Abstract
Phospholipase C isozymes (PLCs) catalyze the conversion of the membrane lipid phosphatidylinositol 4,5-bisphosphate (PIP2) into two second messengers, inositol 1,4,5-trisphosphate and diacylglycerol. This family of enzymes are key signaling proteins that regulate the physiological responses of many extracellular stimuli such as hormones, neurotransmitters, and growth factors. Aberrant regulation of PLCs has been implicated in various diseases including cancer and Alzheimer’s disease. How, when, and where PLCs are activated under different cellular contexts are still largely unknown. We have developed a fluorogenic PLC reporter, WH-15, that can be cleaved in a cascade reaction to generate fluorescent 6-aminoquinoline. When applied in enzymatic assays with either pure PLCs or cell lysates, this reporter displays more than a 20-fold fluorescence enhancement in response to PLC activity. Under assay conditions, WH-15 has comparable Km and Vmax with the endogenous PIP2. This novel reporter will likely find broad applications that vary from imaging PLC activity in live cells to high throughput screening of PLC inhibitors.
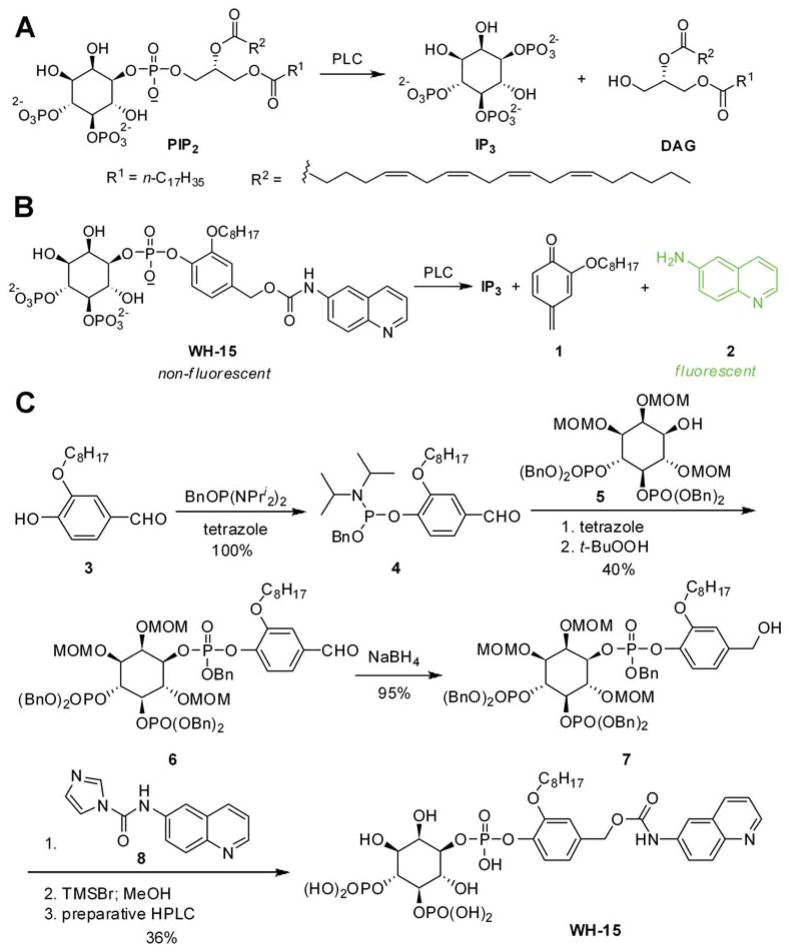
Phospholipase C isozymes (PLCs) catalyze the conversion of the membrane lipid phosphatidylinositol 4,5-bisphosphate (PIP2) into two second messengers (1), inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) (Figure 1A). IP3 mobilizes intracellular stores of Ca2+ while DAG activates protein kinase C (2). Furthermore, depletion of PIP2 alters the membrane association and/or activity of many proteins that harbor phosphoinositide binding domains (3). Consequently, PLC isozymes are key signaling proteins that regulate the physiological responses of many extracellular stimuli such as hormones, neurotransmitters, and growth factors (4). Aberrant regulation of PLCs has been implicated in various diseases including cancer, Alzheimer’s disease, and neuropathic pain (5–11). For example, activation of PLC-γ1 is required for the migration of breast cancer cells in response to epidermal growth factor. Similarly, PLC-γ1 is highly expressed in numerous breast carcinomas and colorectal tumor cell lines; down-regulation of PLC-γ1 expression in MD-MBA-231 breast cancer cells prevents the capacity of these cells to metastasize when injected into nude mice (7). Thus, PLCs are extensively studied and long considered as potential targets for drug development. Unfortunately, two major limitations continue to hinder studies of PLCs.
Figure 1.
Fluorogenic reporter design for mammalian PLCs. (A) PIP2 is cleaved to IP3 and DAG by PLC; (B) WH-15 is cleaved by PLC to form IP3, quinomethide 1, and 6-aminoquinoline 2. When excited at 344 nm and monitored the emission at 535 nm, WH-15 is essentially non-fluorescent while 6-aminoquinoline is highly fluorescent. (C) Chemical synthesis of WH-15.
First, it is impossible to continuously monitor mammalian PLC activity, either in living cells or in enzymatic assays. Typically, cellular PLC activity is measured from the production of radiolabeled inositides after biosynthetic incorporation of radioactivity into phosphoinositide pools (12). These assays are subject to cell-dependent differences in steady-state phosphoinositide metabolism and variable expression of PLCs. Alternatively, cell-permeable dyes that increase in fluorescence upon binding Ca2+ are also routinely used to monitor PLC activity (13). However, these dyes do not directly measure PLC activity and often generate confounding data due to diverse factors known to affect intracellular Ca2+ concentrations. For purified PLCs, radioactive PIP2 is used as the substrate and the enzymatic activity is measured through quantifying radiolabeled IP3. Unfortunately, none of the above assay formats allow for the continuous and direct monitoring of PLC activity.
Second, there are no direct, specific inhibitors of PLCs and this situation cannot easily be addressed with current assays of phospholipase activity since they are not amenable to high throughput screening. Several small molecule PLC inhibitors are indeed reported in the literature, among which U73122 is the most widely used (14). However, U73122 contains an electrophilic maleimide moiety that reacts with diverse nucleophiles including glutathione and various uncharacterized lipid components (15). More importantly, the direct inhibition of phospholipase activity by U73122 has not been demonstrated with purified PLCs. Instead, U73122 inhibits several other proteins including various calcium pumps (16), phosphatidylinositol-4-phosphate kinase (17), and 5-lipoxygenase (18).
Several fluorogenic reporters have been tested to monitor continuously the phospholipase activity of mammalian PLCs. None of them have been widely exploited likely due to limited applicability or ready availability. For instance, PLC-δ1 will efficiently hydrolyze phosphorothiolate analogs of PIP2 (19). However, the resulting thiol products must be coupled to a secondary reaction in order to monitor absorbance changes; such coupled systems increase the potential for artifacts during high-throughput screens or detailed enzymological analyses. Similarly, the fluorescein derivative of phosphoatidylinositol-4-phosphate has been reported to be a substrate of PLC-δ1 with hydrolysis leading to increased fluorescence (20). Unfortunately, it is unclear how efficiently PLC-δ1 hydrolyzes this fluorescein derivative relative to its endogenous phosphatidylinositol substrates. Indeed, PLC-δ1 very poorly hydrolyzed the dibutyl derivative of phosphatidylinositol relative to longer acyl chain derivatives (21) suggesting that the fluorescein derivative might also be a poor substrate since it lacks acyl chains. Regardless, this fluorescein substrate is not commercially available and to the best of our knowledge there have been no subsequent reports of its use to monitor mammalian PLCs. Mammalian PLCs require a phosphate at the 4-position of the inositol ring for efficient hydrolysis of phosphatidylinositols (22) and fluorescent derivatives that do not preserve this phosphate are either inert (23) or not expected to be efficient substrates (24–27).
Here we report a rationally designed small molecule PLC reporter WH-15 (Figure 1B) that features a 4-hydroxybenzyl alcohol linker to bridge the inositol phosphate head group and the fluorophore 6-aminoquinoline. The alkyl group C8H17 is introduced into WH-15 to retain the hydrophobic character of PIP2 while still ensuring it is water soluble. Upon PLC action, the reporter WH-15 is expected to be cleaved in a cascade reaction to generate inositol trisphosphate IP3, quinomethide 1, and 6-aminoquinoline 2 (Figure 1B). The carbamate derivative of the aminoquinoline in WH-15 is predicted to have an emission maximum at 380 nm when excited at 344 nm. In contrast, 6-aminoquinoline 2 has emission maxima at 450 and 530 nm when similarly excited (28–29). Therefore, the enzymatic hydrolysis of WH-15 by a PLC should be readily monitored by fluorescence at ~530 nm.
The synthesis of WH-15 began with compound 3, which was prepared in two steps from commercially available 4-(benzyloxy)-3-hydroxybenzaldehyde (Figure 1C). Phosphorylation of 3 with 1-benzyloxy-N,N,N′,N′-tetraisopropylphosphinediamine generated 4 quantitatively. The benzaldehyde 4 also served as the phosphorylation reagent for enantiomerically and diastereomerically pure inositol phosphate derivative 5 (30), which was converted to 6 after reaction with 4 and oxidation with t-butylperoxide. Reduction of the aldehyde group in 6 to the corresponding benzylalcohol with sodium borohydride (NaBH4) led to 7 in 95% yield. Coupling of 7 with N-(quinolin-6-yl)-1H-imidazole-1-carboxamide 8, which was synthesized from 6-aminoquinoline and 1,1′-carbonyldiimidazole, followed by removal of the protective groups with trimethylsilyl bromide (TMSBr) and MeOH then produced the free phosphatidylinositide WH-15. The reporter is stable for 2 months with storage at −20 °C as judged by analyses with liquid chromatography-mass spectrometry (LC-MS) and nuclear magnetic resonance (NMR).
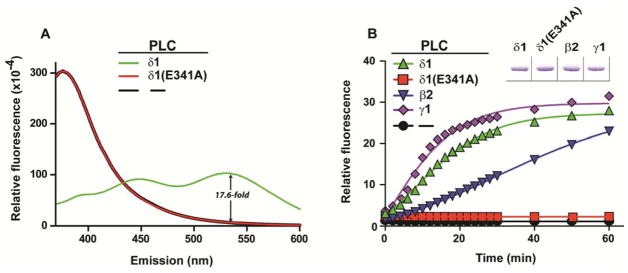
To demonstrate the application of this reporter, WH-15 was incubated with wild type, purified PLC-δ1 at 37 °C for 30 min and the emission spectrum of the reaction mixture was recorded (Figure 2A). Purified PLC-δ1 harboring a single substitution (E341A) within its active site has immeasurable lipase activity and was used in a parallel reaction (31). In addition, the reaction without PLC was also monitored. As shown in Figure 2A, wild type PLC-δ1 generates new emission peaks at 450 nm and 530 nm (green line), consistent with the formation of 6-aminoquinoline and confirmed by LC-MS analyses (Supporting Information, Figure S1). In contrast, the reaction mixture with either PLC-δ1(E341A) or no added PLC showed minimum emission at 530 nm and maximum emission at 380 nm (red and black lines), indicating that WH-15 was not cleaved by either PLC-δ1(E341A) or other components in the assay buffer. These results were further confirmed by LC-MS since no 6-aminoquinoline 2 was detected from the reaction mixture (Supporting Information, Figure S1). To demonstrate that WH-15 can be used to monitor PLC activity, the real-time fluorescence of the reaction mixture was recorded for purified PLC-δ1 (Figure 2B). WH-15 generated approximately a 30-fold increase in fluorescence with PLC-δ1 relative to an identically treated sample containing either catalytically inactive PLC-δ1 (E341A) or BSA. These results suggest that WH-15 specifically reports the enzymatic activity of PLC and the fluorescence-based assay is more sensitive and convenient than the traditional method.
Figure 2.
WH-15 is a reporter for PLC isozymes. (A) WH-15 was incubated with either wild-type (green) or catalytically-inactive (red) PLC-δ1 or no added PLC (black) for 60 min prior to recording emission spectra (λex = 344 nm). The ~17.6-fold increase in emission at 535 nm between reactions with wild-type PLC-δ1 and no added PLC is also shown. (B) Real-time fluorescence of WH-15 cleavage catalyzed by different PLC isoforms and normalized to the initial fluorescence of the reaction without PLC. Equivalent amounts of purified PLCs (inset) were verified by SDS-PAGE followed by staining with Coomassie Brilliant Blue. Initial concentration of WH-15 (44 μM) and PLC isozymes (20 ng/15 μL) are the same in both panels.
The human genome encodes at least 13 distinct PLCs (1). To test whether WH-15 functions as a substrate for other PLC isoforms, the reporter was incubated with either purified PLC-β2 or PLC-γ1 in reactions analogous to that described for PLC-δ1. As shown in Figure 2B, the fluorescence intensity of the reaction mixture with each of the three isoforms increases as reaction proceeds suggesting that WH-15 is a substrate for all three isoforms of PLC tested. Although the kinetics profiles for the three isoforms are different, all reactions reach the same plateau in relative fluorescence intensity after incubation overnight. These results suggest that WH-15 is a general substrate for different PLC isozymes.
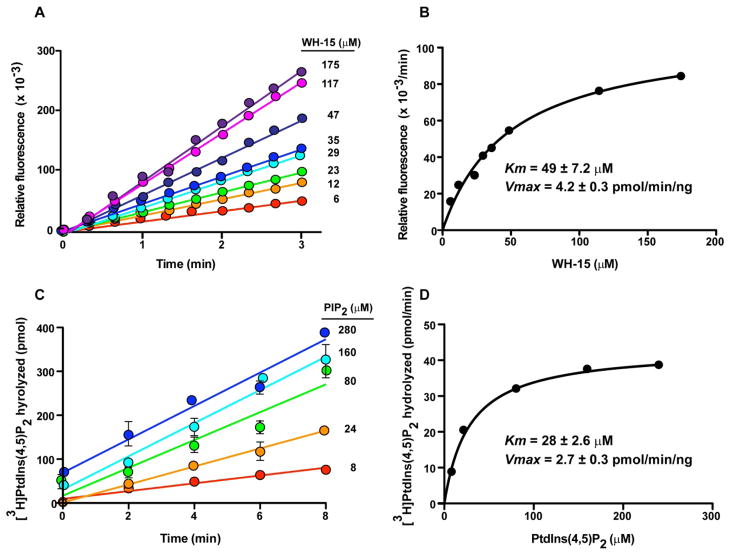
To further characterize WH-15, we measured the kinetic properties of WH-15 with PLC-γ1 (Figure 3). The Km of PLC-γ1 for WH-15 was 49 ± 7.2 μM with a Vmax at 4.2 ± 0.26 pmol/min/ng. For comparison, the endogenous substrate, PIP2, was also applied in the enzymatic reaction under similar conditions. The Km was measured as 28± 2.6 μM with a Vmax of 2.7 ± 0.07 pmol/min/ng. Thus, despite the obvious structural differences between WH-15 and endogenous PIP2, both molecules serve as essentially equivalent substrates for PLC-γ1 under the assay conditions. Furthermore, PLC-δ1 (Km = 30 ± 8.1 μM; Vmax = 1.2 ± 0.11 pmol/min/ng) and -β2 (Km = 86.1 ± 16.9 μM; Vmax = 1.2 ± 0.10 pmol/min/ng) hydrolyze WH-15 with similar kinetics suggesting that all PLC isozymes will cleave WH-15 and PIP2 with similar efficiencies (Supporting Information, Figure S2).
Figure 3.
Kinetics studies of WH-15 (A and B) and endogenous PIP2 (C and D) with PLC-γ1.
The reactive quinomethide intermediate formed upon the hydrolysis of WH-15 is unlikely to modify covalently PLC-γ1 to alter its phospholipase activity. Otherwise, we would expect non-linear rates of WH-15 consumption and this outcome is not observed. Instead, LC-MS analysis of the reaction mixture detected the formation of the products from reaction of quinomethide with dithiothreitol (DTT) and water, suggesting that quinomethide was most likely quenched by nucleophiles in the assay buffer. We also tested substrate specificity of WH-15 for other related lipases. Two other phospholipases, phospholipase A2 (PLA2) and phospholipase D (PLD), did not generate fluorescence enhancement from WH-15 (Supporting Information, Figure S3), indicating that WH-15 is a PLC-selective fluorogenic reporter.
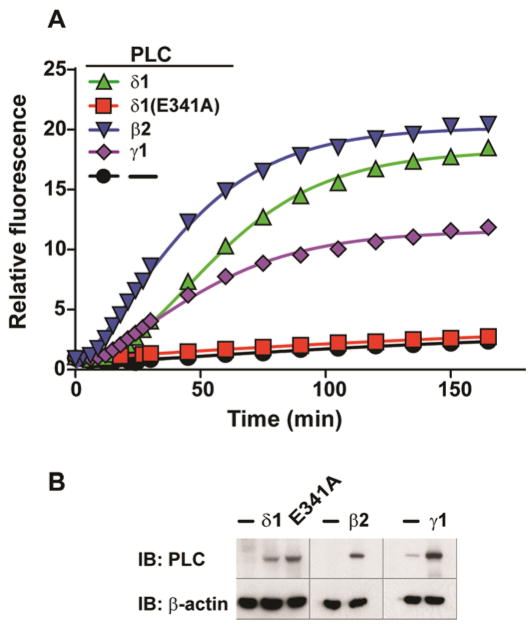
To assess potential non-specific cleavage of WH-15 by the entire repertoire of cellular phosphodiesterases, the stability of WH-15 was evaluated in lysates derived from Human Embryonic Kidney HEK-293T cells (Figure 4). Cells transfected with plasmid encoding either PLC-δ1, PLC-δ1 (E341A), PLC-β2, PLC-γ1, or the parent vector were lysed 24 h after transfection; the resulting lysates were normalized for total protein and tested for capacity to hydrolyze WH-15 (Figure 4A).
Figure 4.
WH-15 reports PLC enzymatic activity in cell lysates. (A) Real-time fluorescence of WH-15 (50 μM) cleavage catalyzed by PLC isozymes in cell lysates and normalized to the initial fluorescence of the reaction with lysates derived from cells transfected with empty vector. (B) HEK-293T cells were lysed and western blotting was performed for the indicated PLC isozymes.
Importantly, lysates from cells transfected with either the parent vector or vector encoding catalytically dead PLC-δ1 (E341A) exhibited minimal capacity to hydrolyze WH-15 as evidenced by minimal increases in fluorescence. These results confirm: i) the expected low basal activities of PLCs prior to upstream stimulation (32) and ii) that the cellular milieu does not contain substantial amounts of non-specific phosphodiesterases capable of hydrolyzing WH-15. Also importantly, cells that were transfected with either PLC-δ1, PLC-β2, or PLC-γ1 show a 10–20 fold increase in fluorescence (Figure 4A) consistent with the overexpression of these isozymes as determined by western blotting (Figure 4B). Taken together, these results unequivocally demonstrate that WH-15 is a sensitive and selective reporter for PLC activities in complex cell lysates. The fact that the fluorescence window is as large as 20-fold highlights the potential application of WH-15 in quantifying PLC activity in different cell lines.
In conclusion, we have developed a fluorogenic reporter WH-15 for mammalian PLCs. This novel reporter functions in both enzymatic assays and cell lysates with high sensitivity, and represents a robust assay that is not based on radioactivity. Given the key roles that PLCs play in cell signaling and diseases, this new PLC reporter will likely find broad applications in profiling different cell types and disease states. Furthermore, the large signal-to-noise ratio of the assay with WH-15 should enable its use in high throughput screens to identify small molecule PLC inhibitors. WH-15 also provides a starting point for developing fluorescent reporters to monitor PLC activities in real-time in cells. Finally, the 4-hydroxybenzyl alcohol linker used in WH-15 should also be applicable to the development of reporters for other enzymes that cleaves a P-O or C-O bond, such as esterases and tyrosine phosphatases.
Methods
Synthesis of the fluorogenic WH-15
The syntheses of WH-15 and intermediates are described in detail in the Supporting Information. The identity and purity of compounds were assessed by LC-MS and nuclear magnetic resonance (NMR) spectroscopy.
Assay with Mammalian PLC Isoforms
All fluorescence assays were performed in Perkin Elmer 384-well Plates and the fluorescence was recorded on a Perkin Elmer Wallac Envision™ 2103 Multilabel Reader with the excitation wavelength of 355 nm (ex filter: 355 nm, 10 nm) and the emission wavelength of 535 nm (em filter: 535 nm, 10 nm). To carry out the assay, the reporter WH-15 (44 μM, final concentration) was dissolved in the assay buffer (15 μL) that contains 133 μg/mL fatty-acid free BSA, 50 mM HEPES (pH 7.2), 70 mM KCl, 3 mM CaCl2, 3 mM EGTA, and 2 mM DTT at 37 °C. Assays were initiated upon addition of 20 ng of purified PLC protein and data were recorded every 2 min. Experiments were repeated at least three times. The excitation and emission spectra were recorded on a QM-4 PTI Spectral Fluorometer. Equivalent amounts of purified PLCs (3 μg, inset) were verified by SDS-PAGE followed by staining with Coomassie Brilliant Blue.
Kinetic Studies of WH-15 and Endogenous PIP2
The reporter WH-15 was dried under a stream of argon and resuspended in 20 mM HEPES which contains 0.5% cholate. This solution was diluted to obtain final assay conditions with 175, 117, 47, 35, 29, 23, 12, or 6 μM WH-15 in the assay buffer (10 mM HEPES, pH 7.4, 120 mM KCl, 10 mM NaCl, 2 mM EGTA, 5.8 mM MgSO4, 0.5% cholate, 160 μg/μL fatty acid-free BSA, and 100 μM free Ca2+) in a final volume of 12 μL. The assays were started by the addition of 4 ng of purified full-length wild type PLC-γ1. The reaction mixtures were then incubated at 30 °C and fluorescence was measured continuously as is described above.
For kinetic studies with the endogenous PIP2, a mixture of PtdIns(4,5)P2 (300 μM, Avanti Polar Lipids) and ~10,000 cpm of [3H]PtdIns(4,5)P2 was dried under a stream of nitrogen and resuspended in 0.5% cholate. The resulting lipid stock was diluted to obtain final assay conditions with either 280, 160, 80, 24, or 8 μM PtdIns(4,5)P2 in the same buffer as described above in a final volume of 60 μL. Assays were initiated by the addition of 17 ng of purified full-length wild-type PLC-γ1. After incubation at 30 °C at time intervals between 0–8 min, reactions were stopped by the addition of 200 μL of 10% (v/v) trichloroacetic acid (TCA) and 100 μL of 10 mg/ml BSA to precipitate uncleaved lipids and protein. Centrifugation of the reaction mixture isolated soluble [3H]Ins(1,4,5)P3, which was quantified using liquid scintillation counting.
Transfected Cell Lysate Assay
Cell lysates were prepared from transiently transfected HEK-293T cells plated in 12-well dishes at a cell density of 65,000 cells/well in Dulbecco’s Modified Eagles Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 10,000 units/mL penicillin, 10,000 units/mL streptomycin, and 25 units/mL amphotericin B. Following incubation for 24 h at 37 °C in an atmosphere of 95% air/5% CO2, cells were transfected with 600 ng of the indicated DNA and 100 ng of empty vector, for a total of 700 ng of DNA per well. DNA was complexed with FuGENE 6 transfection reagent (Roche Applied Sciences) in a 4:1 ratio of FuGENE 6 reagent to DNA prior to transfection. Twenty-four hours post transfection, media was aspirated and replaced with serum-free DMEM for 12–16 h. Subsequently, cells were lysed in 200 μL of RIPA buffer (Sigma) as per manufacturer’s protocol. Lysates were normalized for total protein concentration using a Bio-Rad Protein assay (Bio-Rad Dye Reagent) prior to use in the reporter assay. Reporter assays were initiated by the addition of 50 μL of normalized cell lysate to the mixture (final volume 120 μL) that contains 50 μM reporter WH-15, 83 μg/mL fatty-acid free BSA, 50 mM HEPES (pH 7.2), 70 mM KCl, 3 mM CaCl2, 3 mM EGTA and 2 mM DTT at 37 °C. Data was collected every 3 min using a Wallac Victor2™ 1420 Multilabel Counter (Model: 1420-011, Perkin Elmer Life Sciences) with an excitation wavelength of 340 nm and an emission wavelength of 535 nm. Western blotting was performed on normalized cell lysates to confirm the expression of PLC-β2, -δ1, and -γ1 using monoclonal antibodies (Santa Cruz).
Supplementary Material
Acknowledgments
We thank A. Gresset for the generous gift of PLC-γ1 And Dr. Y. Yu (University of North Carolina) for help on high resolution analysis of mass spectrum of WH-15. Funding is supplied by the University of North Carolina at Chapel Hill (Q.Z.) and National Institutes of Health (J.S., R01-GM057391).
Footnotes
Supporting Information Available: This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Harden TK, Sondek J. Regulation of phospholipase C isozymes by ras superfamily GTPases. Annu Rev Pharmacol Toxicol. 2006;46:355–379. doi: 10.1146/annurev.pharmtox.46.120604.141223. [DOI] [PubMed] [Google Scholar]
- 2.Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol. 2008;9:99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura Y, Fukami K. Roles of phospholipase C isozymes in organogenesisand embryonic development. Physiology (Bethesda) 2009;24:332–341. doi: 10.1152/physiol.00031.2009. [DOI] [PubMed] [Google Scholar]
- 5.Kassis J, Moellinger J, Lo H, Greenberg NM, Kim HG, Wells A. A role for phospholipase C-γ-mediated signaling in tumor cell invasion. Clin Cancer Res. 1999;5:2251–2260. [PubMed] [Google Scholar]
- 6.Cocco L, Manzoli L, Palka G, Martelli AM. Nuclear phospholipase C β1, regulation of the cell cycle and progression of acute myeloid leukemia. Adv Enzyme Regul. 2005;45:126–135. doi: 10.1016/j.advenzreg.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Shepard CR, Kassis J, Whaley DL, Kim HG, Wells A. PLC-γ contributes to metastasis of in situ-occurring mammary and prostate tumors. Oncogene. 2007;26:3020–3026. doi: 10.1038/sj.onc.1210115. [DOI] [PubMed] [Google Scholar]
- 8.Shimohama S, Fujimoto S, Tresser N, Richey P, Perry G, Whitehouse PJ, Homma Y, Takenawa T, Taniguchi T, Suenaga T, et al. Aberrant phosphoinositide metabolism in Alzheimer’s disease. Ann N Y Acad Sci. 1993;695:46–49. doi: 10.1111/j.1749-6632.1993.tb23025.x. [DOI] [PubMed] [Google Scholar]
- 9.Matsushima H, Shimohama S, Fujimoto S, Takenawa T, Kimura J. Reduction of platelet phospholipase C activity in patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 1995;9:213–217. [PubMed] [Google Scholar]
- 10.Matsushima H, Shimohama S, Fujimoto S, Takenawa T, Kimura J. Changesin platelet phospholipase C protein level and activity in Alzheimer’s disease. Neurobiol Aging. 1995;16:895–900. doi: 10.1016/0197-4580(95)02003-9. [DOI] [PubMed] [Google Scholar]
- 11.Shi TJ, Liu SX, Hammarberg H, Watanabe M, Xu ZQ, Hokfelt T. Phospholipase C-β3 in mouse and human dorsal root ganglia and spinal cord is a possible target for treatment of neuropathic pain. Proc Natl Acad Sci U S A. 2008;105:20004–20008. doi: 10.1073/pnas.0810899105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rusten TE, Stenmark H. Analyzing phosphoinositides and their interactingproteins. Nat Methods. 2006;3:251–258. doi: 10.1038/nmeth867. [DOI] [PubMed] [Google Scholar]
- 13.Gee KR, Brown KA, Chen WN, Bishop-Stewart J, Gray D, Johnson I. Chemical and physiological characterization of fluo-4 Ca2+-indicator dyes. Cell Calcium. 2000;27:97–106. doi: 10.1054/ceca.1999.0095. [DOI] [PubMed] [Google Scholar]
- 14.Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, Bunting S. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther. 1990;255:756–768. [PubMed] [Google Scholar]
- 15.Wilsher NE, Court WJ, Ruddle R, Newbatt YM, Aherne W, Sheldrake PW, Jones NP, Katan M, Eccles SA, Raynaud FI. The phosphoinositide-specific phospholipase C inhibitor U73122 (1-(6-((17β-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl)-1H-pyrrole-2,5-dione) spontaneously forms conjugates with common components of cell culture medium. Drug Metab Dispos. 2007;35:1017–1022. doi: 10.1124/dmd.106.014498. [DOI] [PubMed] [Google Scholar]
- 16.Engstrom EM, Ehrhardt DW, Mitra RM, Long SR. Pharmacologicalanalysis of nod factor-induced calcium spiking in Medicago truncatula. Evidence for the requirement of type IIA calcium pumps and phosphoinositide signaling. Plant Physiol. 2002;128:1390–1401. doi: 10.1104/pp.010691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker EM, Bispham JR, Hill SJ. Nonselective effects of the putativephospholipase C inhibitor, U73122, on adenosine A1 receptor-mediated signal transduction events in Chinese hamster ovary cells. Biochem Pharmacol. 1998;56:1455–1462. doi: 10.1016/s0006-2952(98)00256-1. [DOI] [PubMed] [Google Scholar]
- 18.Feisst C, Albert D, Steinhilber D, Werz O. The aminosteroid phospholipaseC antagonist U-73122 (1-[6-[[17β-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione) potently inhibits human 5-lipoxygenase in vivo and in vitro. Mol Pharmacol. 2005;67:1751–1757. doi: 10.1124/mol.105.011007. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Mihai C, Kubiak RJ, Rebecchi M, Bruzik KS. Phosphorothiolateanalogues of phosphatidylinositols as assay substrates for phospholipase C. Chembiochem. 2007;8:1430–1439. doi: 10.1002/cbic.200700061. [DOI] [PubMed] [Google Scholar]
- 20.Rukavishnikov AV, Zaikova TO, Birrell GB, Keana JF, Griffith OH. Synthesis of a new fluorogenic substrate for the continuous assay of mammalian phosphoinositide-specific phospholipase C. Bioorg Med Chem Lett. 1999;9:1133–1136. doi: 10.1016/s0960-894x(99)00166-3. [DOI] [PubMed] [Google Scholar]
- 21.Rebecchi MJ, Eberhardt R, Delaney T, Ali S, Bittman R. Hydrolysis ofshort acyl chain inositol lipids by phospholipase C-δ1. J Biol Chem. 1993;268:1735–1741. [PubMed] [Google Scholar]
- 22.Heinz DW, Essen LO, Williams RL. Structural and mechanistic comparison of prokaryotic and eukaryotic phosphoinositide-specific phospholipases C. J Mol Biol. 1998;275:635–650. doi: 10.1006/jmbi.1997.1490. [DOI] [PubMed] [Google Scholar]
- 23.Rose TM, Prestwich GD. Synthesis and evaluation of fluorogenic substratesfor phospholipase D and phospholipase C. Org Lett. 2006;8:2575–2578. doi: 10.1021/ol060773d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaikova TO, Rukavishnikov AV, Birrell GB, Griffith OH, Keana JF. Synthesis of fluorogenic substrates for continuous assay of phosphatidylinositol-specific phospholipase C. Bioconjug Chem. 2001;12:307–313. doi: 10.1021/bc0001138. [DOI] [PubMed] [Google Scholar]
- 25.Rukavishnikov AV, Smith MP, Birrell GB, Keana JF, Griffith OH. Synthesis of a new fluorogenic substrate for the assay of phosphoinositide-specific phospholipase C. Tetrahedron Lett. 1998;39:6637–6640. doi: 10.1016/s0960-894x(99)00166-3. [DOI] [PubMed] [Google Scholar]
- 26.Scholze H, Stutz H, Paltauf F, Hermetter A. Fluorescent inhibitors for thequalitative and quantitative analysis of lipolytic enzymes. Anal Biochem. 1999;276:72–80. doi: 10.1006/abio.1999.4278. [DOI] [PubMed] [Google Scholar]
- 27.Schmidinger H, Birner-Gruenberger R, Riesenhuber G, Saf R, Susani-Etzerodt H, Hermetter A. Novel fluorescent phosphonic acid esters for discrimination of lipases and esterases. Chembiochem. 2005;6:1776–1781. doi: 10.1002/cbic.200500013. [DOI] [PubMed] [Google Scholar]
- 28.Pez D, Leal I, Zuccotto F, Boussard C, Brun R, Croft SL, Yardley V, Ruiz Perez LM, Gonzalez Pacanowska D, Gilbert IH. 2,4-Diaminopyrimidines as inhibitors of Leishmanial and Trypanosomal dihydrofolate reductase. Bioorg Med Chem. 2003;11:4693–4711. doi: 10.1016/j.bmc.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Lee MR, Baek KH, Jin HJ, Jung YG, Shin I. Targeted enzyme-responsive drug carriers: studies on the delivery of a combination of drugs. Angew Chem Int Ed Engl. 2004;43:1675–1678. doi: 10.1002/anie.200353204. [DOI] [PubMed] [Google Scholar]
- 30.Kubiak RJ, Bruzik KS. Comprehensive and uniform synthesis of all naturallyoccurring phosphorylated phosphatidylinositols. J Org Chem. 2003;68:960–968. doi: 10.1021/jo0206418. [DOI] [PubMed] [Google Scholar]
- 31.Ellis MV, James SR, Perisic O, Downes CP, Williams RL, Katan M. Catalytic domain of phosphoinositide-specific phospholipase C (PLC). Mutational analysis of residues within the active site and hydrophobic ridge of PLC-δ1. J Biol Chem. 1998;273:11650–11659. doi: 10.1074/jbc.273.19.11650. [DOI] [PubMed] [Google Scholar]
- 32.Hicks SN, Jezyk MR, Gershburg S, Seifert JP, Harden TK, Sondek J. General and versatile autoinhibition of PLC isozymes. Mol Cell. 2008;31:383–394. doi: 10.1016/j.molcel.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.