Abstract
Abnormal Wnt signaling and impaired cell–cell adhesion due to abnormal E-cadherin and β-catenin function have been implicated in many cancers, but have not been fully explored in laryngeal squamous cell carcinoma. In this study, β-catenin cellular location and E-cadherin expression levels were analyzed in 16 laryngeal squamous cell carcinomas (LSCCs) (9 glottic and 7 supraglottic) and 11 samples of non-tumoral inflammatory larynx tissue, using immunohistochemical methods. All non-tumoral tissues showed equally strong membranous expression of β-catenin, while cytoplasmic expression was found in only 3 of the 11 samples. By contrast, whereas 8/9 glottic LSCCs exhibited only membranous expression of β-catenin, 6/7 supraglottic LSCCs displayed both membranous and cytoplasmic expression (p = 0.003). Strong E-cadherin staining was observed in 9/11 non-tumoral tissues and 7/9 glottic LSCCs, whereas 4/7 supraglottic LSCCs exhibited weak expression. Reduced membrane expression of E-cadherin and cytoplasmic retention of β-catenin in supraglottic LSCC seems to be related with more aggressive biological behavior which has been described in clinical studies. Further research is required to clarify the involvement of β-catenin in the mechanism associated with malignant transformation in laryngeal tissues.
Keywords: Laryngeal squamous cell carcinoma (LSCC), β-Catenin, E-Cadherin, Wnt signaling
Introduction
Laryngeal squamous cell carcinoma (LSCC), one of the most common tumors of the head and neck, occurs mainly in adult males who abuse tobacco and alcohol, and is characterized by squamous differentiation. Although early-stage glottic cancer has a favorable prognosis, with 5-year survival rates of over 70% [1], many supraglottic and subglottic cancers are not diagnosed until severe signs develop, by which time the 5-year survival rate has dropped to less than 50%. Locoregional recurrence, cervical lymph node metastases and distant metastases are the factors significantly affecting prognosis in LSCC patients [2]. The recognition and identification of tumor markers associated with recurrence and/or metastasis are key elements in predicting the biological behavior of the tumor and deciding on the most appropriate therapeutic strategy.
β-Catenin, originally identified on the basis of its association with cadherin adhesion molecules, is now widely recognized as an essential element in the wingless-Wnt signaling cascade [3]. Wnt proteins bind to receptors belonging to the Frizzled (Fz) family. In the canonical pathway, β-catenin activates an intracellular cascade that involves the inhibition of glycogen synthase kinase 3β (GSK 3β) and the adenomatous polyposis coli (APC) protein, ultimately resulting in the abnormal stabilization of cytoplasmic β-catenin and its translocation to the nucleus. Nuclear β-catenin then interacts with various transcription factors to cause cellular proliferation and differentiation. Abnormal Wnt signaling has been implicated in a number of cancers including head and neck carcinoma, lung cancer, colorectal cancer, melanoma, and leukemia [4]. Although the role of the Wnt pathway in nasopharyngeal cancer has not been fully explored, there is abundant evidence that aberrant Wnt signaling is involved in its development [5–7].
Cytoplasmic β-catenin plays a major role in the normal cell by binding to the intracellular domain of E-cadherin to maintain cell–cell adhesion. The expression of E-cadherin has been found to be downregulated in many cancers [8–12] including nasopharyngeal carcinoma [5, 12]. It has been suggested that E-cadherin downregulation may play a role in tumor progression and metastasis.
Strong β-catenin expression is significantly associated with invasion and metastasis of carcinomas of the head and neck, esophagus, stomach, colon, liver, lung, breast, female genitalia, prostate, bladder, and pancreas, as well as melanomas [11, 14–18]. Recently, several studies have pointed the considerable involvement of β-catenin not only in malignant transformation but also in the regulation of physiological functions, and expression of this adhesion molecule in human nasopharyngeal carcinoma has been investigated [19, 20]; however, it has not yet been thoroughly explored in LSCC.
The present study sought to examine the possible role of molecular cell–cell adhesion mechanisms in the oncogenesis and/or cytodifferentiation of laryngeal squamous cell carcinoma. Expression of β-catenin and E-cadherin was determined in paraffin-embedded LSCC tissues and in non-tumoral tissues from patients with inflammatory diseases of the larynx.
Materials and methods
Patient samples and histological classification
Sixteen patients with well-differentiated LSCCs (12 men and 4 women, aged 46–72) were diagnosed at the Infanta Luisa Hospital in Seville (Spain). The study was approved by the Ethical Committee and informed consent was obtained from all the participants. LSCC samples and non-tumoral inflammatory tissue samples (control group: six male and five female patients aged 19–97) were routinely fixed in 10% buffered formalin and embedded in paraffin. Histological evaluation was performed by two pathologists using hematoxylin/eosin staining.
All patients presented with non-metastatic disease at tumor diagnosis and survived disease-free for at least 5 years.
Immunohistochemical staining
Paraffin-embedded tissue blocks from 16 LSCC patients and 11 patients with non-tumoral inflammation were cut into 3 μm sections and placed on APES pre-coated slides. Sections were deparaffinized in xylene and rehydrated in alcohol and water. Immunohistochemistry (IHC) was performed using the peroxidase-antiperoxidase technique after microwave antigen retrieval procedure. Antibodies for β-catenin and E-cadherin were purchased from ZYMED Laboratories (ZYMED Laboratories, San Francisco, CA, USA). Antibodies against β-catenin (1:200) and E-cadherin (1:100) were overlaid on NPC tissue array sections and incubated overnight at 4°C. Secondary antibody incubation was performed at room temperature for 30 min. Two pathologists independently scored the results of immunohistochemical staining, and any discrepant scores were reexamined to arrive at a consensus score.
For β-catenin, +++ indicates positive staining in membrane, cytoplasm and nucleus; positive staining in membrane and cytoplasm (++); positive staining in membrane (+). For E-cadherin, + indicates 0–50% staining cells, i.e., low staining: ++ 50–100% staining cells, i.e., strong or high staining. Desmoid tumor tissues were used as positive control, and negative controls were obtained by replacing primary antibodies with PBS.
Statistical analysis
Statistical analysis was performed using the SPSS for Windows software package, release 15.0 (SPSS Inc., Chicago, IL, USA). Associations between antigen immunoreactivity, histopathological findings and clinical data were evaluated by the Chi-square test. Differences were regarded as significant if p < 0.05.
Results
Clinical data
Patient clinical data and underlying pathologies are summarized in Table 1. No correlation was found between clinical data (age, sex, location) or mitotic numbers.
Table 1.
Clinical data and diagnosis
| Age (years) | Sex (F/M) | Diagnostic (localization) | |
|---|---|---|---|
| 1 | 19 | M | NT inflammation of larynx |
| 2 | 33 | F | NT inflammation of larynx |
| 3 | 54 | M | NT inflammation of larynx |
| 4 | 67 | M | NT inflammation of larynx |
| 5 | 50 | F | NT inflammation of larynx |
| 6 | 58 | M | NT inflammation of larynx |
| 7 | 35 | F | NT inflammation of larynx |
| 8 | 56 | M | NT inflammation of larynx |
| 9 | 44 | F | NT inflammation of larynx |
| 10 | 33 | F | NT inflammation of larynx |
| 11 | 34 | M | NT inflammation of larynx |
| 12 | 55 | F | Glottic LSCC |
| 13 | 54 | M | Supraglottic LSCC |
| 14 | 49 | M | Supraglottic LSCC |
| 15 | 48 | M | Glottic LSCC |
| 16 | 64 | M | Glottic LSCC |
| 17 | 48 | M | Supraglottic LSCC |
| 18 | 51 | M | Glottic LSCC |
| 19 | 57 | F | Glottic LSCC |
| 20 | 50 | F | Supraglottic LSCC |
| 21 | 61 | M | Glottic LSCC |
| 22 | 57 | M | Supraglottic LSCC |
| 23 | 60 | F | Glottic LSCC |
| 24 | 62 | M | Glottic LSCC |
| 25 | 71 | M | Glottic LSCC |
| 26 | 63 | M | Supraglottic LSCC |
| 27 | 72 | M | Supraglottic LSCC |
F female, M male, NT Non-tumoral
Immunohistochemical analysis
Protein expression was analyzed by immunohistochemical staining for anti-β-catenin and anti-E-cadherin antibodies in LSCCs and non-tumoral tissues. The 11 non-tumoral tissues showed equally strong membranous expression of β-catenin, which was detected in both cytoplasm and membrane in only 23% of cases (3/11). In contrast, 8/9 (89%) glottic LSCCs exhibited only membranous expression of β-catenin, whereas 6/7 (86%) supraglottic LSCCs displayed both membranous and cytoplasmic expression (p = 0.003; Table 2; Fig. 1).
Table 2.
β-Catenin: cellular localization
| No. of cases | Nucleusa | Cytoplasm and membranea | Membranea | P value (Chi-square test) | |
|---|---|---|---|---|---|
| Non-tumoral tissues of larynx | 11 | 0 | 3 (27%) | 8 (73%) | 0.384 |
| LSCC tissues | 16 | 0 | 7 (44%) | 9 (56%) | |
| Glottic | 9 | 0 | 1 (11%) | 8 (89%) | 0.003 |
| Supraglottic | 7 | 0 | 6 (86%) | 1 (14%) |
aThe values are no. (%)
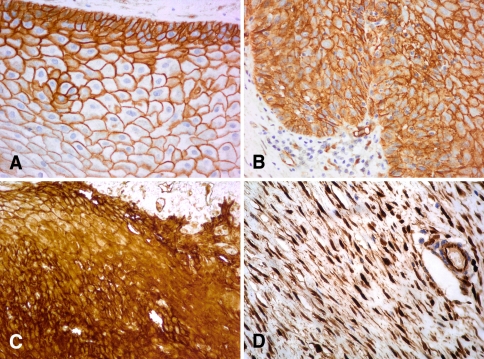
Fig. 1.
Immunohistochemical staining for β-catenin expression. Membranous expression of β-catenin. a Non tumoral tissue (×40). b Glottic laryngeal squamous cell carcinoma (×40). Membranous and cytoplasmic expression of β-catenin. c Supraglottic laryngeal squamous cell carcinoma (×40). d Desmoid tumor (positive control for nuclear β-catenin) (×40)
Strong E-cadherin staining was observed in 9/11 (82%) of non-tumoral tissues, and weak expression in 2/11 (18%). Strong expression was observed in 10/16 (62%) LSCC tissues (Fig. 2; Table 3), including 7/9 (77%) glottic LSCCs, whereas in 4/7 (57%) supraglottic LSCCs, expression was weak (Table 3); however no significant association was found (Fig. 2).
Fig. 2.
Immunohistochemical staining for E-cadherin expression. Strong staining: a (×40) Non tumoral tissue. b (×40) Glottic laryngeal squamous cell carcinoma. Low staining: c (×40) Supraglottic laryngeal squamous cell carcinoma
Table 3.
E-Cadherin expression
| No. of cases | Low E-cadherin expressiona | High E-cadherin expressiona | P value (Chi-square test) | |
|---|---|---|---|---|
| Non-tumoral tissues of larynx | 11 | 2 (18%) | 9 (82%) | 0.250 |
| LSCC tissues | 16 | 6 (37.5%) | 10 (62.5%) | |
| Glottic | 9 | 2 (22%) | 7 (78%) | 0.152 |
| Supraglottic | 7 | 4 (57%) | 3 (43%) |
aThe values are no. (%)
Discussion
β-Catenin contributes both to cell–cell adhesion and to the Wnt signaling pathway [21, 22]. The present study noted no alteration in β-catenin protein expression between LSCCs and non-tumoral tissues. However, differences were found in the cellular location of β-catenin expression: in 89% of glottic LSCCs, β-catenin was expressed only in the cell membrane, a percentage similar to that found for non-tumoral larynx tissue (73%), whereas in 86% of supraglottic LSCCs, β-catenin was expressed both in membrane and in cytoplasm. Similar findings have recently been reported by Goiliomus et al. [25] in a series of 97 LSCCs comprising 63 glottic and 34 supraglottic carcinomas. The cadherin–catenin complex is a group of membrane proteins that are important in cell–cell adhesion, tumor suppression, cell differentiation and cell migration. Some reports have suggested that downregulated expression of β-catenin might play a role in early and late tumor invasion and metastasis [23, 24]. Downregulation of β-catenin has been found to be closely related to advanced clinical disease and short survival in other head and neck carcinomas, including nasopharyngeral carcinoma [12]. These results suggest that β-catenin may in itself be a major factor for supraglottic LSCC tumor cell invasion and metastasis, and that differences in β-catenin expression between glottic and supraglottic carcinomas may be linked to differences in clinical behavior. Here, β-catenin protein levels were evaluated using inmunohistochemical staining, a semi-quantitative technique enabling assessment of proteins in paraffin-embedded tissues. Further research will evaluate β-catenin expression levels using the Western blot technique, with a view to measure possible alterations in β-catenin protein levels in fresh tissue.
β-Catenin is also involved in the Wnt canonical pathway as a transcriptional activator [21, 22]. The inhibition of glycogen synthase kinase (GSK-3β) and the APC protein ultimately results in the stabilization and nuclear translocation of cytoplastic β-catenin. Finally, nuclear β-catenin interacts with various transcription factors to cause proliferation and differentiation. But further research is required into the link between altered β-catenin levels and the development of supraglottic LSCC. Here, β-catenin was detected in membrane and cytoplasm, but not in the nucleus. Goiliomus et al. [25] in a study of 97 LSCCs, detected nuclear β-catenin in only one sample, perhaps due to differences in tissue processing or to the immunohistochemical staining method used. In the present study, failure to detect nuclear β-catenin suggests that the canonical Wnt pathway may be inactivated in this type of cancer. This cannot be categorically confirmed, since stabilized β-catenin was detected in cytoplasm. Further research is required to determine the possible role of cytoplasmic β-catenin retention in the pathogenesis of LSCC supreglottic and whether the Wnt pathway is activated by these retention, overexpression of its receptors or silencing of its suppressors, and also to investigate the possible activation of the non-canonical Wnt pathway that includes signaling through calcium flux, JNK and heterotrimeric G proteins.
Although none of the LSCC samples expressed Wnt pathway activators such as Wnt-1 and Wnt-5a, strong expression of the inhibitors WIF-1 and Dkk-1 was found in all cases. This would suggest that the Wnt pathway is inactive in this type of tumor. These results enable a preliminary approach to the study of the Wnt pathway in LSCC; future research will analyze the expression of different components of the Wnt pathway using quantitative techniques (Western-blot and real-time PCR), thus overcoming the limitations of immunohistochemical analysis and providing a more accurate view of the activation or inactivation of this signaling pathway.
E-Cadherin is essential for normal cell function, and is downregulated in processes such as wound healing, allowing epithelial cells to move and cover denuded tissue [26]. E-Cadherin, localized to the zonula adherens, complexes with catenins β and γ; β- and γ-catenins in turn bind to γ-catenin which attaches to actin filaments in the cell cytoskeleton [27]. E-Cadherin is thought to act as a tumor suppressor, since it suppresses invasion and metastasis [28]. Low expression of E-cadherin has been shown to predict poor outcome in oral cavity and laryngeal carcinomas [29, 30]. In the present study, strong E-cadherin expression was detected in 82% of non-tumoral tissues and only in 62.5% of glottic and supraglottic LSCCs. In contrast, weak E-cadherin expression was detected in 57% of supraglottic LSCC tissues, while only 22% of glottic LSCCs exhibited weak expression; the remaining 78% displayed strong E-cadherin expression, a percentage similar to that found in non-tumoral tissues. Downregulation of E-cadherin expression in supraglottic LSCC tissues may indicate a greater capacity for invasion and metastasis, and thus account for the distinct clinical behavior of these tumors. Indeed, a positive correlation was observed between E-cadherin expression levels and the presence of lymph node metastasis in supraglottic LSCC tissues [31, 32]. In this respect, several studies have shown that diminished E-cadherin expression is a good predictor of concurrent lymph node metastasis in laryngeal carcinoma and head and neck squamous cell cancer [13, 33–36]. Kurtz et al. [13] noted a correlation between reduced E-cadherin expression and decreased survival rates and vascular invasion in LSCC patients. These findings may be due to mutations of the E-CADHERIN gene [37] or to loss of heterozygosity of that gene [7]. In some cancer cells, aberrant gene promoter methylation of E-cadherin may be responsible for the decreased expression of E-cadherin [33, 34]; future research will investigate E-cadherin expression using quantitative RT-PCR, in order to obtain a clearer view of potential differences between sites, and thus focus on possible epigenetic changes due to promoter hypermethylation in LSCC tissues.
Finally, no association was observed between E-cadherin protein levels and β-catenin localization, although low E-cadherin levels were more common among LSCCs exhibiting cytoplasmic β-catenin (40 vs. 23.5%). Si et al. [5] observed a significant relationship between abnormal E-cadherin and β-catenin expression and clinical stage and lymph-node metastasis, although they do not distinguish between glottic and supraglottic LSCCs. Other studies of head and neck squamous cell carcinomas report loss of membranous E-cadherin and β-catenin expression and increased cytoplasmic expression, irrespective of the primary carcinoma or nodal carcinoma involved [38]. Impairment of cell–cell adhesion is an essential step in the progression from localized malignancy to stromal and vascular invasion and metastatic disease. This impairment is achieved through a variety of mechanisms involving the cadherin/catenin complex [39].
The results obtained here suggest an enhancement of the potential ability of cancer cells to disperse, a preliminary step in local invasion. A better knowledge of the regulation of the cadherin–catenin system is required for the prevention or treatment of laryngeal carcinoma.
In conclusion, reduced membrane expression of E-cadherin and cytoplasmic retention of β-catenin in supraglottic LSCC seems to be related with more aggressive biological behavior which has been described in clinical studies. Further research is required to clarify the involvement of β-catenin in the mechanism associated with malignant transformation in laryngeal tissues.
Conflict of interest
The authors have no conflict of interests to declare.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Karatzanis AD, Psychogios G, Zenk J, Waldfahrer F, Hornung J, Velegrakis GA, et al. Comparison among different available surgical approaches in T1 glottic cancer. Laryngoscope. 2009;119:1704–1708. doi: 10.1002/lary.20537. [DOI] [PubMed] [Google Scholar]
- 2.Cosetti M, Yu GP, Schantz SP. Five-year survival rates and time trends of laryngeal cancer in the US population. Arch Otolaryngol Head Neck Surg. 2008;134:370–379. doi: 10.1001/archotol.134.4.370. [DOI] [PubMed] [Google Scholar]
- 3.Mc Crea PD, Gumbiner BM. Purification of a 92-kDa cytoplasmic protein tightly associated with the cell–cell adhesion molecule E-cadherin (uvomorulin) J Biol Chem. 1991;266:1359–1361. [PubMed] [Google Scholar]
- 4.Mazieres J, He B, You L, Xu Z, Jablons D. Wnt signalling in lung cancer. Cancer Lancet. 2005;222:1–10. doi: 10.1016/j.canlet.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 5.Si WF, Sun W, Liu H, Liu J, Sun Y, Chen Z. Expression and clinical significance of E-cadherin and beta-catenin proteins in human laryngeal cancer. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008;22(10):459–461. [PubMed] [Google Scholar]
- 6.Lopez-Gonzalez SJ, Cristerna-Sánchez L, Vazquez-Manriquez ME, Jimenez-Orci G, Aguilar-Cazares D. Localization and level expression of beta-catenin in human laryngeal squamous cell carcinoma. Otolaryngol-Head Neck Surg. 2004;130:89–93. doi: 10.1016/j.otohns.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Pècina-Slaus N, Kljai′c M, Nikuseva-Marti C. Loss of heter-ozygosity of APC and CDH1 gene in laryngeal squamous cell carcinoma. Pathol Res Pract. 2005;201(8–9):557–563. doi: 10.1016/j.prp.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Zeng ZY, Zhou YH, Zhang WL, et al. Gene expression profiling of nasopharyngeal carcinoma reveals abnormally regulated Wnt signalling pathway. Human Pathol. 2007;38:120–133. doi: 10.1016/j.humpath.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 9.Hoteiya T, Hayashi E, Satomura K, et al. Expression of E-cadherin in oral cancer cell lines and its relationship to invasiveness in SCID mice in vivo. J Oral Pathol Med. 1999;28:107–111. doi: 10.1111/j.1600-0714.1999.tb02006.x. [DOI] [PubMed] [Google Scholar]
- 10.del Muro XG, Torregrosa A, Munoz J, et al. Prognostic value of the expression of E-cadherin and beta-cateninin bladder cancer. Eur J Cancer. 2000;36:357–362. doi: 10.1016/S0959-8049(99)00262-2. [DOI] [PubMed] [Google Scholar]
- 11.Kim HC, Kim HJ, Kim JC. Reduced E-cadherin expression as a cause of distinctive signet-ring cell variant in colorectal carcinoma. J Korean Med Sci. 2002;17:23–28. doi: 10.3346/jkms.2002.17.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng Z, Pan J, Chu B, Wong YC, et al. Downregulation and abnormal expression of E-cadherin and beta-catenin in nasopharyngeal carcinoma: close association with advanced disease stage and lymph node metastasis. Hum Pathol. 1999;130:458–466. doi: 10.1016/S0046-8177(99)90123-5. [DOI] [PubMed] [Google Scholar]
- 13.Kurtz KA, Hoffman HT, Zimmerman B, Robinson RA. Decreased E-cadherin but not β-catenin expression is associated with vascular invasion and decreased survival in head and neck squamous carcinomas. Otolaryngol-Head Neck Surg. 2006;134:142–146. doi: 10.1016/j.otohns.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 14.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- 15.Osterheld MC, Bian YS, Bosman FT, Benhattar J, Fontolliet C. Beta-catenin expression and its association with prognostic factors in adenocarcinoma developed in Barrett esophagus. Am J Clin Pathol. 2002;117:451–456. doi: 10.1309/1DB6-GFVH-RA6W-Q07Y. [DOI] [PubMed] [Google Scholar]
- 16.Sugio K, Kase S, Sakada T, Yamazaki K, Yamaguchi M, Ondo K, et al. Micrometastasis in the bone marrow of patients with lung cancer associated with a reduced expression of E-cadherin and beta-catenin: risk assessment by immunohistochemistry. Surgery. 2002;131:226–231. doi: 10.1067/msy.2002.119793. [DOI] [PubMed] [Google Scholar]
- 17.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;12:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery E, Torbenson MS, Kaushal M, Fisher C, Abraham SC. Beta-catenin immunohistochemistry separates mesenteric fibromatosis from gastrointestinal stromal tumor and sclerosing mesenteritis. Am J Surg Pathol. 2002;26:1296–1301. doi: 10.1097/00000478-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Chou J, Yun-Ching L, Kim J, You L, Zhidong X, Biao H, Jablons MD. Nasopharyngeal carcinoma-review of the molecular mechanism of tumorogénesis. Head Neck. 2008;230(7):946–963. doi: 10.1002/hed.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jou T, Stewart D, Stappert J, Nelson W, Marrs J. Genetic and biochemical dissection of protein linkages in the cadherin–catenin complex. Proc Natl Acad Sci USA. 1995;92:5067–5071. doi: 10.1073/pnas.92.11.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clements WM, Wang J, Sarnaik A, et al. Beta-catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res. 2002;62:3503–3506. [PubMed] [Google Scholar]
- 22.Gumbiner BM. Carcinogenesis: a balance between betactenin and APC. Curr Biol. 1997;7:443–446. doi: 10.1016/S0960-9822(06)00214-4. [DOI] [PubMed] [Google Scholar]
- 23.Joo YE, Rew JS, Choi SK, et al. Expression of E-cadherin and catenins in early gastric cancer. J Clin Gastroenterol. 2002;35:35–42. doi: 10.1097/00004836-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Kallakury BV, Sheehan CE, Winn-Deen E, et al. Decreased expression of catenin (alpha and beta), p120 CTN, and E-cadherin cell adhesion proteins and E-cadherin gene promoter methylation in prostatic adenocarcinomas. Cancer. 2001;92:2786–2795. doi: 10.1002/1097-0142(20011201)92:11<2786::AID-CNCR10128>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 25.Gouliomous AK, Varakis J, Goumas P, Papadaki H. Differential β-catenin expression between glottis and supraglottic laryngeal carcinoma. Eur Arch Otorhinolaryngol. 2010;267:1573–1578. doi: 10.1007/s00405-010-1249-4. [DOI] [PubMed] [Google Scholar]
- 26.Dogan A, Wang ZD, Spencer J. E-Cadherin expression in intestinal epithelium. J Clin Pathol. 1995;48:143–146. doi: 10.1136/jcp.48.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hajra KM, Fearon ER. Cadherin and catenin alterations in humancancer. Genes Chromosomes Cancer. 2002;34:255–268. doi: 10.1002/gcc.10083. [DOI] [PubMed] [Google Scholar]
- 28.Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol. 1993;5:806–811. doi: 10.1016/0955-0674(93)90029-P. [DOI] [PubMed] [Google Scholar]
- 29.Chow V, Yuen APW, Lam KY, et al. A comparative study of the clinicopathological significance of E-cadherin and catenins (α, β, γ) expression in the surgical management of oral tongue carcinoma. J Cancer Res Clin Oncol. 2001;127:59–63. doi: 10.1007/s004320000177. [DOI] [PubMed] [Google Scholar]
- 30.Mattijssen V, Peters HM, Schalkwijk L, et al. E-Cadherin expression in head and neck squamous-cell carcinoma is associated with clinical outcome. Int J Cancer. 1993;55:580–585. doi: 10.1002/ijc.2910550411. [DOI] [PubMed] [Google Scholar]
- 31.Li Jj, Zhang Gh, Yang Xm, Li Ss, Liu X, Yang Qt, Ye J (2011) Reduced E-cadherin expression is associated with lymph node metastases in laryngeal squamous cell carcinoma. Auris Nasus Larynx. doi: 10.1016/j.anl.2011.04.003 [DOI] [PubMed]
- 32.Zhang S, Ji W, Liu C, Yue L, Jiang Y, Wang J, Yuan Y, Liu Y. The relation of metastasis and prognosis with transforming growth factor-beta1 and E-cadherin expression in supraglottic larynx squamous cell carcinoma. Lin Chuang Er Bi Yan Hou Za Zhi. 2006;20(12):534–537. [PubMed] [Google Scholar]
- 33.Franchi A, Gallo O, Boddi V, et al. Prediction of occult neck metastases in laryngeal carcinoma: role of proliferating cell nuclear antigen, MIB-1, and E-cadherin immunohistochemical determination. Clin Cancer Res. 1996;2:1801–1808. [PubMed] [Google Scholar]
- 34.Rodrigo JP, Dominguez F, Alvarez C, et al. Expression of E-cadherin in squamous cell carcinomas of the supraglottic larynx with correlations to clinicopathological features. Eur J Cancer. 2002;38:1059–1064. doi: 10.1016/S0959-8049(01)00399-9. [DOI] [PubMed] [Google Scholar]
- 35.Takes RP, de Jong RJB, Alles MJRC, et al. Markers for nodal metastasis in head and neck squamous cell cancer. Arch Otolaryngol Head Neck Surg. 2002;128:512–518. doi: 10.1001/archotol.128.5.512. [DOI] [PubMed] [Google Scholar]
- 36.Massarelli E, Brown E, Tran NK, Liu DD, Izzo JG, Lee JJ, El-Naggar AK, Hong WK, Papadimitrakopoulou VA. Loss of E-cadherin and p27 expression is associated with head and neck squamous tumorogenesis. Cancer. 2005;103:952–959. doi: 10.1002/cncr.20879. [DOI] [PubMed] [Google Scholar]
- 37.Li L, Guo X, Du B. E-Cadherin gene mutation in recurrent and metastasic carcinoma of larynx. Lin Chuang Er Bi Yan Hou Za Zhi. 2002;16(3):114–116. [PubMed] [Google Scholar]
- 38.Andrews NA, Jones AS, Helliwell TR, et al. Expression of the E-cadherin–catenin cell adhesion complex in primary squamous cell carcinomas of the head and neck and their nodal metastases. Br J Cancer. 1997;75:1474–1480. doi: 10.1038/bjc.1997.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beavon IRG. The E-Cadherin–catenin complex in tumour metastasis: structure, function and regulation. Eur J Cancer. 2000;36:1607–1620. doi: 10.1016/S0959-8049(00)00158-1. [DOI] [PubMed] [Google Scholar]