Abstract
Background
Highly active antiretroviral therapy (HAART) results in partial immune restoration for people with AIDS, but its impact on cancer risk among children is unknown.
Methods
Data from the U.S. HIV/AIDS Cancer Match Study were used to evaluate cancer risk for people diagnosed with AIDS as children (diagnosed with AIDS at ages 0–14 years, during 1980–2007, followed for up to 10 years; N=5,850). We calculated standardized incidence ratios (SIRs) to compare cancer risk to the general population. Poisson regression evaluated changes in cancer incidence between the pre-HAART (1980–1995) and HAART eras (1996–2007).
Results
There were 106 cancers observed with significantly elevated risks for the 2 major AIDS-defining cancers: Kaposi sarcoma (KS) (N=20, SIRs 1694, 95% confidence interval [CI], 986–2712 and 1146; 95%CI, 236–3349) during the pre-HAART and HAART eras, respectively), and non-Hodgkin lymphoma (NHL) (N=64, SIRs 338; 95%CI, 242–458 and 116; 95%CI, 74–175). Incidence of both cancers declined 87% and 60%, respectively, in the HAART era (P<0.05). Of non-AIDS-defining cancers, leiomyosarcoma risk (N=9) was elevated during both time periods (SIRs 863; 95%CI, 235–2211 and 533; 95%CI, 173–1243).
Conclusion
People diagnosed with AIDS during childhood remain at elevated risk for KS, NHL, and leiomyosarcoma in the HAART era. Incidence of KS and NHL declined relative to widespread HAART use, but there was no change in the incidence of other cancers.
Impact
People diagnosed with AIDS during childhood remain at elevated risk for certain cancers. Continued monitoring is warranted as this immunosuppressed population ages into adulthood where cancer risks generally increase.
Keywords: AIDS, cancer, children, HIV, leiomyosarcoma, lymphoma
Introduction
From the beginning of the human immunodeficiency virus (HIV) epidemic in the U.S. through 2009, an estimated 10,769 persons <15 years of age have been diagnosed with advanced HIV infection (i.e., acquired immunodeficiency syndrome [AIDS]), 80% of whom acquired HIV through perinatal transmission (1). Combination highly active antiretroviral therapy (HAART) has been widely available since 1996 and has conferred improved survival among adults and children with AIDS. HAART has also resulted in a steep decline in the rate of mother-to-child transmission of HIV (2). In recent years, there has been a shift to older ages among AIDS diagnoses (1, 3), probably reflecting, at least in part, improvements in access to HAART and delays in disease progression. The overall effect of these trends is that, by the end of 2008, there were approximately 10,596 people aged 24 years or younger living with an AIDS diagnosis (1).
Children and adults infected with HIV have an elevated cancer risk relative to the general population. While HIV-infected adults have been evaluated in numerous studies (4–11), fewer studies have described cancers in children with HIV/AIDS (12–15). HIV-induced immunosuppression and coinfection with oncogenic viruses are risk factors for malignancy among HIV-infected people, especially those with AIDS (13, 16). There are 3 AIDS-defining cancers: Kaposi sarcoma (KS) due to KS-associated herpesvirus; non-Hodgkin lymphoma (NHL), many cases of which are due to Epstein Barr virus (EBV); and cervical cancer due to human papillomavirus (HPV) (17). All other malignancies are considered non-AIDS-defining cancers.
A prior analysis of data from the population-based HIV/AIDS Cancer Match (HACM) Study found an excess risk of KS, NHL, and Hodgkin lymphoma, among children with AIDS relative to the general population (12). The study also reported an excess of leiomyosarcomas, rare smooth muscle tumors linked to EBV infection, but the study was limited to children diagnosed with AIDS through 1996, with no data on those diagnosed subsequently in the HAART era. More recently, an Italian study of HIV-infected children reported significant declines in cancer incidence during 2000–2004 relative to earlier periods, consistent with increasing population-level use of HAART (14), however, the impact of HAART on cancer risk among U.S. children with AIDS is unknown.
Current estimates of cancer risk for HIV-infected children are necessary as they live longer with immunosuppression and age into adolescence and young adulthood. Such information can be useful to clinicians treating this population. We assessed long-term cancer risk among people diagnosed with AIDS during childhood (PWAC) using updated data from the HACM Study.
Materials and Methods
Study design
The HACM Study links records of people with HIV or AIDS diagnosed between 1980 and 2007 from 15 U.S. states and metropolitan regions to corresponding cancer registry records (4, 18). Following linkage of registry databases, only de-identified data were retained. Institutional review boards at participating sites approved the study.
The current study focuses on long-term cancer risk among PWAC. AIDS onset date was recorded as the date a person met the 1993 Centers for Disease Control and Prevention (CDC) AIDS case definition (17). The study was limited to the most common racial/ethnic groups (non-Hispanic whites, non-Hispanic blacks, and Hispanics) to allow for stable expected cancer counts. Of 663,750 people with AIDS in the HACM Study, we restricted the analyses to subjects <15 years of age at AIDS onset, and who contributed person-time at-risk for cancer during the 4–120 months (10 years) after AIDS diagnosis (N=5,850). Person-time was tabulated from the start of the 4th month after AIDS onset or the start of cancer registry coverage (whichever occurred last), until death, 10 years after AIDS onset, or end of cancer registry coverage (whichever occurred first). Only subjects with AIDS (as opposed to those with HIV infection alone) were evaluated, since AIDS reporting in the U.S. extends back to the start of the epidemic (1980) and the date a subject met the AIDS surveillance case definition is known.
Malignancies reported to cancer registries were coded according to the International Classification for Diseases for Oncology (19), and categorized by site and histology using the Surveillance, Epidemiology, and End Results (SEER) program’s “site recode with Kaposi sarcoma and mesothelioma” (20). This classification was slightly modified by grouping some rare cancer types and expanding NHL into subtypes of interest. Cases of leiomyosarcoma (histology code 8890, regardless of topography) were separated from other soft tissue malignancies.
Statistical methods
We describe cancers occurring before AIDS onset (from 60 to 7 months prior to AIDS diagnosis) and during the AIDS onset period (from 6 months prior to 3 months after AIDS). The former are prevalent cancers, occurring before AIDS. The latter are also not considered incident cancers due to the intensive surveillance occurring in those with AIDS in the months immediately before and after an AIDS diagnosis. Cancers occurring during this period can also be the events that define the onset of AIDS, making it difficult to consider their risk relative to the general population.
The main analysis focused on cancers occurring from 4–120 months (10 years) after AIDS diagnosis. To understand the role of aging and its impact on time at-risk for cancer, we determined the distribution of follow-up time by attained age groups and calendar years. The maximum age at cancer diagnosis could be 24 years (i.e., a 14 year-old at AIDS diagnosis who was followed for 10 years).
For each cancer type (during the 4–120 month period) we calculated a standardized incidence ratio (SIR), defined as the ratio of observed to expected number of cancer cases, as a measure of risk relative to the general population. Expected counts were obtained by applying gender, age, race/ethnicity, calendar year, and registry-specific incidence rates to person-time among PWAC. Because virtually all cases of KS and central nervous system (CNS) lymphoma are AIDS-related, we used expected rates for these cancers from SEER registries prior to the AIDS epidemic (1973–1979) (21). We calculated 2-sided exact Poisson 95% confidence intervals (CIs) for the SIRs. Analyses were conducted separately for 2 attained calendar periods: the pre-HAART era (1980–1995) and the HAART era (1996–2007).
To determine the impact of widespread HAART use on cancer risk among PWAC, we evaluated changes in the incidence of each cancer between the pre-HAART and HAART eras with a rate ratio (RR) obtained from Poisson regression. To account for under-dispersion or over-dispersion in the models, standard errors of the coefficients were adjusted by the Pearson χ2 divided by the degrees of freedom. P-values <0.05 were considered significant.
Results
Of 5,850 subjects included in the analysis, 4,480 (76.6%) were diagnosed with AIDS during the pre-HAART era (1980–1995), and 1,370 (23.6%) were diagnosed during the HAART era (1996–2007). The proportion of male subjects declined across the calendar periods from 52.6% to 49.1%. By race/ethnicity, most subjects were non-Hispanic black during both time periods (60.1% and 66.7%, respectively). The proportion of subjects 0–4 years old at AIDS onset declined from 73.3% during 1980–1995 to 48.3% during 1996–2007. This reflects declines in perinatal HIV transmission over time and the effectiveness of HAART in delaying AIDS onset to older ages.
During the pre-AIDS period (60 to 7 months prior to AIDS diagnosis), there were 2 cases of lymphocytic leukemia, 1 ganglioneuroblastoma, and 1 poorly-specified cancer. There were 44 cancers observed during the AIDS onset period (6 months prior to AIDS to 3 months after AIDS). Most malignancies observed during this period were NHLs (n=35, 80%).
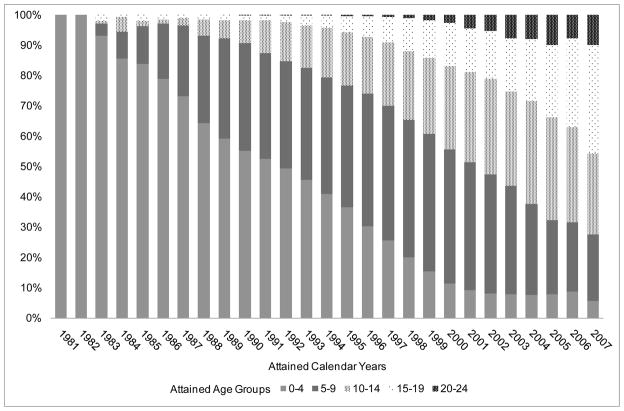
Figure 1 shows the distribution of person-time at-risk for cancer after AIDS diagnosis, by attained year and age-groups. The proportion of person-time contributed by subjects aged 0–4 years declined from 100% in 1981 to 52% in 1991. Subsequently, person-time contributed by people aged 5–9 years increased from 35% in 1991 to 42% in 2001. During 2007 more than half of the person-time was contributed by people with a current age of 10–24 years, but only 10% of person-time was among subjects aged 20 years or older.
Figure 1.
Distribution of person-years for cancer risk by attained age-group and year among people diagnosed with AIDS during childhood
A total of 106 cancer cases were observed during the 10 years after AIDS onset (Table 1). Relative to the general population, overall cancer risk was strongly elevated during both the pre-HAART (SIR 40; 95%CI, 31–51) and HAART eras (SIR 17; 95%CI, 12–23). AIDS-defining cancers were the most common cancer type during both the pre-HAART (84%) and HAART periods (70%). Risks were significantly elevated during both periods for KS (SIRs 1694; 95%CI, 986–2712 and 1146; 95%CI, 236–3349, respectively). Of the 20 KS cases, the median age at cancer diagnosis increased from 2 years in the pre-HAART era to 10 years in the HAART era.
Table 1.
Cancer risk among people diagnosed with AIDS during childhood relative to the general population
| Cancer type | Pre-HAART era 1980–1995 | HAART era 1996–2007 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| No. cases | SIR | 95%CI | No. cases | SIR | 95%CI | |
| All cancer | 69 | 40 | (31–51) | 37 | 17 | (12–23) |
| AIDS-defining cancers overall | 58 | 441 | (335–570) | 26 | 130 | (85–190) |
| Kaposi sarcoma | 17 | 1694 | (986–2712) | 3 | 1146 | (236–3349) |
| Non-Hodgkin lymphoma | 41 | 338 | (242–458) | 23 | 116 | (74–175) |
| Diffuse large B-cell lymphoma | 7 | 280 | (112–576) | 14 | 235 | (128–394) |
| Burkitt lymphoma | 10 | 304 | (146–559) | 3 | 84 | (17–246) |
| CNS lymphoma | 5 | 1994 | (647–4656) | 5 | 1088 | (354–2541) |
| Other/unspecified NHL | 24 | 378 | (242–562) | 6 | 59 | (22–127) |
| Cervix | 0 | 0 | (0–8111) | 0 | 0 | (0–916) |
| Non-AIDS-defining cancers overall | 8 | 5 | (2–10) | 11 | 6 | (3–10) |
| Liver | 1 | 47 | (1.2–263) | 0 | 0 | (0–196) |
| Lung | 0 | 0 | (0–1230) | 1 | 108 | (3–602) |
| Soft tissue (other than leiomyosarcoma) | 2 | 21 | (3–77) | 1 | 9 | (0.22–48) |
| Leiomyosarcoma | 4 | 863 | (235–2211) | 5 | 533 | (173–1243) |
| Thyroid | 1 | 90 | (2.3–502) | 0 | 0 | (0–64) |
| Hodgkin lymphoma | 0 | 0 | (0–70) | 1 | 7 | (0.17–38) |
| Lymphocytic leukemia | 0 | 0 | (0–9) | 2 | 5 | (0.64–19) |
| Miscellaneous | 0 | 0 | (0–13) | 1 | 4 | (0.11–25) |
| Poorly specified | 3 | 86 | (18–251) | 0 | 0 | (0–125) |
Bolded values are significant at P<.05. Abbreviations: CI, confidence interval; CNS, central nervous system; NHL, non-Hodgkin lymphoma; SIR, standardized incidence ratio. The overall NHL category includes diffuse large B-cell lymphoma, Burkitt lymphoma, and other/unspecified NHL. The CNS lymphoma category includes any histologic subtype of NHL occurring in the central nervous system and so overlaps with other NHL categories. The poorly specified category includes cancers of any topography with a poorly specified histology (ICD-O histology codes 8000–8005). The all non-AIDS-defining cancers category includes miscellaneous cancers but excludes poorly specified cancers.
In the 10 years after AIDS onset, NHL was the most common cancer overall (60%) and the most common AIDS-defining cancer (76%). Risk of NHL was elevated during both time periods (SIRs 338; 95%CI, 242–458 and 116; 95%CI, 74–175, respectively). By subtype, risk of CNS lymphoma was especially elevated during the pre-HAART (SIR 1994; 95%CI, 647–4656) and HAART eras (SIR 1088; 95%CI, 354–2541, Table 1). Similar to KS, the median age at NHL onset increased from 3 years during the pre-HAART era to 12 years in the HAART era. No cases of cervical cancer were observed.
There were 19 non-AIDS-defining cancers diagnosed during the study, corresponding to elevated risks during both the pre-HAART (SIR 5; 95%CI, 2–10) and HAART eras (SIR 6; 95%CI, 3–10, Table 1). The most common was leiomyosarcoma (n=9), followed by other soft tissue cancers (n=3), poorly specified cancers (n=3) and lymphocytic leukemia (n=2). Risk of leiomyosarcoma was markedly elevated during both time periods (4 cases in the pre-HAART era, median age 5 years, SIR 863; 95% CI, 235–2211; 5 cases in the HAART era, median age 5 years, SIR 533; 95%CI, 173–1243). There was an elevated risk for soft tissue cancers (other than leimyosarcoma) observed in the pre-HAART era (SIR 21; 95%CI, 3–77). The 2 observed cases were a rhabdomyosarcoma (in a 6 year-old) and a neurofibrosarcoma (in a 3 year-old). The 1 soft tissue cancer in the HAART era (neuroblastoma, age 9 years at cancer diagnosis) did not manifest as a significantly elevated risk relative to the general population.
Other non-AIDS-defining cancers represented single occurrences but contributed to the elevated overall risk. The case of Hodgkin lymphoma was of the nodular sclerosis subtype and occurred in a 16 year-old. The lung cancer was a spindle cell sarcoma in a 4 year-old.
Cancer incidence rates are presented in Table 2. Overall incidence declined 62% relative to widespread HAART use (RR 0.38; 95%CI, 0.23–0.64). This decrease was related to steep declines in the incidence of KS (138 per 100,000 person-years during the pre-HAART era vs. 17 per 100,000 person-years during the HAART era; RR 0.13; 95%CI, 0.02–0.74) and NHL (332 vs. 132 per 100,000 person-years; RR 0.40; 95%CI, 0.21–0.75). There was no apparent decline in the incidence of diffuse large B cell (DLBCL) NHL, although incidence of unspecified NHL declined 82% (RR 0.18; 95%CI, 0.06–0.49). Incidence of Burkitt NHL and CNS NHL declined; however, these decreases were not significant.
Table 2.
Cancer incidence among people diagnosed with AIDS during childhood, by current calendar year period
| Cancer type | Incidence per 100,000 person-years
|
RR | 95%CI | |
|---|---|---|---|---|
| Pre-HAART era 1980–1995 | HAART era 1996–2007 | |||
| All cancer | 559 | 213 | 0.38 | (0.23–0.64) |
| AIDS-defining cancers overall | 470 | 150 | 0.32 | (0.17–0.59) |
| Kaposi sarcoma | 138 | 17 | 0.13 | (0.02–0.74) |
| Non-Hodgkin lymphoma | 332 | 132 | 0.40 | (0.21–0.75) |
| Diffuse large B-cell lymphoma | 57 | 81 | 1.42 | (0.46–4.39) |
| Burkitt lymphoma | 81 | 17 | 0.21 | (0.04–1.04) |
| CNS lymphoma | 41 | 29 | 0.71 | (0.18–2.77) |
| Other/unspecified NHL | 194 | 35 | 0.18 | (0.06–0.49) |
| Non-AIDS-defining cancers overall | 65 | 63 | 0.98 | (0.33–2.86) |
| Soft tissue (other than leiomyosarcoma) | 16 | 6 | 0.35 | (0.01–8.98) |
| Leiomyosarcoma | 32 | 29 | 0.89 | (0.22–3.63) |
Data are for the combined period 1–10 years after AIDS onset. During the pre-HAART era (attained years 1980–1995) there were 12,348 person-years of follow-up, and during the HAART era (attained years 1996–2007) there were 17,394 person-years of follow-up. Rate ratios (RRs) comparing incidence in the two periods were obtained using Poisson regression, Bolded values are significant at P<.05. Abbreviations: CI, confidence interval; CNS, central nervous system; HAART, highly active antiretroviral therapy; NHL, non-Hodgkin lymphoma.
Overall, the incidence of non-AIDS-defining cancers did not change (65 per 100,000 person-years in the pre-HAART era vs. 63 per 100,000 person-years in the HAART era; RR 0.98; 95%CI, 0.33–2.86). There was a non-significant decline in the incidence of soft tissue cancers other than leiomyosarcoma but no change in the incidence of leiomyosarcoma (Table 2).
Discussion
To our knowledge, this is the first U.S. study to document long-term trends in cancer risk among PWAC and changes relative to widespread HAART use. Notably, we found continued elevated risks for 2 AIDS-defining cancers (i.e., KS and NHL) and leiomyosarcoma in the HAART era. Our findings in a large and nationally representative U.S. cohort mirror more limited recent data for HIV-infected children in Italy and Spain (14, 15). Similar to the cancer profile among adults (10, 22), AIDS-defining cancers accounted for the majority of cancers observed among PWAC in the current study. Nonetheless, there was a notable decline in AIDS-defining cancers, likely due to partial immune restoration associated with increasing HAART use (16).
In the U.S., development of KS during childhood is uncommon, due to the rarity of KS-associated herpesvirus (KSHV) infection in early life (23). We found that KS incidence among children with AIDS declined markedly in 1996 and onwards. This could reflect improved control of KSHV through HAART-related immune reconstitution, or perhaps a lower prevalence of KSHV infection. The mechanisms of KSHV transmission during childhood remain unclear and warrant further investigation (24, 25).
The majority of malignancies observed among PWAC were NHL and leiomyosarcoma, linked to EBV. Among the NHLs evaluated, risk was highest for CNS lymphoma. The most common specified NHL subtype was DLBCL and although the incidence of DLBCL did not change significantly in the HAART era, there was a sharp decrease in other/unspecified NHLs, most of which were unspecified and probably cases of DLBCL. The median age at DLBCL onset also increased from 3 years to 10 years, reflecting the aging of this cohort and that PWAC are now surviving long enough to develop NHLs with an extended latency period.
Leiomyosarcoma was the most common non-AIDS-defining cancer observed in this study. Leiomyosarcoma risk was extremely high relative to the general population and did not change with widespread HAART use. Notably, we saw no change in the age at leiomyosarcoma onset across calendar periods (5 years of age), despite the aging of this population, suggesting risk of this malignancy is restricted to the early childhood period among those with AIDS. Almost all leiomyosarcomas in HIV-infected people are EBV positive (26), and tumors express CD21, the EBV receptor (27). In contrast, in the general population, incidence of leiomyosarcoma increases with age, and cases are rarely EBV-positive (28).
A single case of nodular sclerosis Hodgkin lymphoma occurred in an adolescent with AIDS. Among adults with AIDS, risk is most elevated for the mixed cellularity subtype of Hodgkin lymphoma, although the nodular sclerosis subtype is also common, and most cases are EBV positive (29). The subject with Hodgkin lymphoma in this report was exposed to HIV through male-to-male sexual contact, and may have developed primary EBV infection during early adolescence. EBV infection in adolescence manifests as infectious mononucleosis, which is a strong risk factor for EBV-positive Hodgkin lymphoma (30). Among HIV-infected individuals, HAART does not appear to effectively reduce Hodgkin lymphoma risk (29), and Hodgkin lymphoma incidence may rise among HIV-infected adolescents and young adults as more are exposed to EBV.
The observation that many of the malignancies observed in the current analysis are related to EBV warrants additional comment. Both adults and children with AIDS are at elevated risk for EBV-related CNS lymphoma. However, while CNS lymphoma among adults with AIDS has declined appreciably (more than 80%) with the introduction of HAART, we saw no such decline among children (10). Likewise, we did not see a decline among children in the incidence of leiomyosarcoma, another EBV-related tumor. We speculate that PWAC are especially vulnerable to EBV-related malignancies, because primary EBV infection can occur in the setting of marked immunosuppression, and that subsequent HAART may not be effective in allowing control of EBV replication. Additional research is needed to develop prevention paradigms for EBV, including vaccines, which might provide immunity to EBV before primary infection.
The incidence of other non-AIDS-defining cancers was low in our study and did not show a clear pattern. There were 3 miscellaneous cases that contributed to an elevated risk of soft tissue malignancies other than leiomyosarcoma. The single case of lung cancer with an unusual histology (spindle cell sarcoma) is likely unrelated to smoking (as it occurred in a 4-year old) and could be a misclassified case of pulmonary KS.
The relatively young age of subjects included in the current study is notable (the maximum age at cancer diagnosis was 24 years), and none had yet reached ages where lifestyle (e.g., tobacco use, obesity, and alcohol intake) or acquisition of oncogenic viruses (e.g., hepatitis B or C viruses or human papillomavirus) would meaningfully affect risk of non-AIDS-defining cancers. Cancer incidence among PWAC may increase as young people age into adulthood where these factors rise in prominence. Further, clinicians treating young HIV-infected people should counsel them regarding safe sex practices and avoidance of needle sharing, and provide hepatitis B vaccination (31). Although not of proven efficacy in the setting of HIV infection, vaccination against HPV should be provided to girls (and is available for boys) (31). Pap testing of HIV-positive women should also be conducted (32).
Strengths of this study include its large size and representative nature, including most PWAC in the U.S. across the span of the HIV/AIDS epidemic. The characteristics of subjects included in this study accurately reflect the overall distribution of gender, race/ethnicity and mode of HIV transmission of subjects included in the CDC’s national HIV surveillance database (data not shown) (1). A shortcoming is the absence of individual-level information on immune suppression, HIV treatment, and cancer risk factors. Despite these limitations, our extended follow-up well into the current HAART era provides the most recent estimates of cancer risk among young people with AIDS today. Also, our findings are only applicable to young people with AIDS, because we did not study HIV-infected children who had not yet developed AIDS. With early use of HAART (33), cancer risk among children with less advanced HIV infection would be expected to be lower than we observed. Finally, due to the limited number of cancer outcomes, particularly for non-AIDS-defining cancers, we could not perform analyses stratified by demographic characteristics (e.g., age at AIDS or attained age).
Owing to routine HIV testing of pregnant women and provision of appropriate antiretroviral regimens to infants born to HIV-positive mothers, new cases of AIDS among those <15 years of age are at an all-time low (an estimated 71 cases in 2009) (1). Additional research should focus on describing cancer risk in sub-Saharan Africa, where most cases of AIDS among children now occur. Cancer risks are likely to differ dramatically in Africa as HIV therapies have only recently been made available, and the profile of cancers in the general population is different from that of western countries (34). Childhood transmission of KSHV and EBV is common in this region, leading to higher risks of childhood KS and NHL (35–37).
In conclusion, long-term cancer risks among PWAC have changed with access to HAART. Partial immune restoration associated with increasingly effective HIV therapies has resulted in dramatic declines in the incidence of KS and NHL, although PWAC remain at elevated risk for these malignancies. Notably, the majority of the malignancies observed in this study are etiologically linked to KSHV and EBV, suggesting the need for additional research to elucidate routes of transmission during early childhood. These findings can inform clinicians who care for young HIV-infected patients, as an increased awareness may aid detection of some of these malignancies. We suggest that additional non-AIDS-defining cancers may emerge in this population as it continues to age. This possibility points to the increasing importance of cancer prevention and screening in this population.
Acknowledgments
We thank the staff at the HIV/AIDS and cancer registries at the following locations: the states of Colorado, Connecticut, Florida, Illinois, Georgia, Massachusetts, Michigan, New Jersey, Texas, and Washington, D.C.; and the metropolitan regions of New York City, New York; Los Angeles, San Diego and San Francisco, California; and Seattle, Washington. We also thank Mr. Tim McNeel (Information Management Systems, Rockville, MD) for database management.
Grant Support
This study was funded by the Intramural Research Program of the National Cancer Institute.
Footnotes
All authors declare no conflicts of interest.
Note: Data from this manuscript were presented at the 18th Annual Meeting of the Conference on Retroviruses and Opportunistic Infections. Boston, MA (February 2011).
References
- 1.Centers for Disease Control and Prevention. HIV Surveillance Report 2009. National Center for HIV, STD, and TB Prevention; 2011. p. 21. [Google Scholar]
- 2.Lindegren ML, Byers RH, Jr, Thomas P, Davis SF, Caldwell B, Rogers M, et al. Trends in perinatal transmission of HIV/AIDS in the United States. JAMA. 1999;282:531–8. doi: 10.1001/jama.282.6.531. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Epidemiology of HIV/AIDS--United States, 1981–2005. MMWR Morb Mortal Wkly Rep. 2006;55:589–92. [PubMed] [Google Scholar]
- 4.Goedert JJ, Cote TR, Virgo P, Scoppa SM, Kingma DW, Gail MH, et al. Spectrum of AIDS-associated malignant disorders. Lancet. 1998;20:351, 1833–9. doi: 10.1016/s0140-6736(97)09028-4. [DOI] [PubMed] [Google Scholar]
- 5.Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20:1645–54. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 6.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 7.Crum-Cianflone N, Hullsiek KH, Marconi V, Weintrob A, Ganesan A, Barthel RV, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23:41–50. doi: 10.1097/QAD.0b013e328317cc2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;20:148, 728–36. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 9.Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–94. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 10.Simard EP, Pfeiffer RM, Engels EA. Spectrum of cancer risk late after AIDS onset in the United States. Arch Intern Med. 2010;170:1337–45. doi: 10.1001/archinternmed.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simard EP, Pfeiffer RM, Engels EA. Cumulative incidence of cancer among people with AIDS in the United States. Cancer. 2010 [Google Scholar]
- 12.Biggar RJ, Frisch M, Goedert JJ. Risk of cancer in children with AIDS. AIDS-Cancer Match Registry Study Group. JAMA. 2000;284:205–9. doi: 10.1001/jama.284.2.205. [DOI] [PubMed] [Google Scholar]
- 13.Pollock BH, Jenson HB, Leach CT, McClain KL, Hutchison RE, Garzarella L, et al. Risk factors for pediatric human immunodeficiency virus-related malignancy. JAMA. 2003;289:2393–9. doi: 10.1001/jama.289.18.2393. [DOI] [PubMed] [Google Scholar]
- 14.Chiappini E, Galli L, Tovo PA, Gabiano C, Lisi C, Giaquinto C, et al. Cancer rates after year 2000 significantly decrease in children with perinatal HIV infection: a study by the Italian Register for HIV Infection in Children. J Clin Oncol. 2007;25:97–101. doi: 10.1200/JCO.2006.06.6506. [DOI] [PubMed] [Google Scholar]
- 15.Alvaro-Meca A, Micheloud D, Jensen J, Diaz A, Garcia-Alvarez M, Resino S. Epidemiologic Trends of Cancer Diagnoses Among HIV-infected Children in Spain From 1997 to 2008. Pediatr Infect Dis J. 2011 doi: 10.1097/INF.0b013e31821ba148. [DOI] [PubMed] [Google Scholar]
- 16.Biggar RJ, Chaturvedi AK, Goedert JJ, Engels EA. AIDS-related cancer and severity of immunosuppression in persons with AIDS. J Natl Cancer Inst. 2007;20(99):962–72. doi: 10.1093/jnci/djm010. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 18.National Cancer Institute. U.S. HIV/AIDS Cancer Match Study. 2011 [cited 11/2/2011]; Available from: http://hivmatch.cancer.gov/
- 19.World Health Organization. International Classification of Diseases for Oncology. 3. Geneva: World Health Organization; 2000. [Google Scholar]
- 20.National Cancer Institute. SEER Cancer Statistics Review, 1975–2002. 2009 [cited 10/1/2011]; Available from: http://seer.cancer.gov/csr/1975_2002/
- 21.Chaturvedi AK, Mbulaiteye SM, Engels EA. Underestimation of relative risks by standardized incidence ratios for AIDS-related cancers. Ann Epidemiol. 2008;18:230–4. doi: 10.1016/j.annepidem.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Shiels MS, Pfeiffer RM, Gail MH, Hall HI, Li J, Chaturvedi AK, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103:753–62. doi: 10.1093/jnci/djr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson LA, Li Y, Graubard BI, Whitby D, Mbisa G, Tan S, et al. Human herpesvirus 8 seroprevalence among children and adolescents in the United States. Pediatr Infect Dis J. 2008;27:661–4. doi: 10.1097/INF.0b013e3181691740. [DOI] [PubMed] [Google Scholar]
- 24.Gnann JW, Jr, Pellett PE, Jaffe HW. Human herpesvirus 8 and Kaposi’s sarcoma in persons infected with human immunodeficiency virus. Clin Infect Dis. 2000;30(Suppl 1):S72–6. S72–S6. doi: 10.1086/313841. [DOI] [PubMed] [Google Scholar]
- 25.Plancoulaine S, Abel L, van BM, Tregouet DA, Joubert M, Tortevoye P, et al. Human herpesvirus 8 transmission from mother to child and between siblings in an endemic population. Lancet. 2000;356:1062–5. doi: 10.1016/S0140-6736(00)02729-X. [DOI] [PubMed] [Google Scholar]
- 26.Purgina B, Rao UN, Miettinen M, Pantanowitz L. AIDS-Related EBV-Associated Smooth Muscle Tumors: A Review of 64 Published Cases. Patholog Res Int. 2011;2011:561548. doi: 10.4061/2011/561548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McClain KL, Leach CT, Jenson HB, Joshi VV, Pollock BH, Parmley RT, et al. Association of Epstein-Barr virus with leiomyosarcomas in children with AIDS. N Engl J Med. 1995;332:12–8. doi: 10.1056/NEJM199501053320103. [DOI] [PubMed] [Google Scholar]
- 28.Berwick M. Soft Tissue Sarcoma. In: Schottenfeld D, Fraumeni JF, editors. Cancer Epidemiology and Prevention. New York: Oxford; 2006. pp. 959–74. [Google Scholar]
- 29.Biggar RJ, Jaffe ES, Goedert JJ, Chaturvedi A, Pfeiffer R, Engels EA. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood. 2006;108:3786–91. doi: 10.1182/blood-2006-05-024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hjalgrim H, Askling J, Rostgaard K, Hamilton-Dutoit S, Frisch M, Zhang JS, et al. Characteristics of Hodgkin’s lymphoma after infectious mononucleosis. N Engl J Med. 2003;349:1324–32. doi: 10.1056/NEJMoa023141. [DOI] [PubMed] [Google Scholar]
- 31.Mofenson LM, Brady MT, Danner SP, Dominguez KL, Hazra R, Handelsman E, et al. Guidelines for the Prevention and Treatment of Opportunistic Infections among HIV-exposed and HIV-infected children: recommendations from CDC, the National Institutes of Health, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. MMWR Recomm Rep. 2009;58:1–166. [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1–207. [PubMed] [Google Scholar]
- 33.Panel on antiretroviral therapy medical management of HIV infected children. Guidelines for the use of antiretroviral agents in pediatric HIV infection. 2010 [cited 10/01/2011]; Available from: aidsinfo.nih.gov/contentfiles/pediatricguidelines.pdf.
- 34.Sasco AJ, Jaquet A, Boidin E, Ekouevi DK, Thouillot F, Lemabec T, et al. The challenge of AIDS-related malignancies in sub-Saharan Africa. PLoS One. 2010;5:e8621. doi: 10.1371/journal.pone.0008621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mutalima N, Molyneux EM, Johnston WT, Jaffe HW, Kamiza S, Borgstein E, et al. Impact of infection with human immunodeficiency virus-1 (HIV) on the risk of cancer among children in Malawi - preliminary findings. Infect Agent Cancer. 2010;5:5. doi: 10.1186/1750-9378-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stefan DC, Stones DK, Wainwright L, Newton R. Kaposi sarcoma in South African children. Pediatr Blood Cancer. 2011;56:392–6. doi: 10.1002/pbc.22903. [DOI] [PubMed] [Google Scholar]
- 37.Stefan DC, Wessels G, Poole J, Wainwright L, Stones D, Johnston WT, et al. Infection with human immunodeficiency virus-1 (HIV) among children with cancer in South Africa. Pediatr Blood Cancer. 2011;56:77–9. doi: 10.1002/pbc.22672. [DOI] [PubMed] [Google Scholar]