Abstract
It has been demonstrated that restoration of function to compromised tissue can be accomplished by transplantation of bone marrow stem cells (BSC) and/or embryonic stem cells (ESC). One limitation to this approach has been the lack of non-invasive techniques to longitudinally monitor stem cell attachment and proliferation. Recently, murine ESC lines that express green fluorescent protein (GFP), luciferase (LV) and herpes simplex thymidine kinase (HVTK) were developed for detection of actively growing cells in vivo by imaging. In the present study, we investigated use of these ESC lines in a burned mouse model using Integra® as a delivery scaffolding/matrix. Two different cell lines were used: one expressing GFP and LV and the other expressing GFP, LV and HVTK. Burn wounds were produced by application of a brass block (2 × 2 cm kept in boiling water prior to application) to the dorsal surface of SV129 mice for 10 seconds. 24 hrs after injury, Integra® with adherent stem cells was engrafted onto a burn wound immediately after excision of eschar. The stem cells were monitored in vivo by measuring bioluminescence with a CCD camera and immunocytochemistry of excised tissue. Bioluminescence progressively increased in intensity over the time course of the study and GFP positive cells growing into the Integra® were detected. These studies demonstrate the feasibility of using Integra® as a scaffolding, or matrix, for the delivery of stem cells to burn wounds, as well as the utility of bioluminescence for monitoring vivo cellular tracking of stably transfected ESC cells.
INTRODUCTION
Transplantation of bone marrow derived stem cells (i.e. mesenchymal stem cells [MSCs]), as well as embryonic stem cells (ESCs) have previously been shown to restore function to compromised tissue.
Although there are very few studies of stem cell therapy in animal models of burn injury, important observations have been reported by Shumakov et al (1). In this investigation, tissue regeneration in deep burn wounds after transplantation of allogenic and autogenic fibroblast-like bone marrow mesenchymal stem cells and embryonic fibroblasts on burn surfaces in Wistar rats was evaluated. Deep thermal injury was produced by exposure of the skin to hot water. Animals with full thickness burns were divided into four groups and were treated with: allogenic fibroblast-like mesenchymal stem cells, autogenic fibroblast-like mesenchymal stem cells, allogenic embryonic fibroblasts or nothing (controls). Regeneration was determined by the speed of wound closure. Transplantation of allogenic and autogenic fibroblast-like bone marrow mesenchymal stem cells and embryonic fibroblasts decreased cell infiltration of the wound and accelerated the formation of new vessels and granulation tissue in comparison with the controls (burn wounds without cell transplantation). Regeneration processes were most active after transplantation of fibroblast-like bone marrow mesenchymal stem cells, in particular, autogenic cells, which was confirmed by more rapid decrease in burn surface area. Wound healing after transplantation of fibroblast-like bone marrow mesenchymal cells and embryonic fibroblasts was associated with prolonged functioning of the transplanted cells (as shown by staining for beta-galactosidase, in cells that were transfected with an adenovirus vector carrying this marker gene). It was hypothesized that more rapid regeneration of burn wounds after transplantation of fibroblast-like bone marrow mesenchymal stem cells was due to lower differentiation of these cells compared with embryonic fibroblasts.
There have also been studies with stem cells in burn injured patients. In a recent study by Mansilla et al (2) blood samples from acute burn patients and healthy donors were analyzed by flow cytometry with a large panel of monoclonal antibody (MoAbs). Cells expressing the mesenchymal stem cell (MSC) phenotype were detected in the peripheral blood of both groups. However, compared with samples from healthy donors, blood obtained from burn patients showed a higher MSC percentage (0.1643 ± 0.115 vs. 0.0078 ± 0.0044; p < 0.001). The percentage of MSCs correlated with the size and severity of the burn. The authors concluded that MSCs have an important role in regenerative processes of human tissues. They found cells phenotypically identical to MSCs circulating in physiological number in normal subjects, but significantly higher amounts during acute large burns. Therefore, these cells may represent a previously unrecognized circulatory component to the process of skin regeneration.
In a study by Burd et al (3), autologous bone marrow was applied to a chronic unhealed burn wound, a donor sited that had repeatedly failed to heal, and a chronic wound at the extremity of a free muscle flap where the graft was being traumatized by foot ware. The burn wound changed from being chronic and non-healing to re-epithelializing and was closed with a graft. The donor site that had failed to heal and also repeatedly failed to take a graft, healed without grafting. The chronically healed burn wound became more vascular and was finally closed with a skin graft.
Another recent report (4) described the use of allogenic bone marrow mesenchymal stem cells for the treatment of a patient with deep skin burns. In this report, a female patient with extensive skin burn was treated using transplantation of allogenic fibroblast-like bone marrow mesenchymal stem cells onto the surface of the wound. This study of wound healing dynamics after transplantation of allogenic fibroblast-like mesenchymal stem cells showed extensive wound regeneration in the presence of active neoangiogenesis. The authors suggested that autodermoplasty of burn wounds could be carried out with good results as early as on day 4 after transplantation of fibroblast-like mesenchymal stem cells and this led to more rapid healing of donor zones and accelerated rehabilitation of the patient.
Until recently our ability to longitudinally monitor the in vivo dynamic changes in these cell lines after engraftment has been limited. The development of “molecular imaging markers” using fluorescence and bioluminescence has allowed researchers to overcome this limitation. Recently, stably transfected mouse ESC lines that express green fluorescent protein (GFP), luciferase (LV) and herpes simplex thymidine kinase (HVTK) were developed. These cell lines allow for the detection of actively growing cells in vivo, using standard optical and mPET imaging techniques. It has been well established that a scaffold is required for the use of cultured cells in wound healing (5) and Integra® has been used for this purpose (6).
In this study a dermal regeneration template (Integra®) was used as a scaffold to deliver modified ESCs to an experimentally induced burn wound. Imaging studies were then performed to locate and follow ESCs and their progeny over time.
MATERIALS and METHODS
Stem Cell Lines
Two different embryonic stem cell lines were used in this study. One utilized SV129 embryonic stem cells (ESCs) with firefly luciferase (LV or fluc) and Green Fluorescent protein (eGFP) Fluc-eGFP (7) under the control of a beta-actin promoter. The other utilized SV129 ESCs with firefly luciferase (LV or fluc), Green Fluorescent protein (eGFP) and herpes simplex thymidine kinase (HVTK) (8) under the control of a human ubiquitin-C promoter. Both cell lines were produced with a lenti-viral vector and were initially cultured as described elsewhere (1,2). The ESC’s were kept in an undifferentiated, pluripotent state with 1000 IU/mL leukemia inhibitory factor (LIF; Millipore). The cells were cultured on 0.1% gelatin-coated plastic dishes in ES media containing Dulbecco modified Eagle medium supplemented with 15% fetal calf serum, 0.1 mmol/L-β-mercaptoethanol, 2 mmol/L-glutamine, and 0.1 mmol/L nonessential amino acids as described previously (1,2). The culture medium was changed daily, and the cells were passaged every 1 or 2 days. Flow cytometry demonstrated that the cells were positive for Sca-1, C-Kit, CD150, and CD48.
Preparation of the Integra®/Stem Cell Construct
Sheets of Integra® (3 cm × 3 cm) were placed in culture dishes and stem cells from cell lines of SV129 ESCs expressing LV and eGFP (Fluc-eGFP) or LV, eGFP and HVTK were added at a concentration of 100,000 cells/mL. After 5 days, the cells were visually observed to be seeded on the collagen/glycosaminoglycan layer of the Integra® template and had covered ~80% of the sheets.
Experimental Model of Burn Injury and Wound Treatment
SV129 male mice (28–30 grams) were anesthetized with diethyl ether (Fisher Scientific Co.), shaven, and a burn wound created using a brass block (2 × 2 cm, 100 grams total mass) held in place on the dorsal surface for 10 seconds (block previously kept in boiling water). 24 hrs later the animals (n = 6 in each group) were again anesthetized, the eschars were surgically debrided, removed, and the Integra® with ESCs from the two cell lines were sutured over the burn wounds using 6 “0” Ethicon® sutures. The animals were housed in separate cages and their general health and the condition of the wounds were monitored daily for the duration of the study. Imaging studies were conducted on days 7, 14 and 21.
The study was approved by the Subcommittee on Research Animal Care of the Massachusetts General Hospital. Animals were cared for in accordance with the procedures outlined by the National Institutes of Health Guide for Care and Use of Laboratory Animals (Department of Health and Human Services Publication 85-23), Bethesda, MD, National Institutes of Health, 1996.
Imaging of Stem Cells In Vivo
The longitudinal dynamics of the stem cells in the grafts after transplantation were monitored in vivo by imaging luciferase activity. At the time of imaging, the mice were anesthetized with ketamine (50 mg/kg) and injected IP with luciferin (1 mg per 200 µL of isotonic saline). Imaging was conducted using a CCD camera within 30 min after injection. The details of the of low-light imaging system (Hamamatsu Photonics KK, Bridgewater, NJ) and the procedures for its use have been described in detail elsewhere. (9). Briefly, the imaging system employs an intensified CCD camera mounted in a light-tight specimen chamber, fitted with a light-emitting diodes. This set-up allows acquisition of a background gray-scale image of the entire mouse. In the photon-counting mode, an image of the emitted light from the stem cells is captured using an integration time of 2 min, at the maximum setting on the image-intensifier control module. Using ARGUS software (Hamamatsu Photonics KK, Bridgewater, NJ), the luminescence images are presented as a false-color display superimposed on the gray-scale reference image. The image-processing component of the software is used to calculate total pixel values from the luminescence images of the wound area and the data are expressed as relative luminescence units (RLU’s)
Histological Techniques for Analyzing the Cells In Vitro
At the completion of the final imaging session, the wounds and Integra® with attached ES cells were excised, immersed in 10 % formalin and fixed for 24 hours for histology examination. The tissues were block sectioned, inserted into cassettes, processed to paraffin blocks, microtome sectioned to 6 microns and stained with H&E (hematoxylin and eosin) for microscopic examination. The H&E slides were evaluated microscopically for histological changes.
In another set of experiments, 6 micron sections were examined for the presence of Green Fluorescent Protein (GFP) using immunochemistry. Briefly, the sections were deparaffinized, treated with blocking solution followed by antibody to GFP (rabbit polyclonal, Abcam, Inc.). The cells positive for GFP were determined with a Vectastain abc kit (Vector Laboratories) using appropriate positive and negative controls. With this technique, cells expressing GFP (and hence were the stem cells) stained a dark blue. In contrast host cells stained brown due to endogenous peroxidase activity.
Statistical Analysis
The results were analyzed statistically by one and two-way Analysis of variance followed by Duncan’s multiple range test. P values of <0.05 were considered to be statistically significant.
RESULTS
ESCs could be grown on the Integra® in vitro where they became firmly attached. Because of this firm attachment, it was possible to remove the Integra® sheet with ES cells from the dishes, invert the sheet, and place the Integra® sheet seeded with ESCs on to the surface of a burn wound where it could be sutured firmly in place. The sutured Integra® stayed on the wound for the three-week duration of the experiments. No signs of sloughing of the ESC-seeded Integra® sheets were observed.
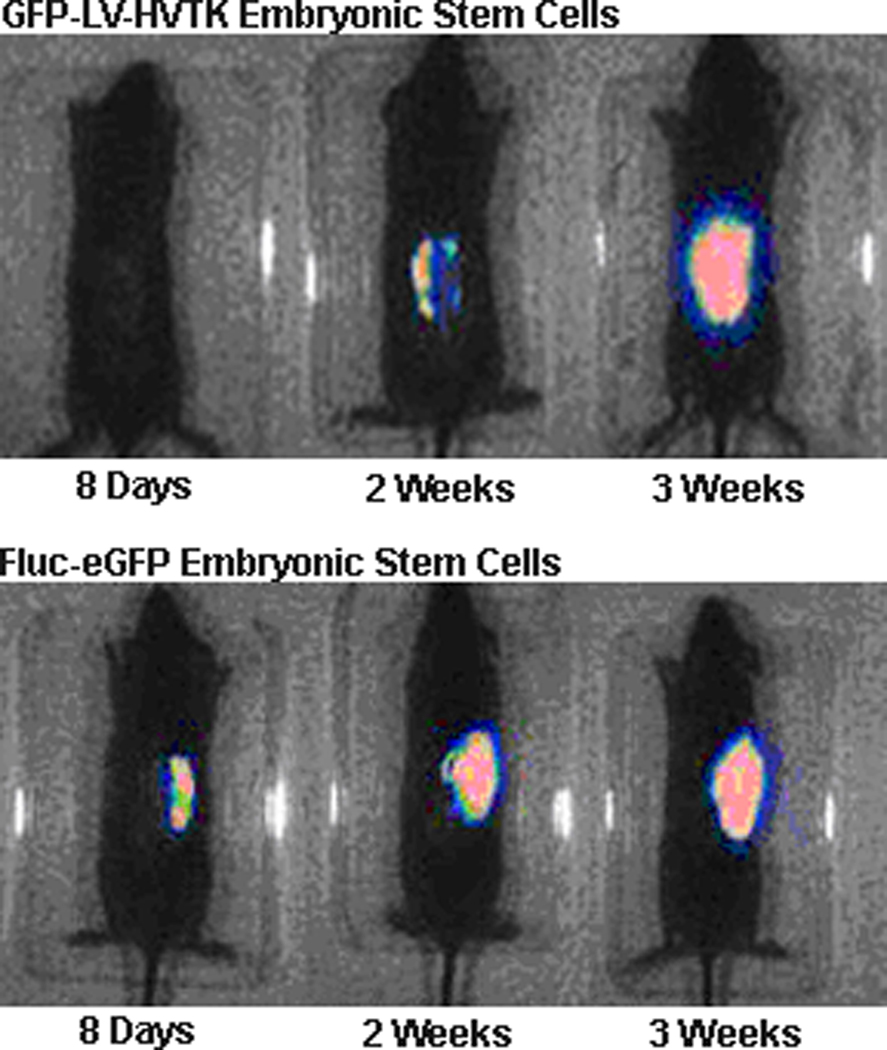
In vivo imaging demonstrated that both, stem cell lines cultured on Integra® and transplanted to escharectomy-debrided wounds produced bioluminescence signals at the site of the wound. Bioluminescence intensity increased progressively over the time course of healing. Figure 1 illustrates the changes observed with both cell lines of ESC in individual animals. The results obtained with all of the animals that were studied are summarized in Figure 2. Obviously, the intensity of bioluminescence continued to increase during the entire course of three weeks of observation. Since two-way ANOVA of the RLU data failed to demonstrate a significant effect of cell line, the results obtained with the two lines were pooled. One way ANOVA of the pooled data demonstrated a significant main effect of time, F2,17=6.61, p<0.003. Mean RLU at 3 weeks was significantly (p<0.05) greater than at 1 week and tended to be higher than at 2 weeks (p<0.10).
Figure 1.
Bioluminescence imaging of Fluc-eGFP or GFP-LV-HVTK Embryonic Stem Cells cultured on Integra® and placed on SV129 mice with burn injury. Two mice treated as described in the methods, one with each cell line, were imaged over the time course shown.
Figure 2.
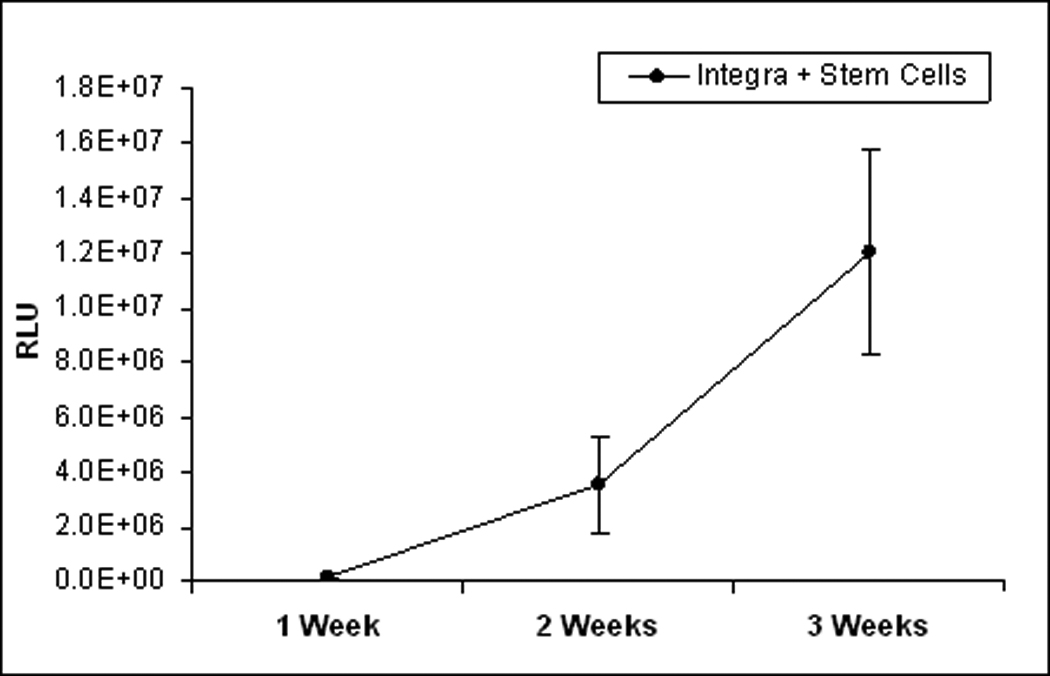
Bioluminescence of Fluc-eGFP or GFP-LV-HVTK Embryonic Stem Cells cultured on Integra® and placed on SV129 mice with burn injury. Each point represents the mean ± sem for 6 mice. *p<0.05
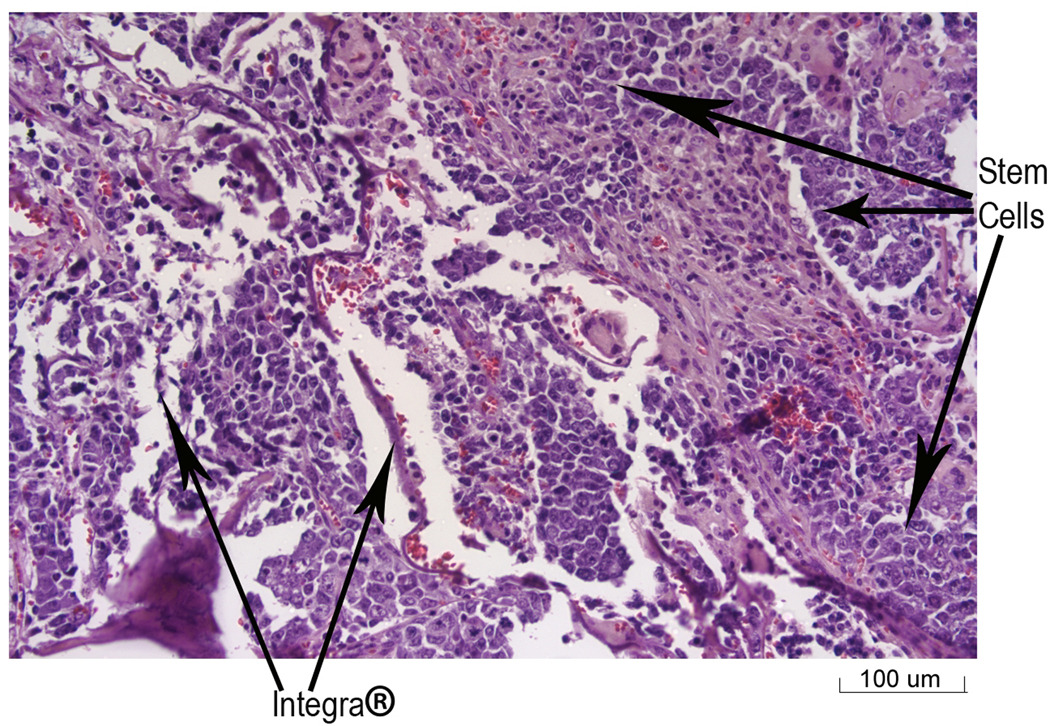
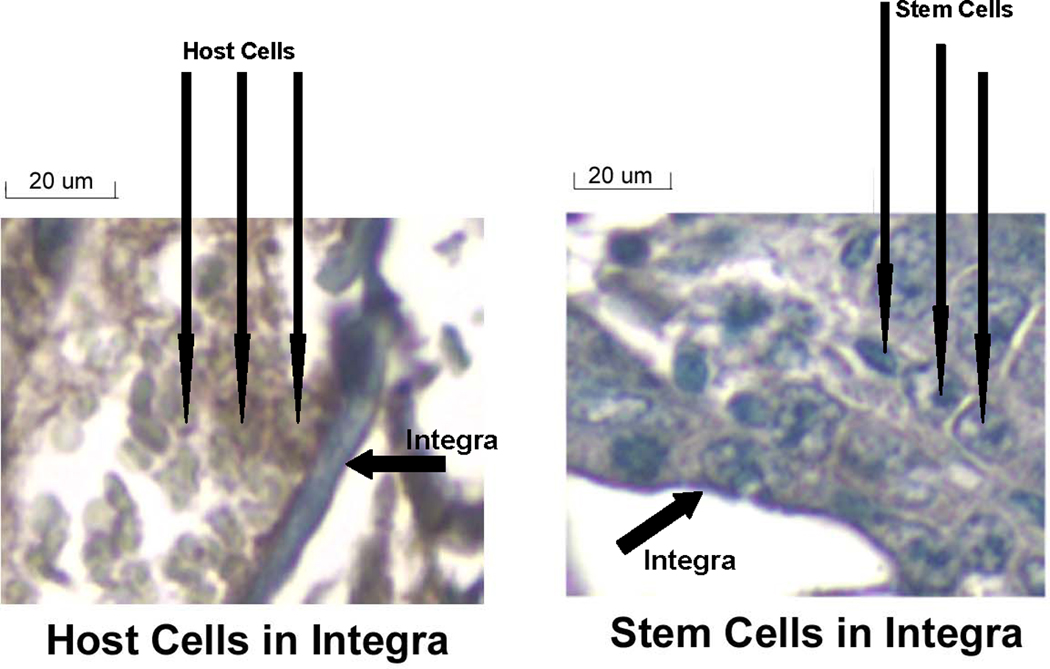
Histological analysis of tissue harvested at the end of three weeks demonstrated that the stem cells had grown into the Integra® that was placed over the burn wound (Figure 3). In addition, native cells from the mice were also observed to be growing into the Integra® dermis (Figure 4). Immunochemical staining was performed on the tissue samples to determine if the stem cells were associated with the wounds. With the technique used here, cells with GFP (stem cells) stained blue, while host cells stained brown due to endogenous peroxidase activity. As can be seen (Figure 5), the wound tissue of the burned mice with the Integra® did show obvious blue stained nuclei. Brown stained cells were seen in areas of the Integra®, which represent host cells that had grown into the Integra®. The presence of stem cells could be observed in the Integra® (Figure 5) at the edges of the wound.
Figure 3.
Stem cells growing into Integra® in tissue harvested from burned mice with GFP-LV-HVTK stem cells. The tissue was harvested at three weeks after application of the Integra®/stem cell graft. The arrows indicate the stem cells (identified by histological appearance) and the Integra® (20× magnification).
Figure 4.
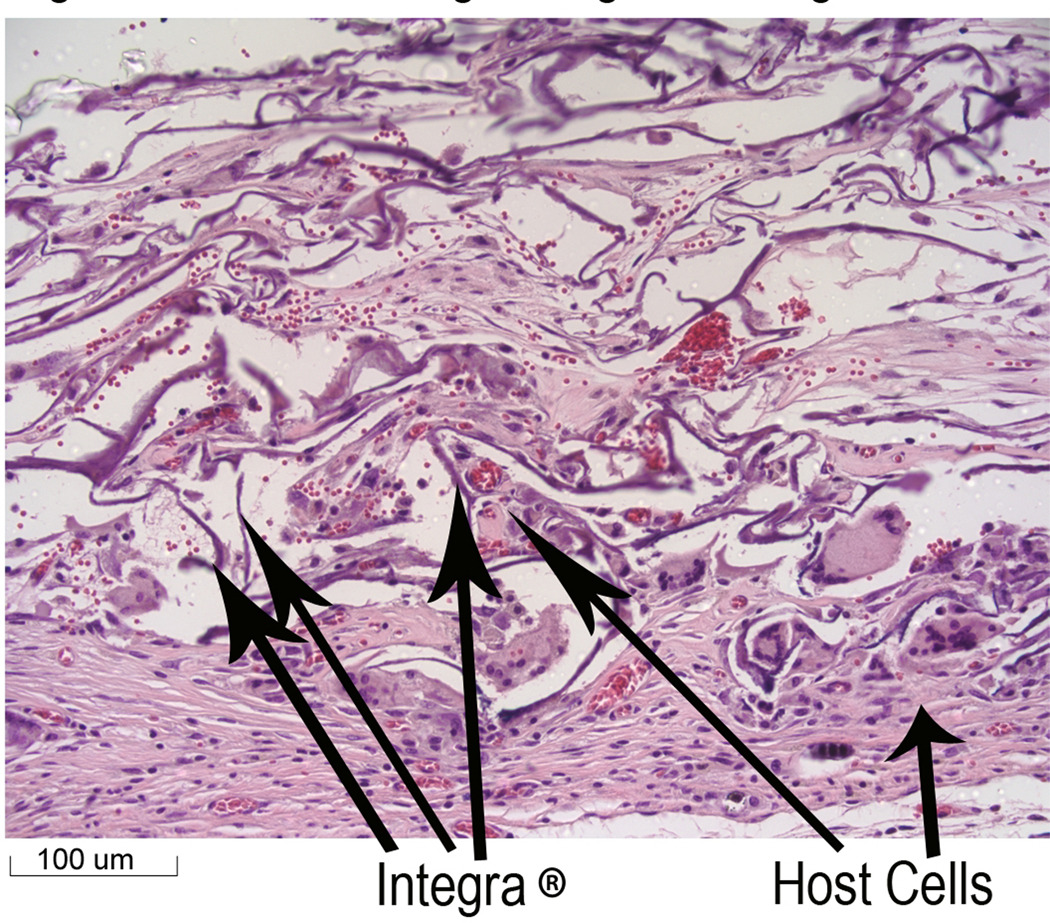
Host Cells growing into Integra®. This figure represents a different section of the sample in Figure 3. The arrows indicate the host cells (identified by histological appearance) and the Integra®. (20× magnification)
Figure 5.
Histochemical Identification of GFP-LV-HVTK Stem Cells (20× magnification). This figure illustrates a section of the sample in Figure 3 where GFP containing stem cells have blue staining nuclei. (20× magnification).
DISCUSSION
Over the past two decades, improvements in critical care and burn resuscitation have drastically reduced overall mortality following burn injury. This has fostered increasing emphasis on the importance of optimizing functional and cosmetic outcomes. The use of stem cell technology offers the potential to fulfill these needs.
At least three distinct mechanisms exist by which stem cells may act to restore normal cellular function. MSCs may hone to the site of injury, at which point they differentiate into the specific cell types that have been damaged; thereby replacing these cells (3). Stem cells can also act in a paracrine fashion through the release of factors that stimulate differentiation of resident stem cells to replace the damaged tissue and recruit MSCs from the blood and bone marrow to the site of injury (8). Additionally, stem cells may hone to the site of injury, and fuse with the damaged cells to form normally functioning hybrid cells which can proceed to replace the damaged tissue (8). An important question in research aimed at establishing potential applications of stem cells to burn care is, how to monitor the real time behavior of the stem cells in vivo.
The present study established methods for applying stem cells with fluorescent and bioluminescent markers to burn wounds and for monitoring cell replication by in vivo imaging. These techniques allow monitoring, tracking, and quantification of cell growth over time. This information is essential to our understanding of how stem cell technology can be utilized in the treatment of burn injury. The results of the study clearly demonstrated that cells that express luciferase can be successfully imaged in vivo. The presence of the herpes simplex thymidine kinase gene also provides a method for terminationg the to growth of stem cells in vivo by administration of Ganciclovir without affecting the replication of the host cells. This should make it possible to determine if there is a point after which the stem cells are applied that their replication should be stopped to promote better healing and/or replication in burns. In addition, although not performed in this study, herpes simplex thymidine kinase allows cell tracking to be performed by positron emission tomography (PET).
There are at least three situations where stem cells could be used in the treatment of burn patients. Stem cells could be used to promote wound healing. Current treatments utilize karotinocytes obtained from the patient’s uninjured skin to provide a covering that is placed over the wound bed after escharectomy. Although this approach has had beneficial effects for the recovery of burn patients and has revolutionized care, the hair follicles and sweat glands destroyed by full thickness burns are not replaced. Stem cells might be able to replace these skin appendages and thus lead to more normal skin (3). Stem cells have also been shown to correct the lung injury produced by beomycin treatment (10). Since lung injury produced by smoke inhalation combined with dermal burn wound is associated with high rates of mortality, stem cell treatment of burn victims with smoke inhalation injury might be of great benefit. Finally, stem cells have been shown to prevent sepsis induced mortality produced in mice by lipopolysaccharide (LPS) (11). Sepsis is still the major cause of morbidity and mortality in burned patients.
Stem cells can be delivered to the burn patients in several ways. The cells can be injected into the wound bed and/or around the edge of the wound. However, injection directly into the wound bed may produce further damage to an already severely injured underlying muscle layer. Injection of cells at the edges of the wound may not allow the cells to get to the surface of the injured underlying muscle layer. In the present study, stem cells were delivered to wounds by seeding of an Integra® dermal regeneration template. This approach is based on a consensus that replacement of connective tissues is required for healing skin wounds (12,13). The beneficial effects of Integra® as a scaffold for seeded cells in the treatment of burn wounds have been experimentally and clinically established (14,15,16). The present study confirmed that stem cells can be cultured on Integra®, applied to the burned wound bed, and monitored noninvasively in vivo.
In summary, our data suggests that it is possible to image Integra® delivered ESCs to a burn wound longitudinally in vivo using a lenti virus Fluc-eGFP or GFP-LV-HVTK construct. This study suggests the possibility of tracking stem cells and their progeny in vivo, which is critical to understanding the mechanisms by which these primitive cells lines may act to repair tissue damage. Furthermore, this research demonstrates a potential approach for the use of Integra® with stem cells for the treatment of burn wounds.
Acknowledgements
This study was supported in part by grants from the National Institutes of Health (2P50 GM21700-27A) and Shriners Hospitals for Children.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Shumakov VI, Onishchenko NA, Rasulov MF, Krasheninnikov ME, Zaidenov VA. Mesenchymal bone marrow stem cells more effectively stimulate regeneration of deep burn wounds than embryonic fibroblasts. Bull Exp Biol Med. 2003;136(2):192–195. doi: 10.1023/a:1026387411627. [DOI] [PubMed] [Google Scholar]
- 2.Mansilla E, Marin GH, Drago H, Sturla F, Salas E, Gardiner C, Bossi S, Lamonega R, Guzman A, Nunez A, Gil MA, Piccinelli G, Ibar R, Soratti C. Bloodstream cells phenotypically identical to human mesenchymal bone marrow stem cells circulate in large amounts under the influence of acute large skin damage: new evidence for their use in regenerative medicine. Transplant Proc. 2006;38(3):967–969. doi: 10.1016/j.transproceed.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 3.Burd A, Ahmed K, Lam S, Ayyappan T, Huang L. Stem cell strategies in burns care. Burns. 2007;33(3):282–291. doi: 10.1016/j.burns.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 4.Rasulov MF, Vasilchenkov AV, Onishchenko NA, Krasheninnikov ME, Kravchenko VI, Gorshenin TL, Pidtsan RE, Potapov IV. First experience of the use bone marrow mesenchymal stem cells for the treatment of a patient with deep skin burns. Bull Exp Biol Med. 2005;139(1):141–144. doi: 10.1007/s10517-005-0232-3. [DOI] [PubMed] [Google Scholar]
- 5.Boyce ST, Warden GD. Principles and practices for treatment of cutaneous wounds with cultured skin substitutes. Am J Surg. 2002;183(4):445–456. doi: 10.1016/s0002-9610(02)00813-9. [DOI] [PubMed] [Google Scholar]
- 6.Boyce ST, Kagan RJ, Meyer NA, Yakuboff KP, Warden GD. The 1999 clinical research award. Cultured skin substitutes combined with Integra Artificial Skin to replace native skin autograft and allograft for the closure of excised full-thickness burns. J Burn Care Rehabil. 1999;20(6):453–461. doi: 10.1097/00004630-199920060-00006. [DOI] [PubMed] [Google Scholar]
- 7.Li Z, Andrew Lee, Mei Huang, Hyung Chun, Jaehoon Chung, Pauline Chu, Grant Hoyt, Phillip Yang, Jarrett Rosenberg, Robert C Robbins, Joseph C Wu. Imaging survival and function of transplanted cardiac resident stem cells. J Am Coll Cardiol. 2009;53(14):1229–1240. doi: 10.1016/j.jacc.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joseph C Wu, Joshua M Spin, Feng Cao, Shuan Lin, Xiaoyan Xie, Olivier Gheysens, Ian Y Chen, Ahmad Y Sheikh, Robert C Robbins, Anya Tsalenko, Sanjiv S Gambhir, Tom Quertermous. Transcriptional profiling of reporter genes used for molecular imaging of embryonic stem cell transplantation. Physiol Genomics. 2006;25:29–38. doi: 10.1152/physiolgenomics.00254.2005. [DOI] [PubMed] [Google Scholar]
- 9.Hamblin MR, O’Donnell DA, Murthy N, Contag CH, Hasan T. Rapid control of wound infections by targeted photodynamic therapy monitored by in vivo bioluminescence imaging. Photochem. Photobiol. 2002;75:51–57. doi: 10.1562/0031-8655(2002)075<0051:rcowib>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Shinji Abe, Craig Boyer, Xiangde Liu, Fu Qiang Wen, Tetsu Kobayashi, Qiuhong Fang, Xingqi Wang, Mitsuyoshi Hashimoto, J. Graham Sharp, Stephen I Rennard. Cells derived from the circulation contribute to the repair of lung injury. Am J Respir Crit Care Med. 2004;Vol 170:1158–1163. doi: 10.1164/rccm.200307-908OC. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Rey E, Anderson P, Gonzalez MA, Rico L, Buscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58(7):929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 12.Desai MH, Mlakar JM, McCauley RL, et al. Lack of long term durability of cultured keratinocyte burn wound coverage: a case report. J Burn Care Rehabil. 1991;12:540–545. doi: 10.1097/00004630-199111000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Cuono C, Langdon R, Birchall N, et al. Composite autologousallogeneic skin replacement: development and clinical application. Plast Reconstr Surg. 1987;80:626–635. doi: 10.1097/00006534-198710000-00029. [DOI] [PubMed] [Google Scholar]
- 14.Wood FM, Stoner ML, Fowler BV, Fear MW. The use of a non-cultured autologous cell suspension and Integra dermal regeneration template to repair full-thickness skin wounds in a porcine model: a one-step process. Burns. 2007;33(6):693–700. doi: 10.1016/j.burns.2006.10.388. [DOI] [PubMed] [Google Scholar]
- 15.Ojeh NO, Frame JD, Navsaria HA. In vitro characterization of an artificial dermal scaffold. Tissue Eng. 2001;7(4):457–472. doi: 10.1089/10763270152436508. [DOI] [PubMed] [Google Scholar]
- 16.Jones I, James SE, Rubin P, Martin R. Upward migration of cultured autologous keratinocytes in Integra artificial skin: a preliminary report. Wound Repair Regen. 2003;11(2):132–138. doi: 10.1046/j.1524-475x.2003.11209.x. [DOI] [PubMed] [Google Scholar]