino G, Di Carlo P, Titone L, Galati D, Bocchino M, Matarese A, Salerno A, et al. Multifunctional CD4(+)T cells correlate with active Mycobacterium tuberculosis infection. Eur. J. Immunol. 2010;40:2211–2220. doi: 10.1002/eji.201040455. [DOI] [PubMed] [Google Scholar]
- 29.Sutherland JS, Adetifa IM, Hill PC, Adegbola RA, Ota MO. Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur. J. Immunol. 2009;39:723–729. doi: 10.1002/eji.200838693. [DOI] [PubMed] [Google Scholar]
- 30.Sutherland JS, Young JM, Peterson KL, Sanneh B, Whittle HC, Rowland-Jones SL, Adegbola RA, Jaye A, Ota MO. Poly-functional CD4(+) and CD8(+) T cell responses to tuberculosis antigens in HIV-1-infected patients before and after anti-retroviral treatment. J. Immunol. 2010;184:6537–6544. doi: 10.4049/jimmunol.1000399. [DOI] [PubMed] [Google Scholar]
- 31.Sutherland JS, de Jong BC, Jeffries DJ, Adetifa IM, Ota MO. Production of TNF-alpha, IL-12(p40) and IL-17 can discriminate between active TB disease and latent infection in a West African cohort. PLoS ONE. 2010;5:e12365. doi: 10.1371/journal.pone.0012365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Day CL, Mkhwanazi N, Reddy S, Mncube Z, van der Stok M, Klenerman P, Walker BD. Detection of polyfunctional Mycobacterium tuberculosis specific T cells and association with viral load in HIV-1-infected persons. J. Infect. Dis. 2008;197:990–999. doi: 10.1086/529048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalsdorf B, Scriba TJ, Wood K, Day CL, Dheda K, Dawson R, Hanekom WA, Lange C, Wilkinson RJ. HIV-1 infection impairs the bronchoalveolar T-cell response to mycobacteria. Am. J. Respir. Crit. Care Med. 2009;180:1262–1270. doi: 10.1164/rccm.200907-1011OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jalapathy KV, Prabha C, Das SD. Correlates of protective immune response in tuberculous pleuritis. FEMS Immunol. Med. Microbiol. 2004;40:139–145. doi: 10.1016/S0928-8244(03)00303-1. [DOI] [PubMed] [Google Scholar]
- 35.Marchant A, Amedei A, Azzurri A, Vekemans J, Benagiano M, Tamburini C, Lienhardt C, Corrah T, McAdam KP, Romagnani S, et al. Polarization of PPD-specific T-cell response of patients with tuberculosis from Th0 to Th1 profile after successful antimycobacterial therapy or in vitro conditioning with interferon-alpha or interleukin-12. Am. J. Respir. Cell Mol. Biol. 2001;24:187–194. doi: 10.1165/ajrcmb.24.2.4274. [DOI] [PubMed] [Google Scholar]
- 36.Caccamo N, Meraviglia S, Dieli F, Romano A, Titone L, Salerno A. Th0 to Th1 switch of CD4 T cell clones specific from the 16-kDa antigen of Mycobacterium tuberculosis after successful therapy: lack of involvement of epitope repertoire and HLA-DR. Immunol. Lett. 2005;98:253–258. doi: 10.1016/j.imlet.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 37.Dieli F, Singh M, Spallek R, Romano A, Titone L, Sireci G, Friscia G, Di Sano C, Santini D, Salerno A, Ivanyi J. Change of Th0 to Th1 cell-cytokine profile following tuberculosis chemotherapy. Scand. J. Immunol. 2000;52:96–102. doi: 10.1046/j.1365-3083.2000.00744.x. [DOI] [PubMed] [Google Scholar]
- 38.Wilkinson RJ, Vordermeier HM, Wilkinson KA, Sjölund A, Moreno C, Pasvol G, Ivanyi J. Peptide-specific T cell response to Mycobacterium tuberculosis: clinical spectrum, compartmentalization, and effect of chemotherapy. J. Infect. Dis. 1998;178:760–768. doi: 10.1086/515336. [DOI] [PubMed] [Google Scholar]
- 39.Lin PL, Rodgers M, Smith L, Bigbee M, Myers A, Bigbee C, Chiosea I, Capuano SV, Fuhrman C, Klein E, Flynn J. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect. Immun. 2009;77:4631–4642. doi: 10.1128/IAI.00592-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diedrich CR, Mattila JT, Klein E, Janssen C, Phuah J, Sturgeon TJ, Montelaro RC, Lin PL, Flynn JL. Reactivation of latent tuberculosis in cynomolgus macaques infected with SIV is associated with early peripheral T cell depletion and not virus load. PLoS ONE. 2010;5:e9611. doi: 10.1371/journal.pone.0009611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capuano SV, III, Croix DA, Pawar S, Zinovik A, Myers A, Lin PL, Bissel S, Fuhrman C, Klein E, Flynn JL. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect. Immun. 2003;71:5831–5844. doi: 10.1128/IAI.71.10.5831-5844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pawar SN, Mattila JT, Sturgeon TJ, Lin PL, Narayan O, Montelaro RC, Flynn JL. Comparison of the effects of pathogenic simian human immunodeficiency virus strains SHIV-89.6P and SHIV-KU2 in cynomolgus macaques. AIDS Res. Hum. Retroviruses. 2008;24:643–654. doi: 10.1089/aid.2007.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 44.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 45.Alberghini M, Pasquinelli G, Zanella L, Bacchini P, Bertoni F. Desmoplastic fibroblastoma: a light and ultrastructural description of two cases. Ultrastruct. Pathol. 2004;28:149–157. doi: 10.1080/01913120490475761. [DOI] [PubMed] [Google Scholar]
- 46.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 47.Dlugovitzky D, Torres-Morales A, Rateni L, Farroni MA, Largacha C, Molteni O, Bottasso O. Circulating profile of Th1 and Th2 cytokines in tuberculosis patients with different degrees of pulmonary involvement. FEMS Immunol. Med. Microbiol. 1997;18:203–207. doi: 10.1111/j.1574-695X.1997.tb01046.x. [DOI] [PubMed] [Google Scholar]
- 48.Lawn SD, Wilkinson RJ. Primate model to study reactivation of TB associated with retroviral infection. Future Virol. 2010;5:391–395. [Google Scholar]
- 49.Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, Lebedeva M, DeCamp A, Li D, Grove D, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J. Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geldmacher C, Schuetz A, Ngwenyama N, Casazza JP, Sanga E, Saathoff E, Boehme C, Geis S, Maboko L, Singh M, et al. Early depletion of Mycobacterium tuberculosis-specific T helper 1 cell responses after HIV-1 infection. J. Infect. Dis. 2008;198:1590–1598. doi: 10.1086/593017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaufmann DE, Walker BD. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J. Immunol. 2009;182:5891–5897. doi: 10.4049/jimmunol.0803771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Up-regulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 53.Einarsdottir T, Lockhart E, Flynn JL. Cytotoxicity and secretion of gamma interferon are carried out by distinct CD8 T cells during Mycobacterium tuberculosis infection. Infect. Immun. 2009;77:4621–4630. doi: 10.1128/IAI.00415-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.