Abstract
The ductus arteriosus (DA) is a fetal shunt that directs right ventricular outflow away from pulmonary circulation and into the aorta. Critical roles for prostaglandin E2 (PGE2) and the EP4 receptor (EP4) have been established in maintaining both the patency of the vessel in utero and in its closure at birth. Here we have generated mice in which loss of EP4 expression is limited to either the smooth muscle (SMC) or endothelial cells and demonstrated that SMC, but not endothelial cell expression of EP4 is required for DA closure. The genome wide expression analysis of full term wild type and EP4−/− DA indicates that PGE2/EP4 signaling modulates expression of a number of unique pathways, including those involved in SMC proliferation, cell migration, and vascular tone. Together this supports a mechanism by which maturation and increased contractility of the vessel is coupled to the potent smooth muscle dilatory actions of PGE2.
Keywords: Ductus arteriosus, Prostaglandins, Smooth Muscle, Remodeling, EP4, conditional gene knockout
The ductus arteriosus (DA) is a fetal arterial vessel that connects the pulmonary duct to the aorta. It thus allows the majority of right ventricular output to be directed past the fluid filled fetal lungs towards the descending aorta and placental circulatory system where oxygenation takes place. Closure of the vessel is believed to occur in two steps, with the initial constriction of the vessel largely triggered by an increase in arterial oxygen tension (as ventilation of the lung is initiated) and by a precipitous drop in PGE2 levels at birth. Remodeling of the vessels is initiated with the lifting of the endothelium from the internal elastic lamina, fragmentation of the elastin and migration of smooth muscle cells (SMC) into the sub-endothelial space [1]. This is followed by dramatic structural changes that result in the eventual transformation of the vessel into the ligamentum arteriosus.
The patency of the DA, both in utero and immediately after birth, is sensitive to non-steroidal anti-inflammatory drugs (NSAIDs) such as indomethacin, which inhibits cyclooxygenase (COX) activity [2,3]. COX converts arachidonic acid into prostaglandin endoperoxide (PGH2), a rate-limiting step in all downstream prostaglandin (PG), prostacyclin, and thromboxane synthesis [4]. Fetal exposure to indomethacin can result in DA closure in utero [2], and indomethacin treatment can often bring about the closure of a patent ductus arteriosus (PDA) particularly in premature infants [3]. Furthermore, prostaglandins, including PGE2 can relax the pre-constricted ductus in vitro and infusion of PGE2 maintains the patency of the vessel after birth [5,6]. Together these experimental and clinical findings support a model in which the DA is regarded as having intrinsic tone with the patency of the fetal vessel in utero dependent on the dilatory actions of prostaglandins; PGE2 [7]. A dramatic drop in circulating PGE2 levels occurs at birth due to loss of the prostanoid rich placental capillary beds, and the redirection of the right ventricular output to the pulmonary circulation where high levels of the PGE2 catabolizing enzyme hydroxyprostaglandin dehydrogenase 15-(NAD) (HPGD), as well as the PGE2 transporter, PGT are expressed. Transport of PGE2 across the cell membrane is the rate limiting step in PGE2 catabolism. The importance of PGE2 catabolism in DA closure is supported by the report that mice lacking either HPGD or PGT Transport of PGE2 die shortly after birth with a PDA [8,9]. Loss of this vasodilatory mediator would allow the intrinsic tone of the vessel to close the fetal shunt. The importance of the decrease in PGE2 levels at birth in DA closure is supported by the finding that mice lacking HPGD die in the perinatal period with PDA and by a high incidence of PDA in families carrying a mutant allele of this gene [10].
This model in which PGE2 counteracts the normal tone of the vessel is consistent with the known potent dilatory action of PGE2 [11]. One study suggested that PGE2, through the EP4 receptors present on endothelial cells, can increase eNOS activity increasing NO production [12]. NO stimulates soluble guanylate cyclase allowing accumulation of cGMP in the SMC [13]. The dilatory actions of PGE2 have been attributed primarily to two of the four PGE2 receptors, EP2 and EP4 [14]. However, the phenotype of the EP4-deficient (Ptger4−/−) mice suggests a more complex role for PGE2 in the physiology of this vessel, including the possible role for this receptor on SMC.
METHODS
In situ hybridization
The Ptger4 cDNA probe was synthesized by rtPCR amplification using primers Ptger4-1F (5’-GTTTGGCTGATATAACTGGTTAAT-3’) and Ptger4-2R (5’-ACCTGGTGCTTCATCGACTGGACC-3’) and thymus RNA as a template. The S35-labeled probes were prepared using a commercially available kit (Ambion). Transverse sections of 8 to 10µm in thickness were prepared, and in situ hybridization carried out as described [15].
Genetically Engineered Mouse Lines
Animal protocols were approved by the Institutional Animal Care and Use Committee at UNC. See supplemental method section S1A and Figure 2a for description of the generation of a loxP-flanked conditional null Ptger4 allele. The generation of mice lacking EP4 and EP2 has been described previously [16,17].
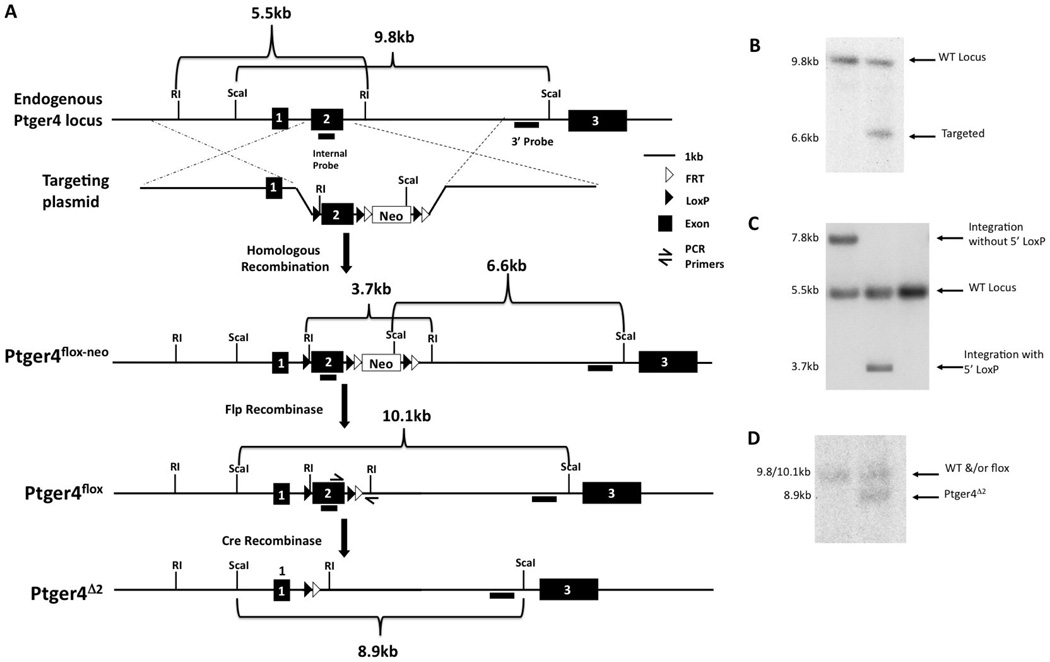
Figure 2.
Schematic depicting the generation of a conditional null Ptger4 allele. A) The structure of, the endogenous Ptger4 locus, the targeting plasmid, and the Ptger4 allele generated by homologous recombination of the plasmid with the wild type locus and the expected structures of these alleles in cells expressing either FLP or Cre recombinase, is shown. Restriction enzymes sites and probes used in identification of cells carrying a correctly targeted allele are indicated. The loxP and FRT sites are represented by filled and open triangles, respectively. EcoRI is abbreviated as RI. Filled, numbered boxes represent exons. B and C Identification of ES cell clones in which the targeting plasmid integrated by homologous recombination and in which this recombination event results in introduction of the loxP site into intron 1. Integration of the targeting plasmid by homologous recombination results in the introduction of an additional ScaI site generating a novel 6.6 kb Sca1 DNA fragment detectable by a probe located just 3’ of the segment of the Ptger4 locus used to assemble the targeting construct. While only the Sca1 fragment corresponding to the endogenous Ptger4 locus is present in DNA shown in lane 1, the presents of a 9.8 and 6.6 kb fragment in lane 2 indicates that in this cell line one of the two Ptger4 alleles has undergone homologous recombination with the targeting plasmid. Recombination between the targeting plasmid and the endogenous locus can occur at any point along regions of homology. To identify those ES cells in which recombination occurred 5’ to the loxP site in intron 1, a second screen was carried out on all targeted ES cells. The presence of an EcoRI site in the linker DNA of the loxP site is expected to reduce the size of the EcoR1 fragment detected by a probe corresponding to exon 2. This probe detects an endogenous fragment of 5.5 kb and either a 7.8 kb or 3.7 kb in targeted ES cells lines, with the later fragment indicative of a crossover event 5’ to the location of the loxP site in intron 1. Although the cell line corresponding to the DNA analyzed in panel C lane 1 carries a targeted Ptger4 allele, this line lacks the loxP site in intron 1. In contrast, the presence of the 3.7 kb EcoR1 fragment in lane three indicates that in this line intron 2 is flanked by loxP sites. The DNA shown in lane 2 was prepared from an untargeted ES cell line. D. Excision of the floxed Ptger4 exon 2 in cells expressing Cre recombinase was verified by preparing DNA from mice generated by intercross of EP4flox mice with mice expressing Cre recombinase in their germline. 8.9 kb Sca1 fragment detected by the 3’ probe in DNA shown in lane 2 is consistent with Cre-mediated excision of exon 2 from the targeted allele. The Sca1 fragment generated from the EP4floxP allele is ~300bp larger than the wild type (WT) allele, due to the presence of the loxP sites and linker sequences. This small difference in size prevents resolution of these two alleles on Southern analysis with Sca1.
The Ptger4+/− mice were maintained on a 129S6 background. C57BL/6 Ptger2+/− mice were crossed for four generations to 129S6. Recombinase transgenic lines, 129S4/SvJaeSor-Gt(ROSA)26Sortm1(FLP1)Dym/J (Stock # 003946) were obtained from Jackson Labs [18]. B6.Cg-Tg(Tek-Cre)129Flv/J lines (Stock #004128), referred to here as Tek-Cre mice, were crossed for seven generations to 129S6 prior to intercross with mice Ptgerfloxed mice [19]. STOCK Tg(Tagln-Cre)1Her/J (Stock #004746), referred to here as Tagln-Cre, were crossed for nine generations to 129S6 mice prior to intercross with Ptgerfloxed [20]. Tg(Wnt1-Cre)11Rth Tg(Wnt1-GAL4)11Rth/J (Stock #003829), referred to here as Wnt1-Cre mice, were crossed for two generations to 129S6 mice prior to intercross with Ptgerfloxed [21]. B6.C-Tg(CMV-Cre)1Cgn/J (Stock # 006054), referred to as CMV-Cre mice, were maintained on a BALB/c genetic background [22]. The presence of the Cre recombinase transgene was identified by using primers specific for Cre recombinase coding sequence (upper 5’-TTACCGGTCGATGCAACGAGT-3’; lower 5’-TTCCATGAGTGAACGAACCTGG-3’).
Quantitative Real-time RT-PCR
DA of identical genotypes were pooled (4–8 per sample) prior to RNA extraction, and cDNA generated using the high-capacity cDNA archive kit (Applied Biosystems). Primers and probes for quantitative real-time RT-PCR (qPCR) were purchased from Applied Biosystems and reactions were performed in duplicate with 10ng of cDNA using the TaqMan PCR Universal Master Mix (Applied Biosystems) and using the Applied Biosystems 7900 HT Fast Real-Time PCR System. Gene expression was normalized to either β-2 microglobulin (B2M) or GAPDH. Due to observed changes in GAPDH expression during DA closure, all genes in DA were normalized to B2M gene. Data were analyzed using the comparative CT method as described by Applied Biosystems.
Indomethacin treatment, Ductus arteriosus collection and scoring
In some experiment, dams were treated with indomethacin (10µg of indomethacin per 1 g of weight, i.v.) 18.5 after mating. 6 h later, dams were euthanized and fetuses were recovered. The patency of DA was determined by visual observation and confirmed by placing slight pressure on the right ventricle and observing movement of blood through the ductus to the aorta. In some cases, closure did not extend the entire length of the vessel, however if blood no longer could cross from the ventricle to the aorta, the vessel was scored as closed. All scoring was done blinded, the genotype of the fetuses becoming available after the documentation of the structure of the DA.
Statistical Analysis
Statistical analysis of qPCR was performed using Prism 4 (GraphPad Software). Comparisons of the distributions were made by unpaired t test unless otherwise noted. Data are shown as median ± S.E.M. Statistical significance was scored as follow: * p<0.05, ** p<0.01, *** p<0.001. Test of proportions was used to calculate statistical significance of survival data. Differences in patency of the ductus were evaluated by Fisher’s exact test.
RESULTS
Expression of the EP4 in the ductus arteriosus
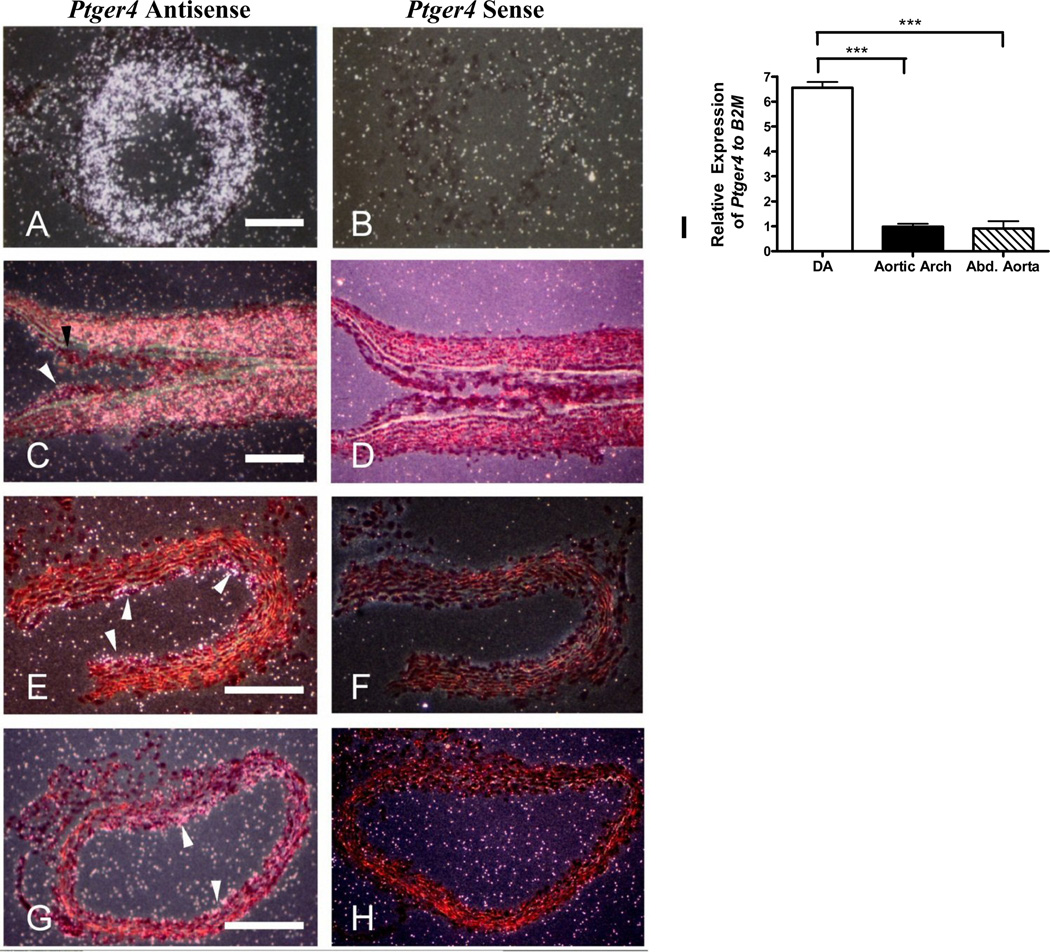
Analysis of transverse sections through the DA isolated from a 18.5 day full term fetus with Ptger4 specific probes showed high levels of expression of Ptger4, the gene encoding EP4, in this vessel, with an intense signal in the layers containing the SMC (Figure 1A–B). Ptger4 expression in the vasculature is generally highest in the endothelial cells. To examine this point further and to determine if expression of EP4 by smooth muscle cells was limited to the DA, longitudinal sections were prepared from the DA of a neonate six hours after birth. At this point the closure and remodeling of the artery assists in defining the transitions between the DA and the aorta. Again, high levels of Ptger4 expression were observed in the SMC layers of the DA (Figure 1C–D). An abrupt change in expression is observed at the junction between the SMC of the DA and the aorta, with little to no expression of Ptger4 in SMC of this vessel. This was confirmed by examination of cross section of the aorta (Figure 1E–F). Similarly, little expression of Ptger4 was detected in the SMC of the pulmonary trunk (Figure 1G–H). In contrast, expression of Ptger4 in endothelial cells was apparent in all vessels. Thus in the DA, unlike other greater vessels, Ptger4 appears to be expressed by both the endothelial cells and the SMC.
Figure 1.
Expression of Ptger4 in the ductus arteriosus. (A, B) Transverse sections of DA from the 18.5 day mouse fetus; (C, D) longitudinal sections of DA from neonate; (E, F) transverse sections of neonatal aorta (6 h after birth); (G, H) transverse section of neonatal pulmonary trunk; sections were hybridized with 35S labeled Ptger4 antisense probe (A,C,E,G) or Ptger4 sense probe (B,D, F,H). Scale bar is 150µm. Black arrowhead points to junction between the SMC of the DA and the aorta; white arrowheads depict Ptger4 expression in endothelial cells. I) Ptger4 transcript levels in abdominal aorta, aortic arch, and DA as measured by qPCR
To quantify the difference in Ptger4 expression in these muscular arteries, RNA was prepared from the DA, the adult aortic arch, and adult abdominal aorta of a full term fetus and subjected to qPCR. Ptger4 transcripts were six times more abundant in the DA than in either the abdominal aorta or aortic arch (Figure 1I).
Generation of the mice carrying a conditional null Ptger4 allele
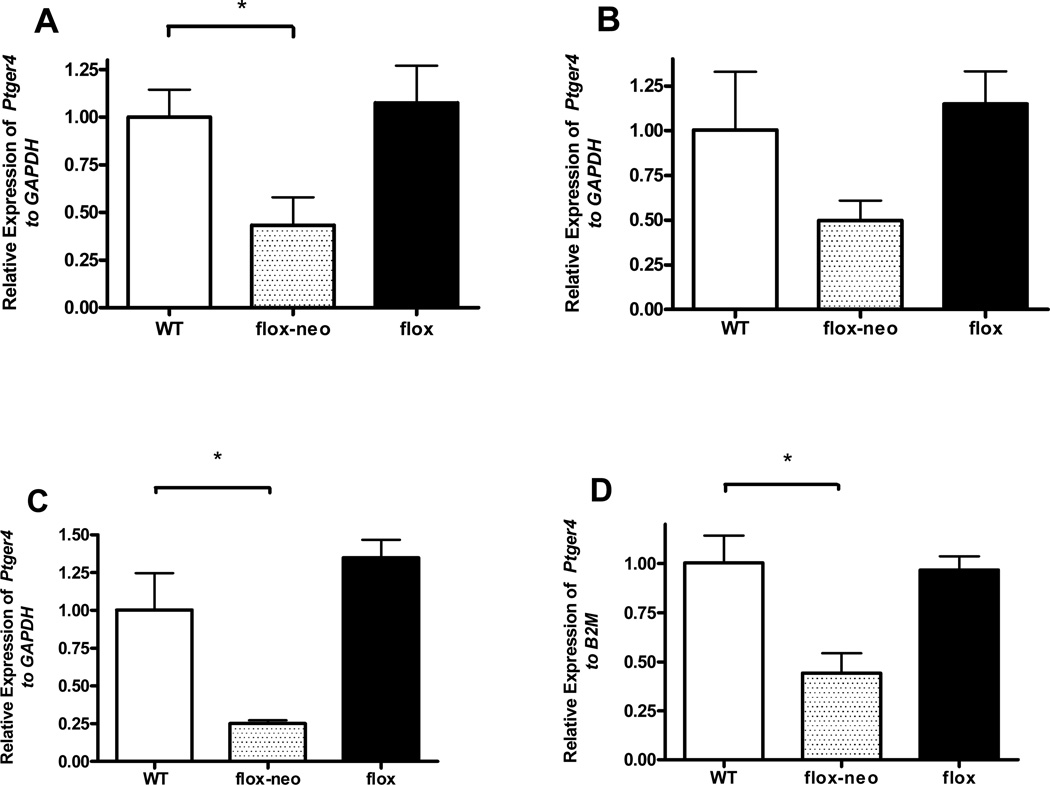
To define the contribution of EP4R expression by smooth muscles cells and endothelial cells to the physiology of the DA we generated a mouse line carrying a loxP-flanked conditional null Ptger4 allele (Figure 2a and supplemental methods S1A). These animals should allow us to test various models for the contribution of EP4 on these cells populations to both the maintenance of the patency of the DA in utero and the constriction and subsequent remodeling of the vessel at birth (Figure 3). The strategy used in generating the mouse line is detailed in methods section. Briefly, homologous recombination was used to introduce loxP sites into the introns flanking exon 2, which encodes the EP4 transmembrane domain, and the neomycin resistance gene, flanked by FRT sites into intron 2. The resulting locus is designated Ptger4flox-neo. Mice homozygous for the Ptger4flox-neo allele are viable with lower levels of Ptger4 transcript lung, aortic arch, abdominal aorta, and DA compare to WT control, although the decrease in the aortic arch did not achieve statistical significance (Figure 4). Despite the hypomorphic levels of Ptger4 transcript, mice undergo normal DA closure. The neomycin gene was excised from intron 2 by breeding to mice expressing FLP recombinase yielding a Ptger4flox locus. Mice homozygous for Ptger4flox locus are viable, undergo DA closure, and express normal levels of Ptger4 in all tissue tested (Figure 4). Mice carrying either a flox or flox-neo Ptger4 allele were bred to CMV-Cre transgenic mice to generate a mouse line carrying a null allele, Ptger4Δexon2. Mice heterozygous for Ptger4Δexon2 mutation were in-crossed to confirm homozygous lethality of the allele generated by Cre-mediated recombination (data not shown).
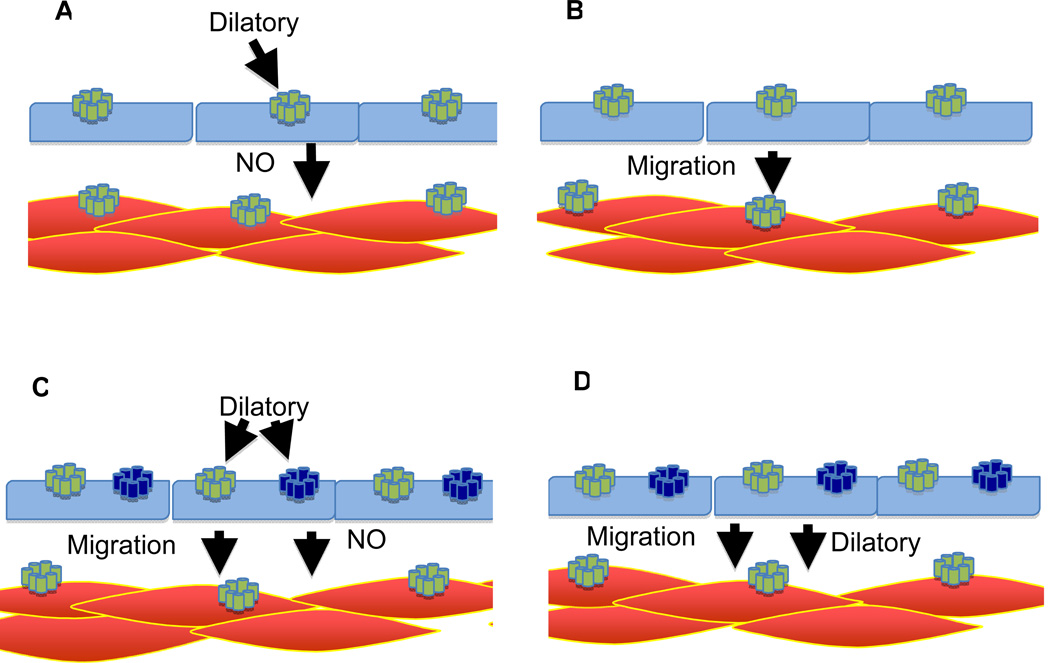
Figure 3.
Models for the role of the EP4 receptor in the maturation and closure of the ductus arteriosus. A) DA has intrinsic tone that is counteracted by PGE2 through endothelial EP4 receptors. This model predicts premature closure in EP4 deficient mice. B) No EP4 dependent dilatory signal is required to maintain patency. PGE2/EP4 is responsible for the maturation of the DA, resulting in increased tone, which is required for closure at birth. However, this model is not consistent with the ability of indomethacin to rescue Hpgd−/− mice. If PGE2 had no dilatory function, the loss of PGE2 would not be expected to cause closure. C) In this model, PGE2 has two roles in the physiology of the DA. First through the smooth muscle EP4 receptors, PGE2 provides a metabolic signal stimulating maturation. At the same time, PGE2, through a dilatory signal via the endothelium, counteracts increasing tone of the DA. However, this model would predict premature closure in mice not expressing either EP4 or EP2 in the endothelium. D) In this model, PGE2/EP4 provides two functions. In its absence, the DA fails to mature. Second, as the vessel matures, PGE2 induced relaxation of the smooth muscle independent of the endothelium prevents premature closure. This model is consistent with the finding that mice lacking expression of EP4 in the smooth muscle die with a PDA, and mice lacking EP4 in the endothelium and EP2 in both endothelium and smooth muscle survive with no naïve phenotype. While in the rodent the source of NO is the endothelium, in larger animals the vasa vasorum, contributes to NO production. Although a number of agents can dilate the ductus, for simplicity we show only PGE2.
Symbols in models:
SMC-red shapes, endothelial cells-blue shapes, EP4-yellow receptors, EP2-blue receptors, activated receptors-yellow highlight.
Figure 4.
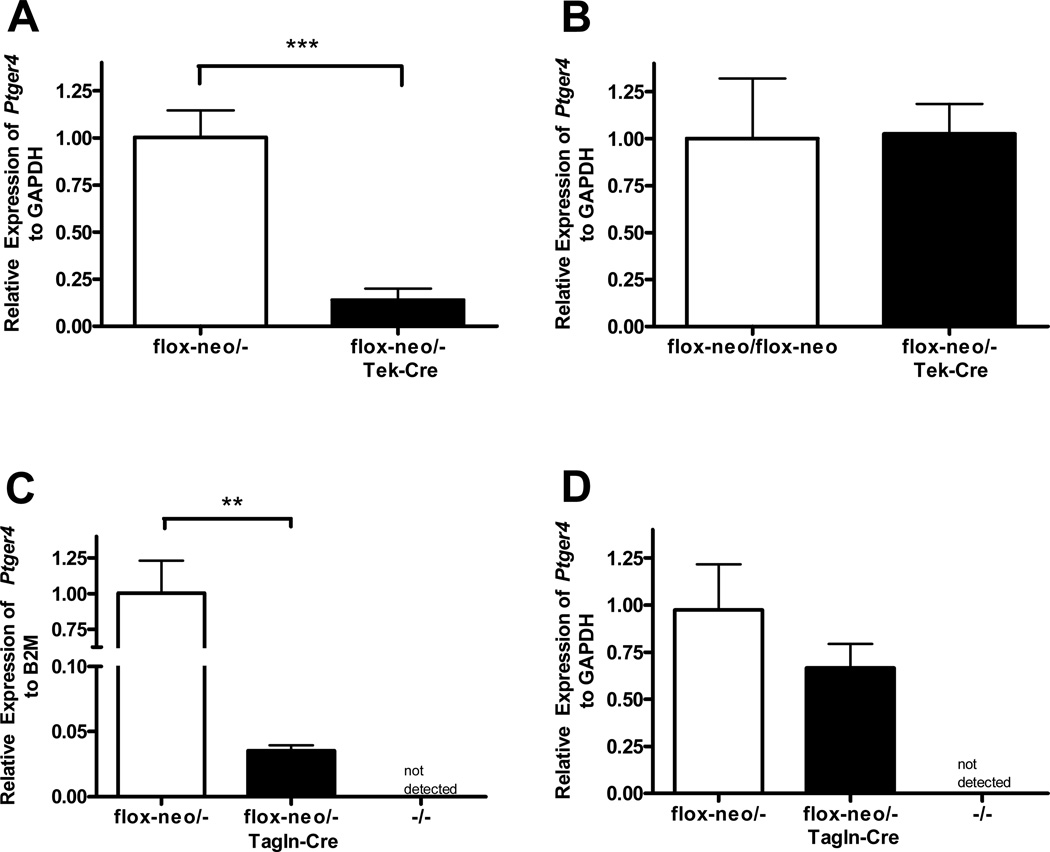
Expression of the Ptger4 receptor in mice homozygous for the conditional null Ptger4 alleles. RNA was prepared from the adult (A) lung (n=5), (B) aortic arch (n=4), and (C) abdominal aorta (n=5), and from the (D) full term (18.5E) DA (n=5) of animals with the indicated genotype and the expression of Ptger4 assessed by qPCR. Gene expression was normalized to either GAPDH (A–C) or β-2 microglobulin (B2M) (D). No difference in expression of Ptger4 was observed between wild type mice and mice carrying the floxed allele. In contrast the presence of the neomycin gene in intron 2 significantly decreased expression of Ptger4 in some tissue. Statistical analysis: * p < 0.05.
Endothelial cell-specific loss of EP4 and DA patency
In situ analysis shows Ptger4 expression by endothelial cells (Figure 1), and this finding is supported by reports indicating that PGE2 can modulate vascular tone through its action on this cell type. It was therefore of interest to determine whether this expression of Ptger4 also played a role in either maintaining the patency of this vessel in utero or in the closure of the vessel after birth. Ptger4flox/flox and Ptger4flox-neo/flox-neo mice were intercrossed with mice heterozygous for the Ptger4 null allele and carrying a Cre transgene controlled by the endothelial specific promoter of the Tek gene to generate mice lacking endothelial Ptger4 expression. Although slightly fewer than expected Tek-Cre Ptger4flox/− pups were identified in the litters, the majority of these underwent DA closure (Table 1).
Table 1.
Survival of mice after cell lineage specific disruption of the Ptger4 floxed allele.
| Tissue Specific |
Breeding | Age, Days |
n | n Cre+ |
Ptger4 Genotype |
Survival Observed / Expected |
p value observed vs expected |
|---|---|---|---|---|---|---|---|
| Smooth Muscle (Tagln-Cre) | TaglnCre/+ Ptger4+/− X Ptger4f/f | P0 | 163 | 72 | f/− | 20/48 | < 0.001 |
| P3 | 143 | 54 | f/− | 2/48 | < 0.001 | ||
| f/+ | 52/48 | - | |||||
| TaglnCre/Cre Ptger4+/− X Ptger4f/f | P0 | 122 | 122 | f/− | 56/66 | - | |
| P3 | 66 | 66 | f/− | 0/66 | < 0.001 | ||
| f/+ | 66/66 | - | |||||
| TaglnCre/+ Ptger4+/− X Ptger4f/+ | P0 | 207 | 119 | f/− | 20/27 | - | |
| P3 | 187 | 99 | f/− | 0/27 | < 0.001 | ||
| +/+ | 33/27 | - | |||||
| +/− | 27/27 | - | |||||
| f/+ | 39/27 | - | |||||
| Neural Crest (Wnt1-Cre) | Wnt1Cre/+ Ptger4+/− X Ptger4f/f | P0 | 249 | 126 | f/− | 56/64 | - |
| P3 | 193 | 70 | f/− | 0/64 | < 0.001 | ||
| f/+ | 70/64 | - | |||||
| Endothelium (Tek-Cre) | TekCre/+ Ptger4+/− X Ptger4f/f | P0 | 285 | 139 | f/− | 60/75 | - |
| P3 | 278 | 132 | f/− | 53/75 | 0.0267 | ||
| f/+ | 79/75 | - | |||||
n = total numbers of pups found after birth. n Cre+ = number of pups with Cre transgene.
Expected # is the Mendelian expectation of Ptger4flox/− Cre+ pups calculated from the numbers of pups observed with at least one intact copy of the Ptger4 locus (wild type or ptger4flox in the absence of Cre). f = floxed locus. P-value calculated with proportion test.
To verify endothelial specific loss of Ptger4 expression in these mice we examined Ptger4 expression via qPCR in the aortic arch (Figure 5A) and abdominal aorta (data not shown), as in situ analysis of Ptger4 in these vessels indicated that expression was limited to the endothelium. Ptger4 expression was significantly reduced in both tissues. In contrast, no difference in expression of EP4 was observed in the kidney, reflecting the relative small contribution of the expression of EP4 by this cell type to the total expression of EP4 in this organ, while in other tissues, including kidney, the level of Ptger4 expression did not changed (Figure 5B). To determine whether the DA of these animals retained their dependence on PGE2 for patency, pregnant dams (18.5E) were treated with NSAID indomethacin as described in method section and DA of fetuses were scored for patency by visual observation. Twelve pregnancies contributed to the 89 fetuses examined in this manner, of which 19 were expected to expression no EP4 receptor on endothelial cells. Of these 19 fetuses, 10 underwent closure of the DA after in utero exposure to indomethacin. 13 of the 17 pups heterozygous for the null and foxed EP4 gene, but lacking the Tek driven Cre gene showed closure of the DA. The frequency of DA closure in Tek-Cre Ptger4flox/− mice was not statistically significant compare the Ptger4flox/− controls (n=12 for dams, 89 for fetuses, P = 0.177).
Figure 5.
Cell lineage restricted loss of EP4R expression. Expression of Ptger4 was examined by qPCR in (A) aortic arch (n=8), (B and D) kidney (n=6), and (C) DA (n=5) isolated from animals (E18.5) heterozygous for Ptger4 null, Ptger4flox-neo, and genetically identical littermates expressing either Tek-Cre (A and B) or Tgln-Cre (C and D). Gene expression was normalized to either GAPDH (A, B, D) or β-2 microglobulin (B2M) (C). Ptger4 levels are decreased by approximately 80% in the aorta of mice expressing the endothelial specific Cre, while no significant change is observed in the kidney. While the majority of Ptger4 expression by the DA is lost in tissue expressing Cre in the SMC, the expression remains higher than that detected in DA from Ptger4−/− mice, reflecting the continued expression of Ptger4 by endothelial cells. . Statistical analysis: ** p < 0.01, *** p<0.001.
In addition to EP4, endothelial cells also express the Gs-coupled PGE2 receptor, EP2. This, together with the slight decrease in the survival of the Tek-Cre Ptger4flox/− suggested the possibility that the EP2 receptor might play a redundant role in this cell type and compensate for EP4 loss. We therefore examined the impact of endothelial- restricted loss of EP4 on an EP2-deficient background. Mice lacking expression of EP2 and EP4 on endothelial cells were present in litters at expected frequency (See Supplemental Table 1) indicating that the loss of this additional receptor did not alter the ability of the vessel to remain patent and support the normal development of the fetus.
Smooth muscle-specific loss of EP4 expression
In situ analysis indicates that the smooth muscle cells of the DA, unlike those of the aorta and pulmonary trunk express EP4 receptors (Figure 1A–D). We therefore generated mice carrying the flox or the flox-neo Ptger4 alleles to Ptger4+/− mice and Cre under the control of the Tagln (Sm22) promoter, which directs Cre expression in both vascular and non-vascular smooth muscle. We expected that mice expressing the Tagln-Cre and the flox alleles would lack expression of the receptor in SMC only, allowing us to isolate the function of EP4 on these cells in DA closure. As seen in table 1, all Ptger4flox-neo/− mice expressing the Tagln-Cre die in the perinatal period with a PDA indistinguishable from that observed in Ptger4−/− mice. Loss of EP4 in SMC was confirmed by qPCR analysis of RNA isolated from the DA of wild type mice, Ptger4flox/− mice, Ptger4flox/− Tagln-Cre mice, and Ptger4−/− pups (Figure 5C). As expected, no expression of the Ptger4 was detected in the Ptger4−/− pups. Low but significant expression of the gene was detected in DA of the Ptger4flox/− Tagln-Cre mice. This is consistent with the undisturbed expression of EP4 on endothelial cells in the Tagln-Cre mice. However, not surprising, given the muscularity of this vessel, the expression of Ptger4 by the Tagln-Cre Ptger4flox/− mice was dramatically lower than that observed in the wild type DA. A much small decrease in Ptger4 expression was observed in kidneys isolated from same animals, (Figure 5D and this change failed to achieve statistical significance. This small change is consistent with the cell type specific loss of Ptger4 expression and reflects the relatively low amount of smooth muscle cell in this organ compared to other cell types. To determine whether the loss of expression of the receptor on the SMC altered the response of the vessel to loss of PGE2, two pregnant dams were treated with indomethacin and the patency of the DA was scored in 18.5-day fetuses. The DA of the two E18.5 day fetuses with SMC restricted loss of EP4 remained patent in the absence of PGE2, while closure was observed in littermates with normal EP4 expression.
Loss of EP4 in Neural crest derived SMC
Tagln drives expression of Cre in all SMC, not only those of the DA. This raises the possibility the PDA observed in the Tagln-Cre Ptger4flox/− mice reflects the combined effect of the loss of expression of this gene on multiple SMC populations. To address this possibility we generated mice in which loss of Ptger4 expression was limited to neural crest-derived cell, the primary embryonic origin of the SMC of the DA and other great arteries of the heart [23]. Mice homozygous for either the flox or flox-neo Ptger4 allele were crossed with Ptger4+/− mice carrying the Cre transgene driven by the Wnt-1 promoter. Neural crest-Ptger4−/− mice succumb to cardiovascular failure secondary to a PDA (Table 1).
Identification of PGE2/EP4 dependent gene expression in the DA
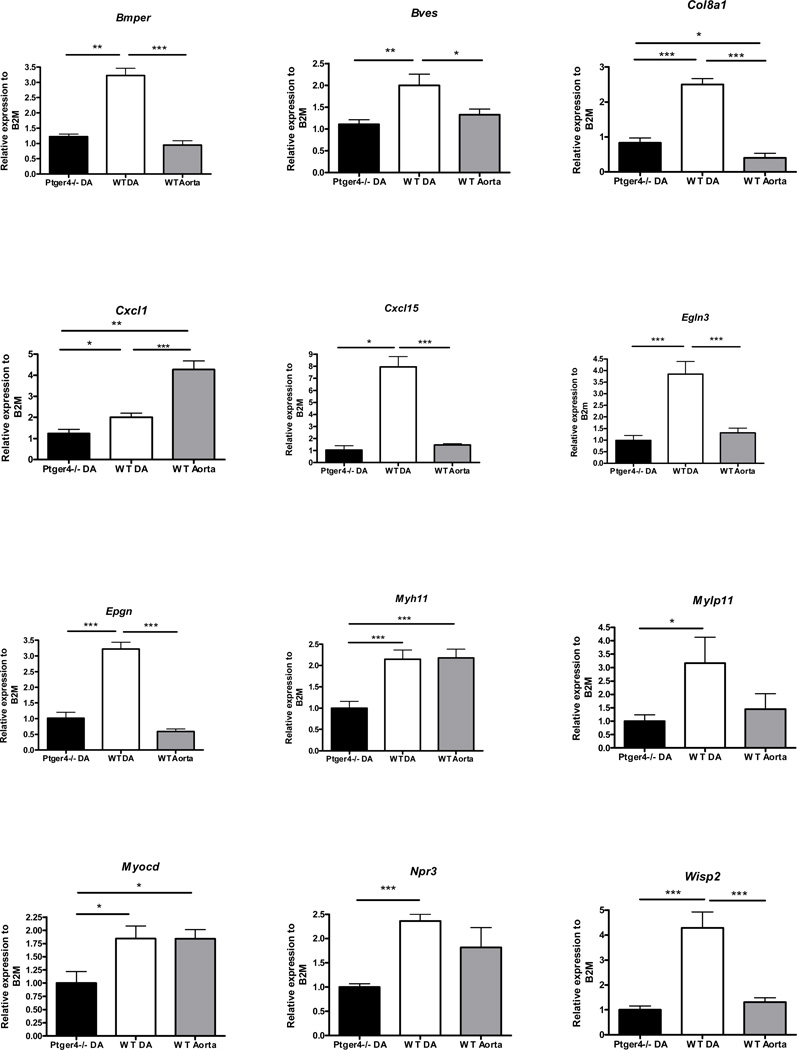
To gain a mechanistic understanding of the contribution of EP4 on the SMC to both closure of the vessel at birth and to the dilatory actions of PGE2 in utero, we carried out expression analysis of DA from full term Ptger4−/− and Ptger4+/+ fetuses. We reasoned that differences in the expression of genes in Ptger4+/+ DA compare toPtger4−/− DA would identify pathways whose activities were either directly or indirectly altered by exposure of the DA to PGE2 and activation of the EP4 receptor. While no gross morphological differences are observed between the DA of Ptger4+/+ and Ptger4−/− fetuses, we were surprised to find that gene expression analysis by Illumina microarray revealed 282 transcripts with statistically significant differential expression between the DA from Ptger4+/+ and Ptger4−/− 18.5 day fetuses [p < 0.05, fold change > 1.25; see Supplemental Data, Table 2 and 3, for a list of all 282 differential expressed transcripts]. Many of the genes identified have been implicated in SMC growth, migration and interestingly in determination of both vascular tone and contractile apparatus (Table 2). We further analyzed expression of 14 as being present at higher levels in the DA of wild type mice compared to those lacking EP4R by quantitative PCR. The genes were chosen based on both the fold change observed in the microarray and the assigned p value. We reasoned that these genes were likely to include genes whose expression was induced by PGE2/EP4 signaling during maturation of the DA. cDNA prepared from the aorta was included in the study for additional comparison. This artery, similar to the DA from the Ptger4−/− animals does not express EP4R in muscle. It would not be unreasonable to expect that the expression of genes induced by PGE2 and thus abundant in the wild type DA would be at similar levels in the aorta and the Ptger4−/− DA, since the smooth muscle of both tissues lack EP4 receptors. We considered the level of expression in the Ptger4−/− DA as “baseline” assigning it a value of one, and asked whether the expression of EP4R significantly increased the expression of the fourteen genes. Significant difference in expression between the Ptger4−/−, DA and wild type DA was observed for 11 of the 14 gene: Bmper, Bves, Col8a1, Cxcl1, Cxcl15, Egln3, Epgn, Myh11, Mylpf, Npr3, and Wisp2. No difference was observed for Pvalb, Ryr3, and Tcfap2b (data not shown). For the majority of the genes examined, higher levels of expression are observed in the Ptger4+/+ DA compared to the Ptger4+/+ aorta consistent with the hypothesis that expression of EP4 by the smooth muscle leads to this increase in gene expression (Figure 6).
Table 2.
Several genes up-regulated in wild type full-term ductus arteriosus versus Ptger4−/− full-term ductus arteriosus are depicted. Fold increase in wild type ductus arteriosus from microarray analysis indicated in (). Underlined genes verified by qPCR.
| Type | Genes |
|---|---|
| Contractile apparatus | Mylpf (2.68), Mylk (1.27), Myh11 (1.27), Myocd (1.84)* |
| Migration | Cxcl15 (6.61), Col8a1 (2.69), Bves (1.93), Adamtsl2 (2.37), Col8a2 (1.66), Cxcl1 (1.42), Cxcl16 (1.29), Cxcr4 (1.28) |
| Growth | Epgn (4.68), Grem1 (2.34), Bmper (2.25), Wisp2 (1.97), Btc (1.59) |
| Vascular Tone | Egln3 (4.68), Npr3 (2.50), Ism1 (1.75), Slc03a1 (1.36) |
| Myocardin sensitive genes | Tpm2 (1.31), Tnnt3 (1.89), Tnni2 (1.73), Tnnc2 (1.71), Tjp1 (1.30), Slc12a2 (1.45), Pdlim3 (1.25), Mylpf (2.68), Myh11 (1.27) |
qPCR identified Myocd as differentially expressed, fold change from qPCR; microarray analysis did not identify Myocd as differentially expressed due to p-value.
Figure 6.
Identification of EP4R-dependent gene expression in the DA. Expression of eleven genes identified by microarray analysis as increased in the wild type DA compared to Ptger4−/− DA were further analyzed by qPCR. Expression of myocardin was also evaluated because of its established role in DA closure. Expression of each of the genes in the full term Ptger4+/+ DA and Ptger4+/+ adjacent aorta was compared to expression in Ptger4−/− DA. N=8 for DA, n=4 for aorta‥ . * p-value < 0.05, **p-value < 0.01, *** p-value<0.001.
Previous studies have identified a critical role for myocardin in expression of contractile genes by the DA. We therefore compared the 11 genes that differed significantly in expression between Ptger4+/+ and Ptger−/− (p>.05) to genes regulated by Myocd pathway using Ingenuity Systems Pathway Analysis (Table 2, myocardin sensitive genes). Since loss of Myocd by the DA results in a PDA in mice [23], we examined expression of this transcription factor. Although, no difference was detected by microarray analysis, qPCR analysis of Ptger4−/− and wild type DA showed a 50% reduction in expression of Myocd in the Ptger4−/− tissue relative to the wild type DA. In the case of these Myocd regulated genes, EP4 expression appears to be required to maintain expression at levels similar to those observed in other muscular arteries.
Discussion
Our studies show that the EP4 is highly expressed by cells located in the media of the DA and that EP4 expression serves as a marker for this great artery, distinguishing it from the adjoining pulmonary truck and aorta. Using mouse lines with SMC and endothelial cell restricted loss of EP4 we provide evidence supporting both a dilatory and developmental function for this receptor, with both of these actions mediated through the EP4 on the SMC.
The expression of EP4 by endothelial cells including those of the DA is consistent with a model in which EP4 activation leads to production of dilatory mediators that act on the underlying SMC maintaining the patency of the vessel (Figure 3). However, while such a model accounts for the ability of PGE2 to maintain the patency of the DA in utero and after birth, it is not consistent with our finding that fetal expression of the EP4 by endothelial cells is not required for the normal development. Pups lacking endothelial EP4 are present in litters at birth and normal DA closure indicates that the vessel has matured and is capable of constriction and remodeling. In addition, maternal exposure to indomethacin results in DA constriction in these fetuses.
PGE2 can mediate relaxation of other smooth muscle through Gs couple receptors. For example, relaxation of airway smooth muscle is mediated through the Gs coupled EP2 [24]. The observation that a rapid decrease in PGE2 levels can lead to rapid closure of the DA, either at birth or in response to maternal indomethacin treatment, suggests that PGE2 does act, at least in part, through similar dilatory pathways in the DA. However, if this represented the only function of SMC EP4 pathways, premature closure of the vessel in utero would be expected in the Tagln-Cre Ptger4flox/− animals.
Taken together, the pharmacological and genetic studies suggest that a more complex model is required to describe the contribution of the EP4 to DA physiology. The SMC of the DA are derived from the neural crest progenitors, cells that also contribute substantially to the SMC of aorta and carotid arteries. However, unlike the SMC of the aorta, DA SMC acquire unique properties including the ability to proliferate and contribute to intimal cushion formation particularly in larger species and the ability to migrate to the sub-endothelial space facilitating the remodeling of the vessel at birth. Concurrent with these changes the vessel must remain patent in utero yet be capable of constricting in response to rise in oxygen tension at birth. Our analysis of genes expressed by DA lacking EP4 indicates that PGE2 signals through this receptor to direct expression of a diverse set of genes, many of which would be expected to contribute to the acquisition of these functional characteristics.
EP4-dependent expression of a number of genes expected to contribute to SMC migration was observed. Notable among these was collagen VIII, which displays chemotaxtic properties and supports migration, adhesion and focal adhesion formation of SMC [25]. One of the most dramatic differences in the expression profile of Ptger4−/− and wild type DA was in the expression of the chemokine Cxcl15. Although, no role for SMC migration has been identified for this chemokine, the related ELR-CXC human chemokine, Cxcl5/ENA-78 has been shown to be inducible by prostanoids and to play a role in mesenchymal cell migration, metastasis and angiogenesis [26,27]. Blood vessel epicardial substance (Bves) is a membrane protein that accumulates at points of cell/cell contact [28]. Introduction of this gene into cells confers adhesive behavior. In addition, its expression is required for coronary vessel development [29]. Bves−/− mice display delayed skeletal muscle regeneration due to impaired migration of satellite cells [30]. The higher expression of Bves in the DA compared to the aorta could reflect a similar function in migration of DA SMC. It is also interesting to note that the wild type DA express higher levels of fibronectin than those lacking the PGE2/EP4 pathway. Although this difference was not verified by PCR analysis it is consistent with early studies showing fibronectin dependent migration of DA smooth muscle cells [31,32].
PGE2 is known to promote growth of many cell types including murine aortic SMC [33]. PGE2 has also been shown to stimulate vascular endothelial growth factors in human airway SMC [34]. However, the mechanism by which PGE2 promotes growth of SMC is not known. A possible mechanism is suggested by our observation of EP4-dependent expression of two EGFR ligands, epithelial mitogen and betacellulin (Btc) by the DA (Supplemental table 2). Expression of epithelial mitogen was approximately three fold higher in the wild type DA compared to both neighboring aorta and Ptger4−/− DA, suggesting positive regulation of an epithelial mitogen by PGE2. While little information is available concerning the functions of epithelial mitogen [35], the related EGFR ligands, EGF, BTC and EPR have been reported to contribute to the transition of differentiated non-proliferative SMC to cells capable of growth and migration [36]. EGFR pathways are believed to contribute both to atherosclerosis and angiogenic changes. The demonstration that PGE2/EP4 can increase expression of EGFR ligand in the DA suggests that similar pathways may be utilized by PGE2 to impact vascular remodeling during other conditions, including wound healing, plaque formation and angiogenesis.
A second possible pathway by which PGE2 could modulate growth and differentiation of SMC is suggested by the demonstration that PGE2 can enhance the Wnt signaling cascade through cAMP/PKA-mediated regulation of β-catenin stability [37]. Consistent with this we note an increase in expression of a number of Wnt transcriptional targets, including Gremlin-1, Wisp2 (Table 2) and Semaphorin 3C (Supplemental table 2) in the wild type DA relative to the Ptger4−/− vessel. The largest increase was noted for the WNT sensitive Gremlin-1 transcript that encodes a secreted bone morphogenic (BMP) protein antagonist [38]. Interestingly, we also noted an increase in expression of Bmper, a more recently identified inhibitor of BMP, suggesting that regulating the actions of BMP may be important in the development of the vessel [39].
The rapid constriction of the DA in the neonate is well documented, and the importance of the contractility of the vessel is demonstrated by the phenotype of a mouse line that lacks myocardin expression in SMC of neural crest origin [23]. These pups die in the perinatal period with a PDA indicating that expression of myocardin and expression of contractile proteins is essential for DA closure. Consistent with this interpretation, mutations in the myocardin regulated contractile protein MYH11 are linked to hereditary PDA, and the loss of MYH11 in mice results in delayed closure of the DA [40,41]. The possibility that the maintenance of the contractile phenotype of the vessel is at least in part dependent on EP4 is suggested by difference in the expression of a number of myocardin sensitive transcripts in Ptger4−/− and wild type animals. Although genome wide analysis did not detect differential myocardin expression, qPCR analysis indicated that the expression of this transcription factor was approximately two times higher in wild type DAs compared to DAs collected from Ptger4−/− pups. This raises the possibility that EP4 expression is required for normal induction and/or maintenance of myocardin expression, and that increased expression of Myh11 and Mylpf in wild type DA compare to Ptger4−/− DA is secondary to increased levels of myocardin. Increased myocardin expression was observed on analysis of fibroblasts after induction of Wnt signaling [42]. This raises the intriguing possibility that PGE2 also contributes to the contractile phenotype of the DA indirectly through stabilization of β-catenin.
While it is possible that all of the dilatory actions of PGE2 are mediated directly through regulation of SMC cAMP levels, our expression analysis raises the possibility that PGE2 might also regulate genes capable of modulate the dilatory/constriction response of the vessel. For example, Egln3 is expressed at high levels in the DA compared to the aorta, in an EP4-dependent manner. Egln3 encodes an intracellular prolyl hydroxylase, which through its ability to hydroxylate the alpha subunit of hypoxia inducible factor can regulate the cellular response to oxygen availability [43]. By induction of this gene PGE2 could contribute to acquisition of an oxygen sensitive contractile response by the DA, enabling the vessel to detect and rapidly constrict upon transitional rise of oxygen at birth. The mechanism for the contractile effects of alterations in oxygen tension on the DA has not been completely defined. A number of studies have suggested that increased oxygen tension leads to smooth muscle cell depolarization and an increase in intracellular Ca++ secondary to closing of oxygen sensitive potassium channels [5]. A possible mechanism by which the change in oxygen tension can mediate constriction of the DA was suggested by the finding that oxygen reversibly inhibited a 4-aminopyridine sensitive potassium channel leading to membrane depolarization and increase in Ca++ by opening of L-type voltage sensitive channels [44]. No alteration in the expression of L type Ca++ channels was observed in the EP4 deficient DA in our analysis. Genome wide analysis showed that loss of EP4 resulted in a decrease in expression of the shaker related potassium channel KCNAB1 whereas expression of KCNJ8 was increased, although these changes were not verified by PCR analysis. KCNJ8 has been shown to play a critical role in dilation of coronary arteries and thus, if this change can be further documented it raises the possibility that EP4 can modulate the physiology of the DA in part by altering expression of potassium channels.
A number of other agents, in addition to PGE2, capable of relaxing the DA have been identified [5]. For example, inhibition of nitric oxide synthase (NOS) was shown to result in contraction of the lamb DA both in vitro and in vivo [45,46]. Further studies using ductuses in which the endothelial cells were removed suggested that the NO could be derived not only from the endothelia cells, which we suggest in one of our models, but also from extraluminal sources [45,47]. Although not confirmed by quantitative PCR, we noted a small increase in the levels of endothelial NOS (eNOS, Supplemental table 3) in the DA from the EP4−/− animals. This increase does not support a role for PGE2 upstream of eNOS. It may in fact represent an attempt of the DA to compensate for the loss of this PGE2 by up-regulation of an alternate dilatory pathway.
The DA is unique among the great arteries in its expression of extremely high levels of EP4. Our studies suggest that this expression pattern evolved to allow for rapid alteration of blood flow at birth, changes required for transition from placental to pulmonary oxygenation of blood. Expression of high levels of EP4 on DA SMC provided a means by which the contractile properties, possibly its sensitivity to oxygen, and the DA’s capacity to undergo remodeling could be modified independent of other arteries. While most studies have focused on the coupling of EP4R to Gαs and thus its ability to increase cAMP, unlike the EP2 receptor, activation of EP4 has been reported to lead to phosphorylation of the extra-cellular signal-regulated kinases (ERKs) by a PI3K dependent mechanism [48]. At this time it is not possible to determine whether all aspects of the function of EP4 in DA physiology are mediated by a single pathway. For example, it is possible that the ability of EP4 to modify gene expression is mediated by the ERK pathway, whereas the dilatory actions are dependent on increase in cAMP. Also it will be important to determine the time course of expression of the EP4 receptor during development and establish whether EP4 functions both to alter gene expression and/or mediate the dilatory actions of PGE2 during all stages of development. It is not unlikely that expression of EP4 might increase during the last few days of gestation but then decrease at birth, and that this decrease contributes to the closure of the vessel.
Mice deficient in EP4 expression in smooth muscle (SMC) and endothelial cells.
EP4 expression only in SMC is required for ductus arteriosus (DA) closure.
Genome wide gene expression analysis of WT and EP4−/− DA.
Identification of PGE2/EP4 dependent gene expression in the DA.
Supplementary Material
Acknowledgements
We thank Anne Latour for assistance in the generation of the Ptger4 mutant mouse line and Tom Coffman and Wendell Jones for helpful discussion. Work was supported by NIH grants DK069896 and HL068141.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.De Reeder EG, Girard N, Poelmann RE, Van Munsteren JC, Patterson DF, et al. Hyaluronic acid accumulation and endothelial cell detachment in intimal thickening of the vessel wall. The normal and genetically defective ductus arteriosus. Am J Pathol. 1988;132:574–585. [PMC free article] [PubMed] [Google Scholar]
- 2.Momma K, Takeuchi H. Constriction of fetal ductus arteriosus by non-steroidal anti-inflammatory drugs. Prostaglandins. 1983;26:631–643. doi: 10.1016/0090-6980(83)90200-9. [DOI] [PubMed] [Google Scholar]
- 3.Heymann MA, Rudolph AM, Silverman NH. Closure of the ductus arteriosus in premature infants by inhibition of prostaglandin synthesis. N Engl J Med. 1976;295:530–533. doi: 10.1056/NEJM197609022951004. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci U S A. 1993;90:11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith GC. The pharmacology of the ductus arteriosus. Pharmacol Rev. 1998;50:35–58. [PubMed] [Google Scholar]
- 6.Elliott RB, Starling MB, Neutze JM. Medical manipulation of the ductus arteriosus. Lancet. 1975;1:140–142. doi: 10.1016/s0140-6736(75)91432-4. [DOI] [PubMed] [Google Scholar]
- 7.Coceani F, Olley PM. The response of the ductus arteriosus to prostaglandins. Can J Physiol Pharmacol. 1973;51:220–225. doi: 10.1139/y73-031. [DOI] [PubMed] [Google Scholar]
- 8.Chang HY, Locker J, Lu R, Schuster VL. Failure of postnatal ductus arteriosus closure in prostaglandin transporter-deficient mice. Circulation. 2010;121:529–536. doi: 10.1161/CIRCULATIONAHA.109.862946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coggins KG, Latour A, Nguyen MS, Audoly L, Coffman TM, et al. Metabolism of PGE2 by prostaglandin dehydrogenase is essential for remodeling the ductus arteriosus. Nat Med. 2002;8:91–92. doi: 10.1038/nm0202-91. [DOI] [PubMed] [Google Scholar]
- 10.Uppal S, Diggle CP, Carr IM, Fishwick CW, Ahmed M, et al. Mutations in 15-hydroxyprostaglandin dehydrogenase cause primary hypertrophic osteoarthropathy. Nat Genet. 2008;40:789–793. doi: 10.1038/ng.153. [DOI] [PubMed] [Google Scholar]
- 11.Clyman RI. Ontogeny of the ductus arteriosus response to prostaglandins and inhibitors of their synthesis. Semin Perinatol. 1980;4:115–124. [PubMed] [Google Scholar]
- 12.Hristovska AM, Rasmussen LE, Hansen PB, Nielsen SS, Nusing RM, et al. Prostaglandin E2 induces vascular relaxation by E-prostanoid 4 receptor-mediated activation of endothelial nitric oxide synthase. Hypertension. 2007;50:525–530. doi: 10.1161/HYPERTENSIONAHA.107.088948. [DOI] [PubMed] [Google Scholar]
- 13.Carvajal JA, Germain AM, Huidobro-Toro JP, Weiner CP. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J Cell Physiol. 2000;184:409–420. doi: 10.1002/1097-4652(200009)184:3<409::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 14.Narumiya S, FitzGerald GA. Genetic and pharmacological analysis of prostanoid receptor function. J Clin Invest. 2001;108:25–30. doi: 10.1172/JCI13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engelhardt JF, Yankaskas JR, Wilson JM. In vivo retroviral gene transfer into human bronchial epithelia of xenografts. J Clin Invest. 1992;90:2598–2607. doi: 10.1172/JCI116155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen M, Camenisch T, Snouwaert JN, Hicks E, Coffman TM, et al. The prostaglandin receptor EP4 triggers remodelling of the cardiovascular system at birth. Nature. 1997;390:78–81. doi: 10.1038/36342. [DOI] [PubMed] [Google Scholar]
- 17.Tilley SL, Audoly LP, Hicks EH, Kim HS, Flannery PJ, et al. Reproductive failure and reduced blood pressure in mice lacking the EP2 prostaglandin E2 receptor. J Clin Invest. 1999;103:1539–1545. doi: 10.1172/JCI6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farley FW, Soriano P, Steffen LS, Dymecki SM. Widespread recombinase expression using FLPeR (flipper) mice. Genesis. 2000;28:106–110. [PubMed] [Google Scholar]
- 19.Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, et al. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J Exp Med. 2001;193:741–754. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, et al. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci U S A. 2002;99:7142–7147. doi: 10.1073/pnas.102650499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- 22.Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23:5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang J, Cheng L, Li J, Chen M, Zhou D, et al. Myocardin regulates expression of contractile genes in smooth muscle cells and is required for closure of the ductus arteriosus in mice. J Clin Invest. 2008;118:515–525. doi: 10.1172/JCI33304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tilley SL, Hartney JM, Erikson CJ, Jania C, Nguyen M, et al. Receptors and pathways mediating the effects of prostaglandin E2 on airway tone. Am J Physiol Lung Cell Mol Physiol. 2003;284:L599–L606. doi: 10.1152/ajplung.00324.2002. [DOI] [PubMed] [Google Scholar]
- 25.Hou G, Mulholland D, Gronska MA, Bendeck MP. Type VIII collagen stimulates smooth muscle cell migration and matrix metalloproteinase synthesis after arterial injury. Am J Pathol. 2000;156:467–476. doi: 10.1016/S0002-9440(10)64751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koch AE, Volin MV, Woods JM, Kunkel SL, Connors MA, et al. Regulation of angiogenesis by the C-X-C chemokines interleukin-8 and epithelial neutrophil activating peptide 78 in the rheumatoid joint. Arthritis Rheum. 2001;44:31–40. doi: 10.1002/1529-0131(200101)44:1<31::AID-ANR5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 27.Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278:8508–8515. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- 28.Hager HA, Bader DM. Bves: ten years after. Histol Histopathol. 2009;24:777–787. doi: 10.14670/hh-24.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wada AM, Reese DE, Bader DM. Bves: prototype of a new class of cell adhesion molecules expressed during coronary artery development. Development. 2001;128:2085–2093. doi: 10.1242/dev.128.11.2085. [DOI] [PubMed] [Google Scholar]
- 30.Andree B, Fleige A, Arnold HH, Brand T. Mouse Pop1 is required for muscle regeneration in adult skeletal muscle. Mol Cell Biol. 2002;22:1504–1512. doi: 10.1128/mcb.22.5.1504-1512.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boudreau N, Turley E, Rabinovitch M. Fibronectin, hyaluronan, and a hyaluronan binding protein contribute to increased ductus arteriosus smooth muscle cell migration. Dev Biol. 1991;143:235–247. doi: 10.1016/0012-1606(91)90074-d. [DOI] [PubMed] [Google Scholar]
- 32.Mason CA, Chang P, Fallery C, Rabinovitch M. Nitric oxide mediates LC-3-dependent regulation of fibronectin in ductus arteriosus intimal cushion formation. FASEB J. 1999;13:1423–1434. doi: 10.1096/fasebj.13.11.1423. [DOI] [PubMed] [Google Scholar]
- 33.Fujino T, Yuhki K, Yamada T, Hara A, Takahata O, et al. Effects of the prostanoids on the proliferation or hypertrophy of cultured murine aortic smooth muscle cells. Br J Pharmacol. 2002;136:530–539. doi: 10.1038/sj.bjp.0704749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradbury D, Clarke D, Seedhouse C, Corbett L, Stocks J, et al. Vascular endothelial growth factor induction by prostaglandin E2 in human airway smooth muscle cells is mediated by E prostanoid EP2/EP4 receptors and SP-1 transcription factor binding sites. J Biol Chem. 2005;280:29993–30000. doi: 10.1074/jbc.M414530200. [DOI] [PubMed] [Google Scholar]
- 35.Kochupurakkal BS, Harari D, Di-Segni A, Maik-Rachline G, Lyass L, et al. Epigen, the last ligand of ErbB receptors, reveals intricate relationships between affinity and mitogenicity. J Biol Chem. 2005;280:8503–8512. doi: 10.1074/jbc.M413919200. [DOI] [PubMed] [Google Scholar]
- 36.Yamanaka Y, Hayashi K, Komurasaki T, Morimoto S, Ogihara T, et al. EGF family ligand-dependent phenotypic modulation of smooth muscle cells through EGF receptor. Biochem Biophys Res Commun. 2001;281:373–377. doi: 10.1006/bbrc.2001.4385. [DOI] [PubMed] [Google Scholar]
- 37.Goessling W, North TE, Loewer S, Lord AM, Lee S, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136:1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maciel TT, Melo RS, Schor N, Campos AH. Gremlin promotes vascular smooth muscle cell proliferation and migration. J Mol Cell Cardiol. 2008;44:370–379. doi: 10.1016/j.yjmcc.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 39.Moser M, Binder O, Wu Y, Aitsebaomo J, Ren R, et al. BMPER, a novel endothelial cell precursor-derived protein, antagonizes bone morphogenetic protein signaling and endothelial cell differentiation. Mol Cell Biol. 2003;23:5664–5679. doi: 10.1128/MCB.23.16.5664-5679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu L, Vranckx R, Khau Van Kien P, Lalande A, Boisset N, et al. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet. 2006;38:343–349. doi: 10.1038/ng1721. [DOI] [PubMed] [Google Scholar]
- 41.Morano I, Chai GX, Baltas LG, Lamounier-Zepter V, Lutsch G, et al. Smooth-muscle contraction without smooth-muscle myosin. Nat Cell Biol. 2000;2:371–375. doi: 10.1038/35014065. [DOI] [PubMed] [Google Scholar]
- 42.Klapholz-Brown Z, Walmsley GG, Nusse YM, Nusse R, Brown PO. Transcriptional program induced by Wnt protein in human fibroblasts suggests mechanisms for cell cooperativity in defining tissue microenvironments. PLoS One. 2007;2:e945. doi: 10.1371/journal.pone.0000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tuckerman JR, Zhao Y, Hewitson KS, Tian YM, Pugh CW, et al. Determination and comparison of specific activity of the HIF-prolyl hydroxylases. FEBS Lett. 2004;576:145–150. doi: 10.1016/j.febslet.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Tristani-Firouzi M, Reeve HL, Tolarova S, Weir EK, Archer SL. Oxygen-induced constriction of rabbit ductus arteriosus occurs via inhibition of a 4-aminopyridine-, voltage-sensitive potassium channel. J Clin Invest. 1996;98:1959–1965. doi: 10.1172/JCI118999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coceani F, Kelsey L, Seidlitz E. Occurrence of endothelium-derived relaxing factor--nitric oxide in the lamb ductus arteriosus. Can J Physiol Pharmacol. 1994;72:82–88. doi: 10.1139/y94-013. [DOI] [PubMed] [Google Scholar]
- 46.Fox JJ, Ziegler JW, Ivy DD, Halbower AC, Kinsella JP, et al. Role of nitric oxide and cGMP system in regulation of ductus arteriosus tone in ovine fetus. Am J Physiol. 1996;271:H2638–H2645. doi: 10.1152/ajpheart.1996.271.6.H2638. [DOI] [PubMed] [Google Scholar]
- 47.Clyman RI, Waleh N, Black SM, Riemer RK, Mauray F, et al. Regulation of ductus arteriosus patency by nitric oxide in fetal lambs: the role of gestation, oxygen tension, and vasa vasorum. Pediatr Res. 1998;43:633–644. doi: 10.1203/00006450-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Fujino H, Xu W, Regan JW. Prostaglandin E2 induced functional expression of early growth response factor-1 by EP4, but not EP2, prostanoid receptors via the phosphatidylinositol 3-kinase and extracellular signal-regulated kinases. J Biol Chem. 2003;278:12151–12156. doi: 10.1074/jbc.M212665200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.