Abstract
PURPOSE/OBJECTIVES
To examine how attentional fatigue changed from the time of simulation to four months after the completion of radiation therapy (RT) and to investigate whether specific variables predicted initial levels and trajectories of attentional fatigue.
DESIGN
Descriptive, longitudinal study.
SETTINGS
Two RT departments.
SAMPLE
73 women with breast cancer who received primary or adjuvant RT.
METHODS
Participants completed questionnaires prior to, during, and after RT. Descriptive statistics and hierarchical linear modeling were used for data analysis.
MAIN RESEARCH VARIABLES
Attentional fatigue; demographic, clinical, and symptom characteristics.
FINDINGS
Large amounts of inter-individual variability were found in the trajectories of attentional fatigue. At baseline, higher levels of attentional fatigue were associated with younger age, not working, a higher number of comorbidities, and higher levels of trait anxiety. The trajectory of attentional fatigue improved over time for women with a higher body mass index at baseline.
CONCLUSIONS
This study is the first to identify predictors of inter-individual variability in attentional fatigue in women with breast cancer undergoing RT. These predictors need to be considered in the design of future correlational and interventional studies.
IMPLICATIONS FOR NURSING
Nurses could use knowledge of these predictors to identify patients at risk for higher levels of attentional fatigue. In addition, nurses could use this information to educate their patients about how attentional fatigue may change during and following a course of RT for breast cancer.
Attentional fatigue is a decreased capacity to direct attention (Cimprich, 1992a). This capacity is defined by three concepts: selectivity, which is the ability to highlight one stimulus while ignoring others; sustained focus, which is the maintenance of selectivity over time; and limited capacity, which is a ceiling on the number of stimuli that can be processed successfully at any one time (Cimprich, 1992a; Kaplan & Kaplan, 1982; Posner & Boies, 1971). Attentional fatigue is not physical fatigue, so a person can experience the former with or without the latter (Cimprich, 1992b). In addition, the cognitive changes associated with chemotherapy that are popularly referred to as “chemo brain” include, but are not limited to, attentional fatigue (Hess & Insel, 2007).
Anatomically, attention is thought to reside in the anterior and posterior attention systems of the frontal and parietal cortices (Cimprich, 1995; Posner & Dehaene, 1994; Posner & Petersen, 1990). This hypothesis is supported by findings from a recent imaging study that evaluated for changes in the prefrontal and anterior cingulate cortices of women with breast cancer prior to chemotherapy (Cimprich et al., 2009) and found significantly larger differences in the activation of the right inferior frontal gyrus compared to healthy controls. In addition, in these women with breast cancer, more areas of the brain were activated during the completion of tasks that required them to direct their attention.
There are two types of attention, involuntary and voluntary (James, 1983; Kaplan & Kaplan, 1982). Some stimuli that originate in our thoughts or in the world around us (i.e., our internal and external environments) engage involuntary attention without effort (Cimprich, 1992a; James, 1983; Kaplan & Kaplan, 1982). These stimuli include nature, things that affect survival, and things that fascinate us (Cimprich, 1992a; James, 1983; Kaplan & Kaplan, 1982). Other stimuli must consciously be selected for processing by voluntary attention, which requires effort that reduces our capacity to direct attention further (Cimprich, 1992a; James, 1983; Kaplan & Kaplan, 1982). Voluntary attention is required to act purposefully (Lezak, 1982), to monitor one’s self, and to inhibit emotional reactions (Cimprich, 1992a). As involuntary attention is drawn to a greater diversity and intensity of sensory information, experienced as distraction, one must expend greater effort to direct voluntary attention (Cimprich, 1992a; Kaplan & Kaplan, 1982).
After diagnosis with breast cancer, involuntary attention is drawn to the threatening information received and to the unfamiliar physical environment in which treatment occurs, both of which pertain to survival (Cimprich, 1992b). The concept of limited capacity suggests that the direction of voluntary attention during the time of diagnosis and treatment would require increased effort, which results in attentional fatigue and its sequelae (e.g., irritability when presented with further demands on one’s attention and a decreased ability to focus on selected stimuli) (Cimprich, 1992b; Kaplan & Kaplan, 1982).
Three cross-sectional studies evaluated the correlates of self-reported attentional fatigue, as measured using the Attentional Function Index (AFI), before treatment in women diagnosed with breast cancer (Cimprich, 1999; Cimprich, So, Ronis, & Trask, 2005; Lehto & Cimprich, 1999). Across these studies with a total of 303 women, significant correlates of higher levels of attentional fatigue included younger age, pre-menopausal status, higher symptom distress scores and greater number of symptoms, greater mood disturbance, and high versus low-to-moderate anxiety. Two papers from the same study described self-reported attentional fatigue in women following breast cancer surgery. In these papers, higher levels of attentional fatigue were reported by women with greater mood disturbance (Cimprich, 1992b) and in those assessed closer to the time of surgery (Cimprich, 1993). In a more recent cross-sectional study of breast cancer survivors (Von Ah, Russell, Storniolo, & Carpenter, 2009), higher levels of attentional fatigue correlated with younger age, higher levels of depression and physical fatigue, and lower levels of psychological well-being and physical functioning. In a longitudinal study that evaluated self-reported attentional fatigue in women undergoing chemotherapy for breast cancer (Jansen, Dodd, Miaskowski, Dowling, & Kramer, 2008), higher levels of attentional fatigue were significantly correlated with the administration of chemotherapy and higher levels of depression.
A number of studies have employed measures other than the AFI to assess self-reported attentional fatigue alone or in combination with other cognitive changes in patients with cancer. In a cross-sectional study of breast cancer survivors (Mehnert et al., 2007), higher levels of attentional fatigue, measured using a German questionnaire for self-perceived deficits in attention (Zimmermann, Merser, Poser, & Sedelmeier, 1991), were associated with higher levels of physical fatigue and lower health-related quality of life. Across two studies (Schagen et al., 1999; van Dam et al., 1998) that used a Dutch questionnaire that assessed for cognitive problems in daily life (Huyser, 1993), higher levels of attentional fatigue were associated with higher levels of anxiety and depression and a lower quality of life. Across three studies (Castellon et al., 2004; Jenkins et al., 2006; Jenkins, Shilling, Fallowfield, Howell, & Hutton, 2004) that used the Cognitive Failures Questionnaire (Broadbent, Cooper, FitzGerald, & Parkes, 1982), higher levels of attentional fatigue were associated with higher levels of depression, trait anxiety, psychological distress, and physical fatigue, as well as a lower quality of life. Finally, in an imaging study (Ferguson, McDonald, Saykin, & Ahles, 2007) that used the Multiple Ability Self-Report Questionnaire (Seidenberg, Haltiner, Taylor, Hermann, & Wyler, 1994), a higher level of attentional fatigue was associated with the administration of chemotherapy. Taken together, the findings from these studies suggest that attentional fatigue is associated with decreased physical functioning, higher levels of mood disturbance, and poorer quality of life. However, it is not known how well the AFI, which was used in the present study, correlates with these other subjective measures of attentional fatigue.
No studies were found that examined the trajectories of self-reported attentional fatigue in women with breast cancer before, during, and after radiation therapy (RT). An increased understanding of the predictors and trajectories of attentional fatigue in women with breast cancer may help clinicians identify patients at risk for more severe attentional fatigue and may guide the development of interventions tailored to their individual experiences. Therefore, the purposes of this study, in a sample of women who underwent RT for breast cancer, were (1) to examine how self-ratings of attentional fatigue changed from the time of simulation to four months after the completion of RT and (2) to investigate whether specific participant, disease, and symptom characteristics predicted initial levels of attentional fatigue and/or characteristics of the trajectories of attentional fatigue.
Methods
Participants and Settings
This descriptive, longitudinal study recruited 73 women with breast cancer who met the following inclusion criteria: were ≥ 18 years of age; had the ability to read, write, and understand English; had a Karnofsky Performance Status score of ≥ 60; and were scheduled to receive primary or adjuvant RT. Participants were excluded if they had metastatic disease, had more than one cancer diagnosis, or had a diagnosed sleep disorder. They were recruited from RT departments located in a comprehensive cancer center and a community-based oncology program. This study was approved by the Human Subjects Committees of the University of California, San Francisco and the second study site.
One hundred thirty-four participants were approached and 73 consented to participate (55% response rate). The major reasons for refusal were being too overwhelmed with their cancer experience or too busy. No differences were found in any demographic or clinical characteristics between participants who did and did not choose to participate.
Instruments
The study instruments included a demographic questionnaire, the Karnofsky Performance Status (KPS) scale (Karnofsky, 1977), the AFI, a descriptive numeric rating scale (NRS) for worst pain intensity from the Brief Pain Inventory, the Center for Epidemiologic Studies-Depression (CES-D) scale, the General Sleep Disturbance Scale (GSDS), the Lee Fatigue Scale (LFS), and the Spielberger State-Trait Anxiety Inventories (STAI-S and STAI-T). The demographic questionnaire provided information on age, living arrangements, marital status, years of education, employment status, race, and whether children were living at home. Additional clinical characteristics were collected, including number of comorbidities, stage of disease, use of hormone replacement therapy prior to diagnosis, treatment with lymph node dissection and/or chemotherapy prior to RT, and total dose of RT. Measurements of weight and height were used to determine body mass index (BMI), which was calculated by dividing weight in kilograms by height in meters squared.
Self-reported attentional fatigue was measured using the AFI (Cimprich, 1992b). Originally developed for use with a visual analogue scale anchored by phrases describing extremes, such as “not at all” and “extremely well,” the 16-item AFI was modified for this study to employ a 0-to-10 NRS. A mean AFI score was calculated, with higher scores indicating greater capacity to direct attention and, therefore, lower levels of attentional fatigue (Cimprich, 1992b). Based on a previously conducted analysis of the frequency distributions of AFI scores, attentional fatigue can be grouped into categories of functional status (i.e., participants who score < 5.0 functioning poorly and experiencing high levels of attentional fatigue, participants who score 5.0 to 7.5 functioning moderately well and experiencing moderate levels of attentional fatigue, and participants who score > 7.5 functioning well and experiencing low levels of attentional fatigue) (Cimprich et al., 2005). The AFI has established reliability and validity (Cimprich, 1992b; Jansen et al., 2008; Jansen, 2006; Tennessen & Cimprich, 1995). In the current study, Cronbach’s alpha for the AFI was 0.95.
Worst pain was evaluated using a descriptive NRS from the Brief Pain Inventory that ranged from 0 (no pain) to 10 (excruciating pain) (Cleeland & Ryan, 1994; Daut, Cleeland, & Flanery, 1983). A descriptive NRS is a valid and reliable measure of pain intensity (Jensen, 2003). Because 51% of participants in this study did not have pain, the symptom was recoded as present or absent for the longitudinal analysis.
The CES-D consists of 20 items selected to represent the major symptoms in the clinical syndrome of depression (Radloff, 1977). Scores can range from 0 to 60, with a score of ≥ 16 indicating the need for an individual to seek a clinical evaluation for depression (Radloff, 1977). The CES-D has well-established reliability and concurrent and construct validity (Carpenter et al., 1998; Radloff, 1977; Sheehan, Fifield, Reisine, & Tennen, 1995). In the current study, Cronbach’s alpha for the CES-D was 0.83.
The GSDS consists of 21 items that evaluate various aspects of sleep disturbance (Lee & DeJoseph, 1992). Each item is rated on a NRS that ranges from 0 (never) to 7 (every day). The 21 items are summed to yield a total score that can range from 0 (no disturbance) to 147 (extreme sleep disturbance). The GSDS has well-established validity and reliability (Lee, 1992; Lee & DeJoseph, 1992; Lee, Portillo, & Miramontes, 2001). In the current study, Cronbach’s alpha for the GSDS total score was 0.81.
The severity of physical fatigue was measured using the 13-item LFS (Lee, Hicks, & Nino-Murcia, 1991). Each item is rated using a 0-to-10 NRS, and a total score is calculated as the mean of the 13 items. Higher scores indicated higher levels of fatigue severity. Respondents were asked to rate each item based on how they felt “right now,” prior to going to bed (i.e., evening fatigue), and within 30 minutes of awakening (i.e., morning fatigue) for two consecutive nights and days. The LFS has been used with healthy individuals, as well as with patients with cancer and HIV (Lee & DeJoseph, 1992; Lee, Portillo, & Miramontes, 1999; Miaskowski & Lee, 1999). The LFS has well-established validity and reliability (Lee et al., 1991; Lee, Lentz, Taylor, Mitchell, & Woods, 1994). In the current study, Cronbach’s alphas for the LFS for evening and morning fatigue were 0.95 and 0.96, respectively.
The STAI-S and STAI-T consist of 20 items each that are rated from 1 to 4 (Bieling, Antony, & Swinson, 1998). The score for each scale is summed and can range from 20 to 80, with a higher score indicating greater anxiety (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983). The STAI-S measures an individual’s transitory emotional state during a stressful situation, while the STAI-T measures an individual’s predisposition to anxiety and estimates how that person generally feels (Kennedy, Schwab, Morris, & Beldia, 2001). The STAI-S and STAI-T have well-established criterion and construct validity and internal consistency reliability coefficients (Bieling et al., 1998; Kennedy et al., 2001; Spielberger et al., 1983). In the current study, Cronbach’s alphas for the STAI-S and STAI-T were 0.91 and 0.86, respectively.
Study Procedures
At the time of the simulation visit (i.e., approximately one week prior to the start of RT), a research nurse approached patients to discuss participation in the study. After obtaining written informed consent, participants were asked to complete baseline study questionnaires. Participants were taught to complete the AFI as part of the collection of study instruments administered at baseline, every other week during RT (four assessments) and for two months after RT, and once a month for an additional two months. The majority of participants completed 11 assessments over six months.
Data Analysis
Descriptive statistics and frequency distributions were generated on the sample characteristics and baseline symptom severity scores using SPSS™ Version 15.0. For each of the 11 assessments, a mean AFI score was calculated for use in the subsequent statistical analyses.
Hierarchical linear modeling (HLM), based on full maximum likelihood estimation, was done using software developed by Raudenbush and colleagues (Raudenbush & Bryk, 2002; Raudenbush, Bryk, Cheong, & Congdon, 2004). Compared with other methods for analyzing change, HLM has two major advantages. First, HLM can accommodate unbalanced designs, which allows for the analysis of data when the number and spacing of assessments vary across respondents (Raudenbush, 2001; Raudenbush & Bryk, 2002). Although every participant was to be assessed on a pre-specified schedule, the actual number of assessments was not the same for all participants due to varying periods of RT and scheduling conflicts. Second, HLM has the ability to model individual change, which helps to identify more complex patterns of change that are often overlooked by other methods (Raudenbush, 2001; Raudenbush & Bryk, 2002).
With HLM, repeated measures of the outcome variable (i.e., attentional fatigue) are conceptualized as being nested within individuals, and the analysis of change in attentional fatigue scores is at two levels: within persons (level one) and between persons (level two). At level one, the outcome is conceptualized as varying within individuals and is a function of person-specific change parameters plus error. At level two, these person-specific change parameters are multivariate outcomes that vary across individuals. Level-two outcomes can be modeled as a function of demographic or clinical characteristics that vary between individuals, plus an error associated with the individual. Combining level one with level two results in a mixed model with fixed and random effects (Li, 2005a, 2005b; Raudenbush & Bryk, 2002).
HLM analysis proceeded in two stages. First, intra-individual variability in attentional fatigue over time was examined. In this study, time in weeks refers to the length of time from the simulation visit to four months after the completion of RT. Three level-one models were compared to determine if the participants’ attentional fatigue levels did not change over time (i.e., no time effect), changed at a constant rate (i.e., linear time effect), or changed at a rate that accelerated or decelerated over time (i.e., quadratic effect). At this point, the level-two model was constrained to be unconditional (i.e., no predictors), and significance tests were used to determine the best model. These analyses answered the first research question and identified the change parameters that best described individual changes in attentional fatigue over time.
The second stage of the HLM analysis, which answered the second research question, examined inter-individual differences in the trajectories of attentional fatigue by modeling individual change parameters (i.e., intercept and linear slope) as a function of proposed predictors at level two. Personal characteristics, disease and treatment characteristics, and symptom severity scores were evaluated as potential predictors of the intercept and linear slope based on a review of the literature of attentional fatigue in women with breast cancer (see Table 1). In addition, other potential predictors (i.e., BMI, presence of pain, baseline level of sleep disturbance) were identified from an analysis of the trajectories of morning and evening fatigue (i.e., physical fatigue) in the same sample (Dhruva et al., 2009).
Table 1.
Potential Predictors of the Intercept (I) and Linear Coefficient (LC) for Attentional Fatigue Using Baseline Characteristics
| I | LC | |
|---|---|---|
| Demographic Characteristics | ||
| Age | ν | |
| Children at home | ν | |
| Employment status | ν | |
| Racial group (white/other) | ||
| Lives alone | ||
| Marital status | ||
| Years of education | ||
|
| ||
| Clinical Characteristics | ||
| Body mass index | ν | |
| Chemotherapy prior to radiation therapy | ||
| Hormone replacement therapy prior to diagnosis | ||
| Karnofsky Performance Status score | ||
| Lymph node dissection prior to radiation therapy | ||
| Number of comorbidities | ν | |
| Stage of disease | ||
| Total dose of radiation | ||
|
| ||
| Symptom Characteristics | ||
| Center for Epidemiologic Studies-Depression scale score | ν | ν |
| General Sleep Disturbance Scale score | ν | ν |
| Lee Fatigue Scale—evening fatigue | ν | |
| Lee Fatigue Scale—morning fatigue | ν | |
| Presence of pain | ||
| Spielberger State Anxiety score | ν | ν |
| Spielberger Trait Anxiety score | ν | ν |
Note. ν = From the exploratory analysis, potential predictors that had a t-value of ≥ 2.0.
To improve estimation efficiency and construct a model that was parsimonious, an exploratory level-two analysis was completed in which each potential predictor was assessed to see if it would result in a better model if it alone were added as a level-two predictor. Predictors with a t-value of < 2.0, which indicated a lack of significant effect, were dropped from subsequent model testing. All potentially significant predictors from the exploratory analyses were entered into the model to predict each individual change parameter, but only predictors that maintained a statistically significant contribution in conjunction with other variables (p-value of < 0.05) were retained in the final model.
Results
Participant Characteristics and Symptom Severity Scores
The demographic and clinical characteristics of the 73 participants are presented in Table 2. On average, participants in this sample were 55 years of age (range 30–85) and well educated, with a KPS score of 87.7 and an average of five comorbidities. Most of the participants self-identified as white (70%). Forty-five percent were employed and 22% were caring for children at home.
Table 2.
Demographic, Clinical, and Symptom Characteristics of the Participants (n=73) at Baseline
| Mean (SD) | |
|---|---|
| Demographic Characteristics | |
| Age (years) | 55.1 (11.0) |
| Education (years) | 16.2 (2.7) |
| Children at home | 22% |
| Employed | 45% |
| Racial group | |
| White | 70% |
| Other | 30% |
| Lives alone | 41% |
| Marital status | |
| Married/partnered | 29% |
| Divorced/separated | 30% |
| Other | 41% |
|
| |
| Clinical Characteristics | |
| Body mass index | 27.4 (7.3) |
| Underweight | 4% |
| Normal weight | 44% |
| Overweight | 23% |
| Obese | 29% |
| Karnofsky Performance Status score | 87.7 (12.4) |
| Number of comorbidities | 5.3 (2.6) |
| Total dose of radiation therapy (cGys) | 5829.0 (438.3) |
| Chemotherapy prior to radiation therapy | 55% |
| Hormone replacement therapy prior to diagnosis | 44% |
| Lymph node dissection prior to radiation therapy | 49% |
| Stage of disease | |
| Localized | 56% |
| Locally advanced | 44% |
|
| |
| Symptom Characteristics | |
| Attentional Function Index score | 6.6 (1.9) |
| Center for Epidemiologic Studies-Depression scale score | 12.0 (9.2) |
| General Sleep Disturbance Scale score | 44.7 (21.7) |
| Lee Fatigue Scale—evening fatigue | 4.9 (1.8) |
| Lee Fatigue Scale—morning fatigue | 2.9 (2.1) |
| Spielberger State Anxiety score | 33.7 (12.9) |
| Spielberger Trait Anxiety score | 36.2 (11.3) |
| Presence of pain | 49% |
Individual and Mean Change in Attentional Fatigue
The first stage of HLM analysis examined how levels of attentional fatigue changed from the time of the simulation visit to four months after the completion of RT. Two models were estimated in which the function of time was linear or quadratic. In the linear model, the test of the linear slope was significant (p=0.003). However, when a quadratic component was added to the model, neither the linear component (p=0.731) nor the quadratic component (p=0.121) was significant. Consequently, the linear model was deemed the better fit.
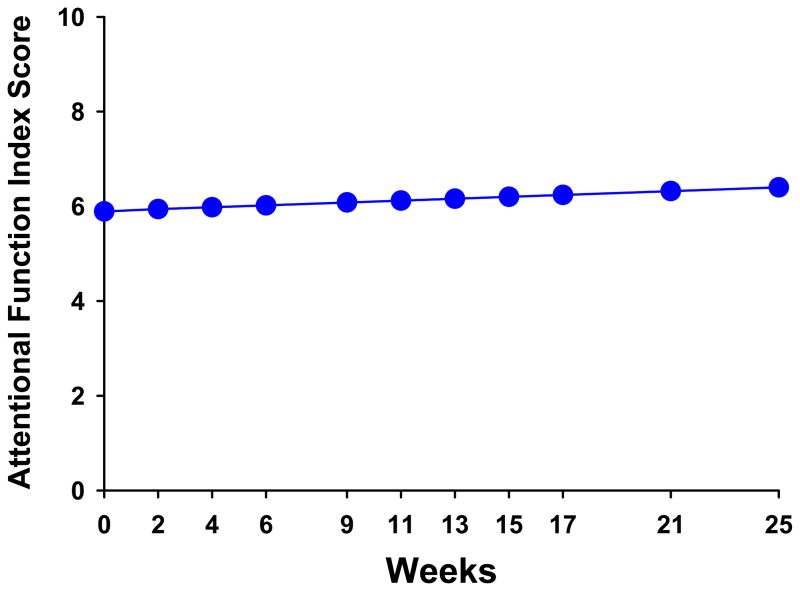
The estimates of the linear change model are presented in Table 3 (unconditional model). Because the model had no covariates (i.e., unconditional), the intercept represents the estimated level of attentional fatigue (i.e., 6.32 on a 0-to-10 scale) at the time of the simulation visit. The estimated linear rate of change in AFI scores, for each additional week, was 0.022 (p=0.003). Figure 1 displays the predicted trajectory for attentional fatigue in the unconditional model from the time of the simulation visit to four months after the completion of RT. During this time, attentional fatigue was projected to improve over the course of RT (i.e., weeks one to nine) and to continue to improve after the completion of RT. It should be noted that the mean scores for the various groups depicted in all figures are estimated or predicted means based on the HLM analysis.
Table 3.
Hierarchical Linear Model of Attentional Fatigue
| Coefficient (SE)
|
||
|---|---|---|
| Unconditional Model
|
Final Model
|
|
| Fixed effects | ||
| Intercept | 6.324 (0.213)** | 5.895 (0.193)** |
| Timea (linear rate of change) | 0.022 (0.007)* | 0.020 (0.006)* |
|
| ||
| Time invariant covariates | ||
| Intercept: Age | 0.036 (0.014)+ | |
| Work | 0.961 (0.290)* | |
| Number of comorbidities | −0.165 (0.058)* | |
| Spielberger Trait Anxiety score | −0.088 (0.013)** | |
| Linear: Body mass index × time | 0.004 (0.001)** | |
|
| ||
| Variance components | ||
| In intercept | 3.028** | 1.185** |
| In linear rate | 0.002** | 0.001** |
|
| ||
| Goodness-of-fit devianceb | 2229.568 (6) | 2157.193 (11) |
| Model comparison (χ2 [df]) | 72.375 (5)** | |
p < 0.0001.
p < 0.01.
p = 0.014.
Time was coded 0 at the time of the simulation visit.
Parameters estimated.
Figure 1.
Trajectory of predicted attentional fatigue, as measured by the Attentional Function Index (AFI), over the 25 weeks of the study. Lower AFI scores indicate higher levels of attentional fatigue.
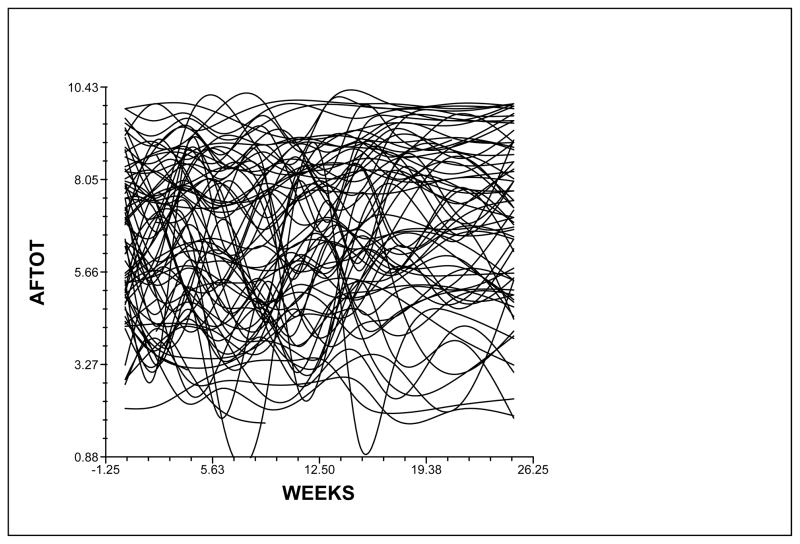
Although the results indicated a sample-wide improvement in attentional fatigue, this does not imply that all participants exhibited the same trajectory. The variance in individual change parameters estimated by the model (i.e., variance components, Table 3) suggested that substantial inter-individual differences existed in the trajectories of attentional fatigue, which are illustrated in Figure 2. These results suggested that further examination of inter-individual differences in the individual change parameters was warranted.
Figure 2.
Spaghetti plot of the 73 participants’ individual trajectories of attentional fatigue (AFTOT) illustrating the large amount of inter-individual variability among study participants. Lower scores indicate higher levels of attentional fatigue.
Inter-Individual Differences in the Trajectories of Attentional Fatigue
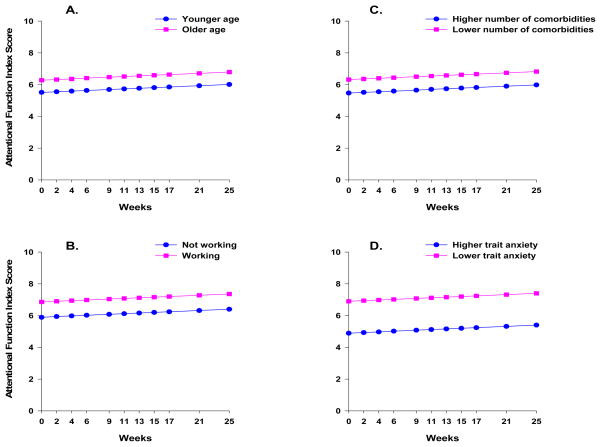
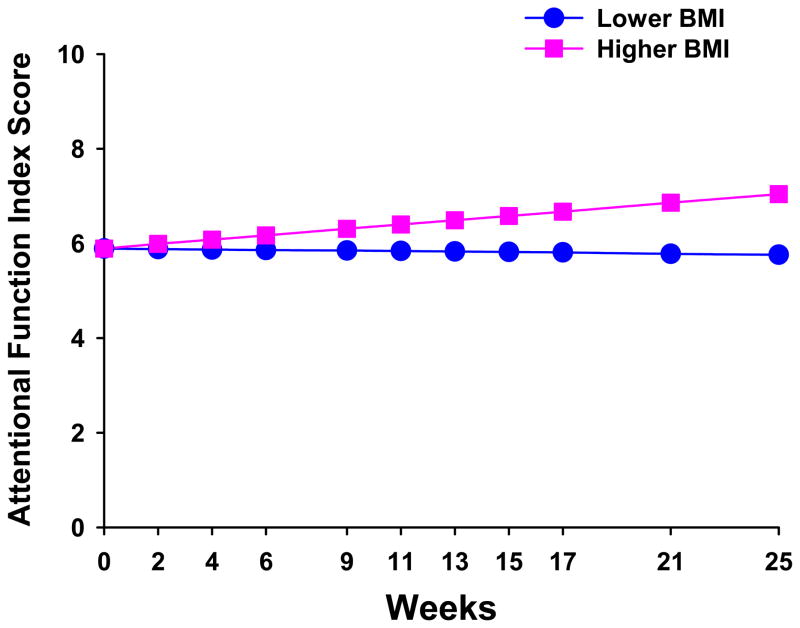
The second stage of HLM analysis tested the hypothesis that the pattern of change over time in attentional fatigue varied based on specific person, disease, treatment, and/or symptom variables that were found to influence the level of attentional fatigue in other studies (see Table 1). As shown in the final model in Table 3, the four variables that predicted inter-individual differences in the intercept for attentional fatigue (i.e., inter-individual differences in baseline levels of attentional fatigue) were age, work, number of comorbidities, and baseline level of trait anxiety (i.e., baseline STAI-T score). The single variable that predicted inter-individual differences in the slope parameter for attentional fatigue was BMI.
To illustrate the effects of the five predictors on participants’ initial levels and trajectories of attentional fatigue, Figures 3 and 4 display the adjusted change curves of attentional fatigue that were estimated based on differences in age (i.e., younger or older calculated based on one standard deviation (SD) below and above the mean age of the participants), employment status (i.e., working or not working), number of comorbidities (i.e., lower or higher number of comorbidities calculated based on one SD below and above the mean number of comorbidities), baseline level of trait anxiety (i.e., lower or higher STAI-T calculated based on one SD below and above the mean baseline STAI-T score), and BMI (i.e., lower or higher BMI calculated based on one SD below and above the mean BMI).
Figure 3.
Trajectories of attentional fatigue, as measured by the Attentional Function Index (AFI), by (A) age (i.e., younger/older), (B) employment status (i.e., working or not working), (C) number of comorbidities (i.e., lower number/higher number), and (D) baseline level of trait anxiety (i.e., lower STAI-T/higher STAI-T). Lower AFI scores indicate higher levels of attentional fatigue.
Figure 4.
Trajectories of attentional fatigue, as measured by the Attentional Function Index (AFI), by body mass index (i.e., lower BMI/higher BMI). Lower AFI scores indicate higher levels of attentional fatigue.
Discussion
To our knowledge, this longitudinal study is the first to evaluate the trajectories of self-reported attentional fatigue in women with breast cancer undergoing RT. In this study, the mean AFI score before treatment was 6.6 (range 2.1 to 9.9), which was similar to baseline means in previous studies (Cimprich, 1992b, 1999; Cimprich et al., 2005; Jansen et al., 2008; Lehto & Cimprich, 1999). Of the 63% of women who reported moderate to high levels of attentional fatigue at baseline, 41% reported moderate levels of attentional fatigue (i.e., an AFI score of 5.0 to 7.5) and 22% reported high levels of attentional fatigue (i.e., an AFI score of < 5.0). The model predicted improvement in attentional fatigue scores from the beginning (5.9) to the end (6.4) of the study. However, at the end of the study, the majority of the women were still experiencing moderate levels of attentional fatigue.
In this sample, younger age was associated with higher levels of attentional fatigue at the time of the simulation visit. This finding is supported by a hypothesis put forward by Cimprich et al. (2005) that younger women may be more distressed by changes in attentional function than older women, who possibly have become accustomed to a diminished capacity to direct attention. Younger women may then rate their attentional fatigue at higher levels than older women. A similar result was noted in a study of breast cancer survivors (Von Ah et al., 2009).
Not working predicted higher levels of attentional fatigue at baseline. While Cimprich (1999) did not find a correlation between employment status and attentional fatigue in women with recent diagnoses of breast cancer, our findings are consistent with a previous report in patients with depression (Williams et al., 2000). In that report, the authors hypothesized that the mechanisms involved in directing attention may be conditioned in a work environment to function more efficiently. Based on this hypothesis, a person who is not working could lack this routine conditioning, which may contribute to the perception of higher levels of attentional fatigue when that person is confronted with a demanding life situation, like RT for breast cancer.
The finding that higher levels of trait anxiety were associated with higher levels of attentional fatigue prior to treatment is consistent with previous reports (Cimprich, 1999; Cimprich et al., 2005; Lehto & Cimprich, 1999). Lehto and Cimprich (Lehto & Cimprich, 1999) proposed that unrelenting anxiety may worsen attentional fatigue by reducing one’s ability to maintain sustained focus. The consistent finding of an association between anxiety and attentional fatigue across multiple studies suggests that clinicians should routinely assess patients undergoing breast cancer treatment for anxiety and attentional fatigue and provide appropriate interventions.
Although not a predictor of inter-individual variability in attentional fatigue in this study, depression was found to correlate with self-reported attentional fatigue in two previous studies (Jansen et al., 2008; Von Ah et al., 2009). In addition, previous studies have found correlations between mood states, which include depression, and attentional fatigue (Cimprich, 1992b, 1999; Cimprich et al., 2005). In the present study, 62% of the participants scored above the cut point of 31.8 (Spielberger et al., 1983) for significant trait anxiety. In contrast, only 33% scored at or above the cut point of 16.0 for significant depressive symptoms (Radloff, 1977). Perhaps in the setting of RT, anxiety contributes more to the development of attentional fatigue than does depression.
Finally, while a previous study of women newly diagnosed with breast cancer found no association between comorbidities and attentional fatigue (Cimprich et al., 2005), in this study a higher number of comorbidities was associated with higher levels of attentional fatigue at baseline. These inconsistent findings may be related to the methods used to evaluate comorbidities. In the study by Cimprich et al. (2005), comorbidities were coded as present or absent, so the total number of comorbidities experienced by the women is not known. While it is interesting that the presence or absence of pain, as separately assessed, did not predict inter-individual differences in attentional fatigue in the current study, the three most frequently reported comorbidities were allergies (59%), back problems (55%), and headaches (44%). It is possible that engagement of the attentional processes needed to manage multiple comorbidities, in light of the concept of limited capacity, fatigues the neurological mechanisms involved in directing attention. In addition, it is possible that participants took allergy medications and analgesics that contributed to attentional fatigue (Banerji, Long, & Camargo, 2007; Palos, 2008). Additional research is warranted to evaluate these relationships in more detail.
Relative to the mean BMI for this sample (27.4 ± 7.3), estimates that used BMI scores of one SD above the mean suggest that a higher BMI at baseline predicted improvement in AFI scores, or lower levels of attentional fatigue, over the six months of the study. The mean baseline BMI for the women in this study is categorized as overweight by the National Heart, Lung, and Blood Institute (NHLBI) (NHLBI, 2009). The estimate for higher BMI at baseline (i.e., one SD above the mean) is categorized as obese, while the estimate for lower BMI (i.e., one SD below the mean) is categorized as normal weight. Additional research is warranted to determine the physiological mechanisms that might explain this finding.
A surprising finding from this study is that in neither the exploratory analyses nor in the final analysis did any of the disease or treatment characteristics predict participants’ trajectories of attentional fatigue. While Von Ah et al. (2009) found a similar lack of correlation in breast cancer survivors, increased levels of attentional fatigue were found in patients who completed four cycles of chemotherapy (Jansen et al., 2008) and in patients in the immediate post-surgical period (Cimprich, 1993). The reasons for these differences are not readily apparent and warrant investigation in future studies.
Results of this study are limited in their generalizability by the characteristics of the sample, especially that most of the women were white, middle-aged, and highly educated. Given that many of the women who declined to participate in this study stated that their reason was being too overwhelmed with the experience of cancer, it is possible that the current study underestimates baseline levels of attentional fatigue in participants with breast cancer prior to RT. This study did not collect data on menopausal status, which has been shown to influence self-reported attentional fatigue (Cimprich et al., 2005). Although previous studies collected data on attentional fatigue using both objective measures and the AFI (Cimprich, 1992b, 1993, 1999; Cimprich et al., 2005; Jansen et al., 2008; Lehto & Cimprich, 1999), the current study used only the AFI. While the sample size for the current study was sufficient for the number of predictors tested, a larger sample would have the potential to identify more predictors and stronger relationships among the variables. The collection of longitudinal data, the avoidance of practice effects by employing a subjective measure of attentional fatigue, and the use of HLM strengthen the findings from this study.
Implications for Future Research
Since this study is the first to identify predictors associated with the trajectories of attentional fatigue in women with breast cancer undergoing RT, replication of these findings is warranted in a larger sample as well as in patients with other cancer diagnoses. In addition, future studies need to identify phenotypic and genotypic characteristics that are associated with higher levels of attentional fatigue. In future studies, a battery of objective measures of attention should be used to supplement the self-report measure. As noted above, future studies need to evaluate the relationship between BMI and attentional fatigue and the relationships between number and types of comorbidities and this symptom. Finally, findings from the present study could be used to inform the adaptation of the current natural restorative environment intervention to improve attentional fatigue in women with breast cancer undergoing surgery (Cimprich & Ronis, 2003) so that it could be tested in women undergoing RT.
Clinical Implications
The capacity to direct attention is essential to the maintenance of purposeful activity (Lezak, 1982). This capacity is especially important for breast cancer patients at a time when attentional demands are high (i.e., at the time of diagnosis and during treatment) (Cimprich, 1992b). Because most participants experienced moderate to severe levels of attentional fatigue at baseline and over the course of RT, these women likely experience a decreased capacity to direct attention to the large amount of information offered by oncology clinicians. Nurses need to evaluate the capacity of their patients to direct attention. In addition, they need to simplify and reinforce the most important pieces of information that patients need to know to be able to effectively manage the physical and psychological effects of their cancer and its treatment. Clinicians need to create a healthcare environment that minimizes distractions, particularly when information is being provided to patients. Oncology nurses could use knowledge of the predictors uncovered in this study to identify patients at risk for higher levels of attentional fatigue. Finally, nurses could use this information to educate their patients about how attentional fatigue may change during and following a course of RT for breast cancer.
Key Points.
Attentional fatigue is defined as a decreased capacity to direct attention. It has been reported to occur in women prior to and following breast cancer surgery.
Approximately 63% of women with breast cancer reported moderate (41%) to high (22%) levels of attentional fatigue prior to the initiation of RT.
The factors that were associated with higher levels of attentional fatigue prior to the initiation of RT included younger age, not working, a higher number of comorbidities, and higher trait anxiety scores.
Oncology nurses need to screen women prior to the initiation of RT for these risk factors and provide education about this symptom.
Acknowledgments
This research was supported by a grant from the National Institute of Nursing Research (NR04835). Additional support for the corresponding author’s program of research was provided through unrestricted grants from Endo Pharmaceuticals; the PriCara division of Ortho-McNeil-Janssen Pharmaceuticals, Inc.; and Purdue Pharma LP.
The assistance of the research nurses on the project—Mary Cullen, Carol Maroten, and LuDene Wong-Teranishi—and the support of the physicians and nurses at the study sites were greatly appreciated.
References
- Banerji A, Long AA, Camargo CA., Jr Diphenhydramine versus nonsedating antihistamines for acute allergic reactions: A literature review. Allergy and Asthma Proceedings. 2007;28(4):418–426. doi: 10.2500/aap.2007.28.3015. [DOI] [PubMed] [Google Scholar]
- Bieling PJ, Antony MM, Swinson RP. The State-Trait Anxiety Inventory, Trait version: Structure and content re-examined. Behavioural Research and Therapy. 1998;36(7–8):777–788. doi: 10.1016/s0005-7967(98)00023-0. [DOI] [PubMed] [Google Scholar]
- Broadbent DE, Cooper PF, Fitzgerald P, Parkes KR. The Cognitive Failures Questionnaire (CFQ) and its correlates. British Journal of Clinical Psychology. 1982;21(1):1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- Carpenter JS, Andrykowski MA, Wilson J, Hall LA, Rayens MK, Sachs B, et al. Psychometrics for two short forms of the Center for Epidemiologic Studies-Depression scale. Issues in Mental Health Nursing. 1998;19(5):481–494. doi: 10.1080/016128498248917. [DOI] [PubMed] [Google Scholar]
- Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. Journal of Clinical and Experimental Neuropsychology. 2004;26(7):955–969. doi: 10.1080/13803390490510905. [DOI] [PubMed] [Google Scholar]
- Cimprich B. A theoretical perspective on attention and patient education. Advances in Nursing Science. 1992a;14(3):39–51. doi: 10.1097/00012272-199203000-00007. [DOI] [PubMed] [Google Scholar]
- Cimprich B. Attentional fatigue following breast cancer surgery. Research in Nursing and Health. 1992b;15(3):199–207. doi: 10.1002/nur.4770150306. [DOI] [PubMed] [Google Scholar]
- Cimprich B. Development of an intervention to restore attention in cancer patients. Cancer Nursing. 1993;16(2):83–92. [PubMed] [Google Scholar]
- Cimprich B. Symptom management: Loss of concentration. Seminars in Oncology Nursing. 1995;11(4):279–288. doi: 10.1016/s0749-2081(05)80009-9. [DOI] [PubMed] [Google Scholar]
- Cimprich B. Pretreatment symptom distress in women newly diagnosed with breast cancer. Cancer Nursing. 1999;22(3):185–194. doi: 10.1097/00002820-199906000-00001. quiz 195. [DOI] [PubMed] [Google Scholar]
- Cimprich B, Reuter-Lorenz P, Nelson J, Clark PM, Therrien B, Normolle D, et al. Prechemotherapy alterations in brain function in women with breast cancer. Journal of Clinical and Experimental Neuropsychology. 2009;99999(1):1–8. doi: 10.1080/13803390903032537. [DOI] [PubMed] [Google Scholar]
- Cimprich B, Ronis DL. An environmental intervention to restore attention in women with newly diagnosed breast cancer. Cancer Nursing. 2003;26(4):284–292. doi: 10.1097/00002820-200308000-00005. [DOI] [PubMed] [Google Scholar]
- Cimprich B, So H, Ronis DL, Trask C. Pre-treatment factors related to cognitive functioning in women newly diagnosed with breast cancer. Psycho-Oncology. 2005;14(1):70–78. doi: 10.1002/pon.821. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Ryan KM. Pain assessment: Global use of the Brief Pain Inventory. Annals Academy of Medicine Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17(2):197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- Dhruva A, Dodd MJ, Paul SM, Cooper BA, Lee K, West C, et al. Trajectories of Fatigue in Patients with Breast Cancer Before, During, and After Radiation Therapy. 2009. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson RJ, McDonald BC, Saykin AJ, Ahles TA. Brain structure and function differences in monozygotic twins: Possible effects of breast cancer chemotherapy. Journal of Clinical Oncology. 2007;25(25):3866–3870. doi: 10.1200/JCO.2007.10.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess LM, Insel KC. Chemotherapy-related change in cognitive function: A conceptual model. Oncology Nursing Forum. 2007;34(5):981–994. doi: 10.1188/07.ONF.981-994. [DOI] [PubMed] [Google Scholar]
- Huyser Y. De Symptomatologie van Depressieve Stoornissen en Angststoornissen. Amsterdam: Benecke Consultants; 1993. [Google Scholar]
- James W. The Principles of Psychology. Cambridge, MA: Harvard University Press; 1983. [Google Scholar]
- Jansen CE, Dodd MJ, Miaskowski CA, Dowling GA, Kramer J. Preliminary results of a longitudinal study of changes in cognitive function in breast cancer patients undergoing chemotherapy with doxorubicin and cyclophosphamide. Psycho-Oncology. 2008;17(12):1189–1195. doi: 10.1002/pon.1342. [DOI] [PubMed] [Google Scholar]
- Jansen DA. Attentional demands and daily functioning among community-dwelling elders. Journal of Community Health Nursing. 2006;23(1):1–13. doi: 10.1207/s15327655jchn2301_1. [DOI] [PubMed] [Google Scholar]
- Jenkins V, Shilling V, Deutsch G, Bloomfield D, Morris R, Allan S, et al. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. British Journal of Cancer. 2006;94(6):828–834. doi: 10.1038/sj.bjc.6603029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins V, Shilling V, Fallowfield L, Howell A, Hutton S. Does hormone therapy for the treatment of breast cancer have a detrimental effect on memory and cognition? A pilot study. Psycho-Oncology. 2004;13(1):61–66. doi: 10.1002/pon.709. [DOI] [PubMed] [Google Scholar]
- Jensen MP. The validity and reliability of pain measures in adults with cancer. Journal of Pain. 2003;4(1):2–21. doi: 10.1054/jpai.2003.1. [DOI] [PubMed] [Google Scholar]
- Kaplan S, Kaplan R. Cognition and Environment: Functioning in an Uncertain World. New York: Praeger; 1982. [Google Scholar]
- Karnofsky D. Performance Scale. In: Kennealey GT, Mitchell MS, editors. Factors That Influence the Therapeutic Response in Cancer. New York: Plenum Press; 1977. pp. 97–101. [Google Scholar]
- Kennedy BL, Schwab JJ, Morris RL, Beldia G. Assessment of state and trait anxiety in subjects with anxiety and depressive disorders. Psychiatric Quarterly. 2001;72(3):263–276. doi: 10.1023/a:1010305200087. [DOI] [PubMed] [Google Scholar]
- Lee KA. Self-reported sleep disturbances in employed women. Sleep. 1992;15(6):493–498. doi: 10.1093/sleep/15.6.493. [DOI] [PubMed] [Google Scholar]
- Lee KA, DeJoseph JF. Sleep disturbances, vitality, and fatigue among a select group of employed childbearing women. Birth. 1992;19(4):208–213. doi: 10.1111/j.1523-536x.1992.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Research. 1991;36(3):291–298. doi: 10.1016/0165-1781(91)90027-m. [DOI] [PubMed] [Google Scholar]
- Lee KA, Lentz MJ, Taylor DL, Mitchell ES, Woods NF. Fatigue as a response to environmental demands in women’s lives. Image—The Journal of Nursing Scholarship. 1994;26(2):149–154. doi: 10.1111/j.1547-5069.1994.tb00935.x. [DOI] [PubMed] [Google Scholar]
- Lee KA, Portillo CJ, Miramontes H. The fatigue experience for women with human immunodeficiency virus. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 1999;28(2):193–200. doi: 10.1111/j.1552-6909.1999.tb01984.x. [DOI] [PubMed] [Google Scholar]
- Lee KA, Portillo CJ, Miramontes H. The influence of sleep and activity patterns on fatigue in women with HIV/AIDS. Journal of the Association of Nurses in AIDS Care. 2001;12(Suppl):19–27. doi: 10.1177/105532901773742257. [DOI] [PubMed] [Google Scholar]
- Lehto RH, Cimprich B. Anxiety and directed attention in women awaiting breast cancer surgery. Oncology Nursing Forum. 1999;26(4):767–772. [PubMed] [Google Scholar]
- Lezak MD. The problem of assessing executive functions. International Journal of Psychology. 1982;17(2/3):281–297. [Google Scholar]
- Li LW. From caregiving to bereavement: Trajectories of depressive symptoms among wife and daughter caregivers. Journal of Gerontology: Psychological Sciences. 2005a;60B(4):P190–198. doi: 10.1093/geronb/60.4.p190. [DOI] [PubMed] [Google Scholar]
- Li LW. Longitudinal changes in the amount of informal care among publicly paid home care recipients. Gerontologist. 2005b;45(4):465–473. doi: 10.1093/geront/45.4.465. [DOI] [PubMed] [Google Scholar]
- Mehnert A, Scherwath A, Schirmer L, Schleimer B, Petersen C, Schulz-Kindermann F, et al. The association between neuropsychological impairment, self-perceived cognitive deficits, fatigue and health related quality of life in breast cancer survivors following standard adjuvant versus high-dose chemotherapy. Patient Education and Counseling. 2007;66(1):108–118. doi: 10.1016/j.pec.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Lee KA. Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: A pilot study. Journal of Pain and Symptom Management. 1999;17(5):320–332. doi: 10.1016/s0885-3924(99)00008-1. [DOI] [PubMed] [Google Scholar]
- NHLBI. Body mass index table. 2009 Retrieved February 22, 2009, from http://www.nhlbi.nih.gov/guidelines/obesity/bmi_tbl.pdf.
- Palos GR. Opioids and cancer survivors: Issues in side-effect management. Oncology Nursing Forum. 2008;35(Suppl):13–19. doi: 10.1188/08.ONF.S1.13-19. [DOI] [PubMed] [Google Scholar]
- Posner MI, Boies SJ. Components of attention. Psychological Review. 1971;78(5):391–408. [Google Scholar]
- Posner MI, Dehaene S. Attentional networks. Trends in Neuroscience. 1994;17(2):75–79. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Raudenbush SW. Comparing personal trajectories and drawing causal inferences from longitudinal data. Annual Review of Psychology. 2001;52:501–525. doi: 10.1146/annurev.psych.52.1.501. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- Raudenbush SW, Bryk AS, Cheong YF, Congdon RT. HLM 6: Hierarchical Linear and Nonlinear Modeling. Lincolnwood, IL: Scientific Software International; 2004. [Google Scholar]
- Schagen SB, van Dam FS, Muller MJ, Boogerd W, Lindeboom J, Bruning PF. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer. 1999;85(3):640–650. doi: 10.1002/(sici)1097-0142(19990201)85:3<640::aid-cncr14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Sheehan TJ, Fifield J, Reisine S, Tennen H. The measurement structure of the Center for Epidemiologic Studies-Depression scale. Journal of Personality Assessment. 1995;64(3):507–521. doi: 10.1207/s15327752jpa6403_9. [DOI] [PubMed] [Google Scholar]
- Seidenberg M, Haltiner A, Taylor MA, Hermann BB, Wyler A. Development and validation of a multiple ability self-report questionnaire. Journal of Clinical and Experimental Neuropsychology. 1994;16(1):93–104. doi: 10.1080/01688639408402620. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (Form Y): (“Self-evaluation questionnaire”) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Tennessen CM, Cimprich B. Views to nature: Effects on attention. Journal of Environmental Psychology. 1995;15(1):77–85. [Google Scholar]
- van Dam FS, Schagen SB, Muller MJ, Boogerd W, vd Wall E, Droogleever Fortuyn ME, et al. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: High-dose versus standard-dose chemotherapy. Journal of the National Cancer Institute. 1998;90(3):210–218. doi: 10.1093/jnci/90.3.210. [DOI] [PubMed] [Google Scholar]
- Von Ah D, Russell KM, Storniolo AM, Carpenter JS. Cognitive dysfunction and its relationship to quality of life in breast cancer survivors. Oncology Nursing Forum. 2009;36(3):326–336. doi: 10.1188/09.ONF.326-334. [DOI] [PubMed] [Google Scholar]
- Williams RA, Hagerty BM, Cimprich B, Therrien B, Bay E, Oe H. Changes in directed attention and short-term memory in depression. Journal of Psychiatric Research. 2000;34(3):227–238. doi: 10.1016/s0022-3956(00)00012-1. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Merser C, Poser U, Sedelmeier P. Ein Fragebogen Erlebter Defizite der Aufmerksamkeit (FEDA) Freiburg: University Institute of Psychology; 1991. [Google Scholar]