Abstract
Objective
The development of abdominal aortic aneurysms (AAA) requires extensive aortic wall matrix degradation. Human AAA lesions express high levels of cathepsin L (CatL), one of the most potent mammalian elastases. Whether this protease participates directly in AAA pathogenesis, however, is unknown.
Methods and Results
We generated experimental AAA with aortic elastase perfusion in mice and established an essential role of CatL in AAA formation. After 14 days postperfusion, most wild-type (Ctsl+/+) mice developed AAA, but none of the CatL-deficient (Ctsl–/–) mice did. AAA lesion macrophage contents, CD4+ T cell numbers, CD31+ and laminin-5 angiogenic fragment γ2+ microvessel numbers, and elastin fragmentation were all significantly lower in Ctsl–/– mice than in Ctsl+/+ mice. While lesions from Ctsl–/– mice contained fewer Ki67+ proliferating cells than did Ctsl+/+ mice, the absence of CatL did not affect lesion apoptotic cell contents or medial smooth-muscle cell loss significantly. Mechanistic studies indicated that the absence of CatL reduced lesion chemokine monocyte chemotactic protein-1 content, macrophage and T-cell in vitro transmigration, and angiogenesis, and altered the expression and activities of matrix metalloproteinases and other cysteinyl cathepsins in inflammatory cells, vascular cells, and AAA lesions.
Conclusion
CatL contributes to AAA formation by promoting lesion inflammatory cell accumulation, angiogenesis, and protease expression.
Keywords: aneurysms, angiogenesis, macrophages, cathepsin L, elastase
The development of abdominal aortic aneurysms (AAA) involves extensive aortic wall remodeling, in which proteases play an essential role.1 Matrix metalloproteinases (MMP) and serine proteases may participate directly in experimental AAA in animals.2–5 Most of the cysteine proteases called cathepsins are regulatory proteases that are expressed in restricted tissues under physiological conditions but are induced in almost all tested tissues or cells by pathological stimuli, particularly by inflammatory cytokines.6 Human AAA lesions are rich in inflammatory cells, including T cells, macrophages, neutrophils, and mast cells.7,8 All of these cells produce inflammatory cytokines that enhance vascular cell cathepsin expression.6,9 Cathepsins (Cat) S, L, and K are potent elastases that are highly expressed in human AAA lesions,6,10–12 suggesting their participation in AAA. Indeed, mice lacking CatC are resistant to aortic elastase perfusion-induced AAA,13 and the absence of CatK also protected mice from AAA in the same experimental AAA model.14 Like CatK, CatL is a potent mammalian elastase that is localized to smooth-muscle cells (SMC), endothelial cells (EC), and macrophages in human AAA lesions.6,10 We showed previously that CatL contributed to atherogenesis in LDL receptor-deficient (Ldlr–/–) mice by promoting aortic wall elastin degradation, intima inflammatory cell accumulation, and inflammatory cell protease expression. The absence of CatL reduced atherosclerosis in Ldlr–/– mice in a gene dose-dependent manner.15 AAA is clinically different but shares many pathophysiologies with atherosclerosis, including inflammatory cell recruitment, extracellular matrix protein degradation, angiogenesis, and apoptosis.7,16,17 Given our demonstrated observations of CatL functions in atherosclerosis and the close association between AAA and atherosclerosis, we hypothesized that increased CatL expression in human AAA lesions is not just secondary to the disease but also participates directly in its pathogenesis. This study used CatL-deficient mice and elastase perfusion-induced experimental AAA to test this hypothesis.
Materials and Methods
Mouse AAA Model and Lesion Characterization
Ten-week-old male CatL-deficient (Ctsl–/–) mice18 and male wild-type (Ctsl+/+) littermates (all in C57BL/6/129S background) underwent aortic elastase perfusion-induced experimental AAA.2 Mouse aortic diameters were measured using a surgical microscope (Zeiss Stemi. SV11) equipped with a micrometer eyepiece (14 mm/0.1, SG02.T0218c, Motic Instruments, Inc, Vancouver, Canada), which allowed us to read the aortic diameters under physiological blood pressure at any time during the surgical procedure or during tissue harvesting.2,13 Mouse aortic diameters were measured and recorded before elastase perfusion and 5 minutes after perfusion restoration. Mouse abdominal aortas were collected at 7 days and 14 days postperfusion. Before mice were euthanized, abdominal aorta diameters were measured and recorded at both the 7-day and 14-day time points. Aortic diameter expansion ≥100% of that before elastase perfusion defined AAA.2 Each mouse aorta was isolated for both frozen section preparation and tissue protein extraction in a pH 5.5 buffer containing 1% Triton X-100, 40 mmol/L sodium acetate, and 1 mmol/L EDTA.19 Frozen sections were used for immunostaining for macrophages (Mac-3), SMC (α-actin), T cells (CD4), monocyte chemotactic protein-1 (MCP-1), EC (CD31), elastin (Verhoeff-van Gieson), apoptotic cells (TUNEL), and proliferating cells (Ki67), as described previously.9,19,20 Corresponding purified IgG isotypes were used routinely as immunostaining negative controls. Elastin fragmentation and SMC content in media were graded with the same keys, as we described recently.20 Numbers of CD4+ T cells, MCP-1+ cells, TUNEL-positive apoptotic cells, Ki67+ proliferating cells, and CD31+ or lamin-5 γ2+ microvessels in AAA lesions were counted blindly by 2 independent observers. Mac-3+ macrophage areas in AAA lesions were measured with computer-assisted image analysis software (Image-Pro Plus; Media Cybernetics, Bethesda, MD).
To produce AAA by periaortic chemical injury, 8-week-old male Ctsl+/+ and Ctsl–/– mice were used for aorta CaCl2 (0.25 mol/L) injury as described.3 Aortic diameters were measured before CaCl2 application and 4 weeks after the injury using the same micrometer eyepiece.
Real-Time Polymerase Chain Reaction
Mouse aortic SMC and mouse heart microvessel endothelial cells (MHEC) were prepared as described elsewhere.19,21 Total cellular RNA was extracted from SMC and MHEC using a TRIzol reagent (Invitrogen, Carlsbad, CA). The RNA samples were then treated with a RNase-free DNase (Ambion, Carlsbad, Cal) to remove genomic DNA contaminants. Equal amounts of RNA were reverse transcribed, and quantitative PCR was performed in a single-color real-time polymerase chain reaction (RT-PCR) detection system (Stratagene, La Jolla, CA). The mRNA levels of CatS, CatK, CatB, MMP-2, and MMP-9 were normalized to those of housekeeping gene β-actin.
MHEC Cysteine Protease Active Site Labeling and Gelatin Gel In Situ Zymogram
MHEC were lysed into a pH 5.5 buffer.19 Five micrograms of protein from each sample were incubated with 12 mmol/L dithiothreitol and 1 μL of biotin-conjugated JPM in 30 μL of a pH 5.5 buffer for 1 hour at 37°C. Protein samples were then separated on a 12% SDS-PAGE, followed by immunoblot detection with horseradish peroxidase-conjugated avidin.4 MMP activity was detected with a gelatin gel zymogram, essentially the same as reported previously.22 Equal protein loading (20 μg protein/ lane) was confirmed by immunoblot analysis with goat anti-mouse GAPDH polyclonal antibodies (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA).
Aortic Ring Assay
An aortic ring assay was used to test the role of CatL in angiogenesis. In brief, a 96-well plate was coated with 50 μL of Matrigel (BD Biosciences, San Diego, CA). A 1-mm long mouse aortic ring from Ctsl+/+ mouse or Ctsl+/+ mouse was laid on top of the solidified Matrigel and covered with 100 μL of Matrigel. After solidification at room temperature, 150 μL of RPMI (with 10% FBS) was added to each well. After 7 to 10 days of culture, the aortas were photographed, and the endothelial outgrowth was analyzed using Image-Pro Plus software and presented as square millimeters. Basic fibroblast growth factor (bFGF) (10 ng/mL; PeproTech, Rocky Hill, NJ) was used as a positive control.
In Situ Elastin Zymography
AAA lesion elastinolytic activity was determined in 8-μM frozen sections using elastin conjugated with quenched fluorescein (DQ elastin; Invitrogen) as a substrate, which requires cleavage by elastinolytic enzymes to become fluorescent. In brief, DQ elastin (1 mg/mL in H2O) was mixed 1:10 with 1% low-melting agarose (Sigma, St. Louis, MO). This mixture (20 μL) was added on top of each section, coverslipped, and gelled at 4°C. Following incubation at 37°C (48 hours), fluorescence was examined under fluorescent microscopy. Cysteine protease activity was determined using an EDTA-containing pH 5.5 buffer with or without 20 μmol/L E64 days.9 Zymographic images were acquired using identical shutter conditions. The percentage of fluorescence intensity of each cross section, excluding the media area due to medial elastin filament autofluorescence, was measured using computer-assisted image quantification (Image-Pro Plus software).
Cell Proliferation Assay
CD4+ T cell and monocyte proliferation was assessed with the Cell Titer 96AQ Assay kit, according to the manufacturer's instructions (Promega, Madison, WI). T cells and monocytes were plated with serial 2-fold dilutions on 96-well plates starting from 2×105 cells in 100 μL of 10% FBS RPMI 1640 per well and cultured 2 days at 37°C. Mouse anti-CD3 monoclonal antibody (1 μg/mL, Pharmingen, San Diego, CA) was used to coat the 96-well plate for T-cell proliferation assay, as described.23 Then 20 μL of a mixture of tetrazolium compound and phenazine methosulfate was added, and the absorbance was determined at 492 nm.
Statistical Analysis
Because of the relatively small sample sizes and data distribution abnormality, we selected the nonparametric Mann-Whitney U test for nonpaired data sets and the Wilcoxon signed-rank test for paired data sets to examine statistical significance throughout this study. Two-way repeated-measures ANOVA test followed by Bonferroni posthoc correction was used for multiple group comparisons. P<0.05 was considered statistically significant.
Results
CatL Deficiency Protected Mice From Elastase Perfusion-Induced AAA
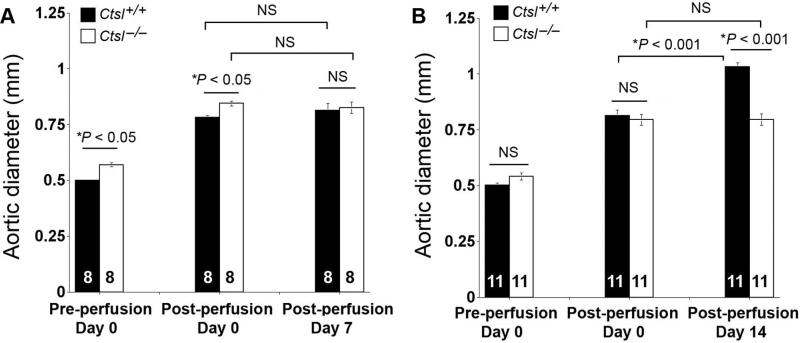
Both Ctsl–/– and Ctsl+/+ littermates underwent aortic elastase perfusion.2,18 For unknown reasons, baseline aortic diameters of Ctsl–/– mice were larger than those of Ctsl+/+ littermates from both the 7-day (0.56±0.01 versus 0.50±0.01 mm, P<0.05) and 14-day (0.54±0.01 versus 0.50±0.01 mm, P>0.05) experimental groups, although all mice were 10-week-old males. In mice used for the 7-day time point, at the time of harvesting (day 7), there was no difference between aortic diameters of Ctsl–/– mice and Ctsl+/+ mice, nor between immediate postperfusion and 7 days postperfusion in Ctsl–/– mice (Figure 1A). At this time point, no mice from either group developed AAA by definition.2 In mice used for the 14-day time point, aortic diameters of Ctsl+/+ mice were significantly enlarged at 14 days postperfusion compared with those from Ctsl–/– mice (P<0.001) or those immediate postperfusion from the same group of mice (P<0.001) (Figure 1B). At this time point, 9 of 11 Ctsl+/+ mice developed AAA, as defined by >100% of aortic diameter increase, but none of the Ctsl–/– mice (n=11) did.
Figure 1.
Aortic expansion in elastase perfusion-induced experimental abdominal aortic aneurysms. Aortic diameters from Ctsl+/+ mice and Cts–/– mice at different time points: preperfusion, immediate postperfusion (day 0), and at harvesting on day 7 (A) and day 14 (B). Data are mean±SE. The number of mice per group is indicated in each bar. P<0.05 was considered statistically significant, 2-way repeated-measures ANOVA test followed by Bonferroni posthoc correction. NS indicates not significant.
CatL Deficiency Reduced AAA Lesion Inflammatory Cell Accumulation
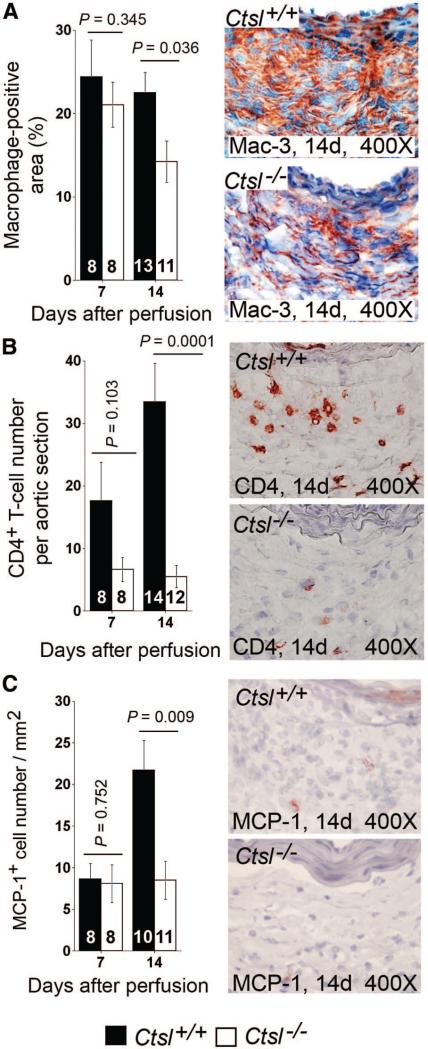
Inflammatory cell infiltration to AAA lesions is an important signature of inflammation in this disease. As in atherosclerotic lesions,15 the absence of CatL reduced Mac-3+ macrophage and CD4+ T-cell accumulation significantly in AAA lesions (Figure 2A/B). Reduced macrophages and CD4+ T cells in Ctsl–/– mouse AAA lesions suggest a role of CatL in leukocyte recruitment or proliferation. In an in vitro transwell migration assay, monocytes or CD4+ T cells from Ctsl–/– mice transmigrated through collagen-coated or EC monolayer-coated transwells much more slowly than did those from Ctsl+/+ mice.15 In AAA lesions, we also detected fewer chemokine MCP-1–positive cells in Ctsl–/– mice than in Ctsl+/+ mice at 14 days postperfusion (Figure 2C). We did not, however, detect significant differences in monocyte or T-cell proliferation between the genotypes (data not shown). These observations suggest that reduced macrophages and CD4+ T cells in Ctsl–/– mouse AAA lesions were caused by impaired inflammatory cell recruitment but not by proliferation.
Figure 2.
Abdominal aortic aneurysms (AAA) lesion inflammatory cell accumulation. Mac-3+ macrophages (A) and CD4+ T cells (B) accumulated less in AAA lesions from Ctsl–/– mice than in those of Ctsl+/+ mice at 14 days postperfusion. At the same time point, MCP-1+ cell numbers were also fewer in AAA lesions from Ctsl–/– mice than in Ctsl+/+ mice (C). Data are mean±SE. The number of mice per group is indicated in each bar. P<0.05 was considered statistically significant; Mann-Whitney U test. Representative images are shown to the right in each panel. All measurements are from the entire lesion including both adventitia and media.
Impaired Angiogenesis in AAA Lesions From Ctsl–/– Mice
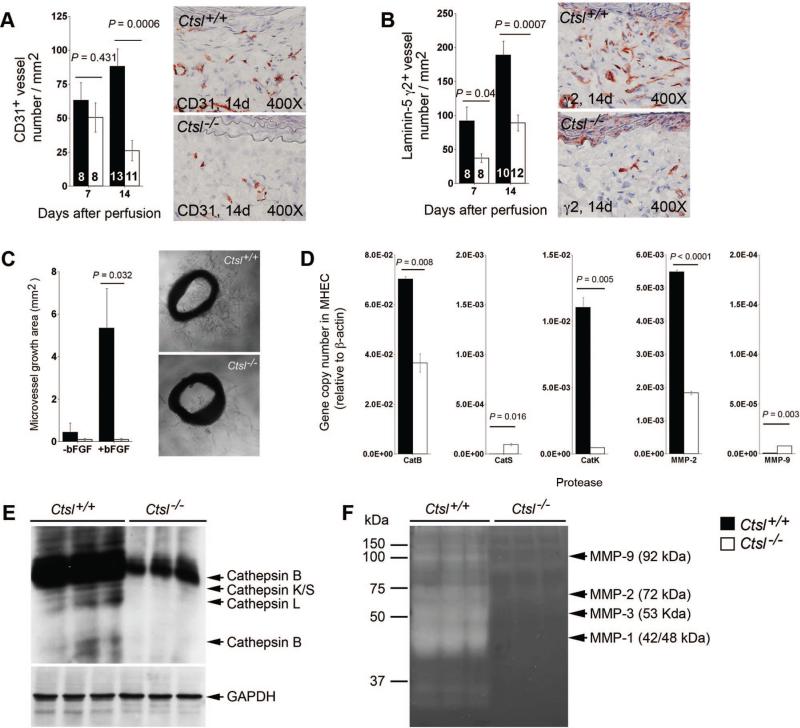
We have shown previously that CatS plays an important role in angiogenesis by degrading antiangiogenic matrix collagen fragments and generating proangiogenic laminin-5 fragments.24 AAA lesions from Ctsl–/– mice contained significantly reduced numbers of CD31+ microvessels at 14 days postperfusion and of proangiogenic laminin-5 γ2+ vessels at both 7 days and 14 days postperfusion, compared with Ctsl+/+ mice (Figure 3A/B). In vitro aortic ring angiogenesis assay showed defects of microvessels sprouting from aortic rings from Ctsl–/– mice with or without angiogenic factor bFGF (Figure 3C). To assess whether the absence of CatL affected the expression or activities of other proteases, we performed RT-PCR in MHEC from Ctsl–/– and Ctsl+/+ mice and demonstrated that the absence of CatL reduced CatB, CatK, and MMP-2 mRNA levels in MHEC (Figure 3D). Cysteinyl cathepsin active site labeling with JPM (Figure 3E) and gelatin gel zymogram (Figure 3F) revealed reduced activities of major cathepsins (cathepsins B, L, K, and S) and MMPs (MMP-1, –2, –3, and –9), suggesting that reduced angiogenesis in Ctsl–/– mice was caused in part by reduced EC protease expression and activities.
Figure 3.
Cathepsin L function in neovascularization in abdominal aortic aneurysms (AAA) lesions. CD31+ (A) and proangiogenic laninin-5 fragment γ2+ (B) microvessel numbers were reduced in AAA lesions from Ctsl–/– mice. The number of mice per group is indicated in each bar. Both measurements are from the entire lesion including adventitia and media. Aortic ring assay in vitro demonstrated impaired microvessel sprouting from Ctsl–/– mouse aortic rings with or without angiogenic factor bFGF (C). Representative images for panels A–C are shown to the right. RT-PCR showed reduced transcripts of cathepsins B and K, and matrix metalloproteinases (MMP)-2 in microvessel endothelial cells (MHEC) from Ctsl–/– mice compared with those from Ctsl+/+ mice (D). Cysteine protease active site labeling with JPM (E) and gelatin gel zymogram (F) demonstrated impaired cathepsin and MMP activities in MHEC from Ctsl–/– mice, respectively. GAPDH immunoblot was used for protein loading control. All data are mean±SE. P<0.05 was considered statistically significant; Mann-Whitney U test.
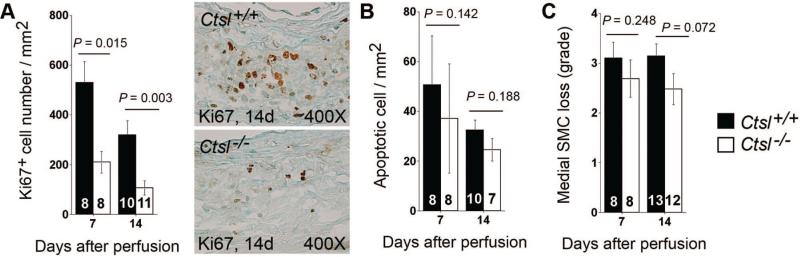
Absence of CatL Did Not Affect AAA Lesion Cell Apoptosis
Although we detected fewer Ki67+ proliferating cells in AAA lesions from Ctsl–/– mice than in that of Ctsl+/+ mice (Figure 4A), the absence of CatL did not affect lesion cell apoptosis (Figure 4B), which causes loss of SMC in AAA.17 Consistent with this observation, medial SMC loss was not different between the 2 genotypes (Figure 4C). These observations suggested that CatL played a negligible role in SMC apoptosis. We tested this hypothesis in cultured aortic SMC. SMC undergo apoptosis after stimulation with antioxidant pyrrolidine dithiocarbamate (PDTC),25 but SMC from Ctsl–/– mice showed no significant differences in apoptosis from those of Ctsl+/+ mice (data not shown).
Figure 4.
Cathepsin L deficiency and abdominal aortic aneurysms (AAA) lesion cell proliferation and apoptosis. AAA lesion Ki67+ proliferating cells were reduced in Ctsl–/– mice at 7 days and 14 days postperfusion (A; representative images are shown to the right), but cathepsin L deficiency did not affect AAA lesion cell apoptosis (B) or lesion medial smooth-muscle cell loss (C). The number of mice per group is indicated in each bar. Measurements in panels A and B are from the entire lesion including adventitia and media. All data are mean±SE. P<0.05 was considered statistically significant; Mann-Whitney U test.
Essential Role of CatL in Medial Elastin Degradation
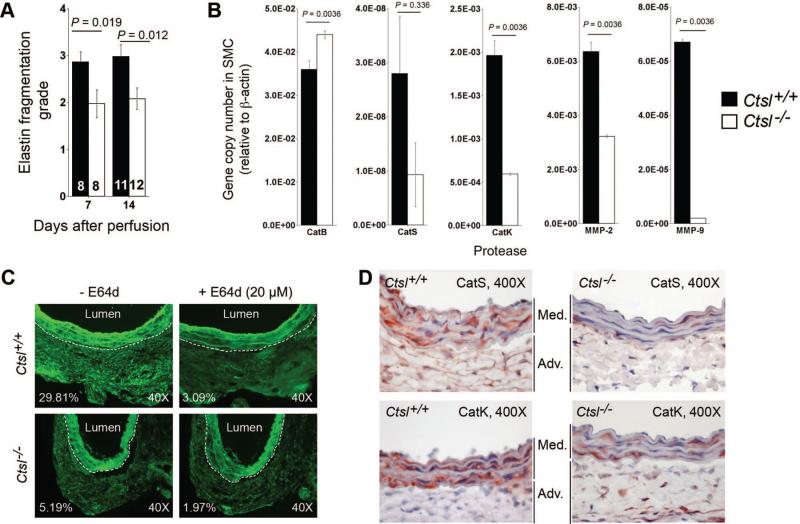
As one of the most potent elastases, CatL may be essential to AAA, in which medial elastin degradation is a major molecular mechanism of pathogenesis. As we anticipated, aortic medial elastin fragmentation at the site of AAA development was significantly impaired in Ctsl–/– mice as compared with Ctsl+/+ mice (Figure 5A). We have shown previously that medial elastin degradation was also impaired in Ctsl–/– and Ctsl± mice in the background of Ldlr–/– after 12 weeks or 26 weeks on an atherogenic diet.15 SMC from Ctsl–/– mice had greatly reduced elastinolytic activities.14 RT-PCR demonstrated that the absence of CatL reduced the mRNA levels of CatS, CatK, MMP-2, and MMP-9 in SMC from Ctsl–/– mice compared with those in Ctsl+/+ mice (Figure 5B), although the reduction of CatS in Ctsl–/– SMC did not reach statistical significance. Insignificance in CatS expression was likely due to its low expression levels and associated low experimental sensitivity (Figure 5B).
Figure 5.
Medial elastin fragmentation and protease expression. A, Medial elastin fragmentation was reduced in abdominal aortic aneurysms (AAA) lesions from Ctsl–/– mice. The number of mice per group is indicated in each bar. B, RT-PCR demonstrated altered cathepsin and matrix metalloproteinases (MMP) transcript levels in aortic smooth-muscle cell from Ctsl–/– mice compared with those of Ctsl+/+ mice. All data are mean±SE. P<0.05 was considered statistically significant; Mann-Whitney U test. C, In situ fluorescent zymogram assay showed high elastinolytic cysteinyl cathepsin activities in the adventitia of Ctsl+/+ mouse AAA lesions (top left) that can be largely inhibited by 20 μmol/L of E64d (top right). Such activities were reduced in AAA lesion adventitia from Ctsl–/– mice (bottom left), and E64d further reduced cathepsin activities (bottom right). The percentages of adventitia fluorescence yielded from fluorogenic elastin degradation represented cysteinyl cathepsin activities. The white dotted lines separate medial elastin autofluorescence from the adventitia. D, Immunohistological analysis showed reduced medial expression of cathepsin S (top panels) and cathepsin K (bottom panels) in AAA lesions from Ctsl–/– mice, compared with those from Ctsl+/+ mice.
Reduced cysteine protease cathepsin expression and activities in SMC (Figure 5B), MHEC (Figure 3D/3E), T cells, and monocytes15 in the genetic absence of CatL strongly suggests that CatL participates in regulating other cathepsin expression and activities. Impaired AAA formation in Ctsl–/– mice may associate with impaired overall protease expressions. To examine this hypothesis, we performed in situ cathepsin elastase zymogram assay. As we showed previously,4,20 AAA lesions from wild-type mice at 14 days postperfusion contained high levels of elastinolytic activities under acidic (pH5.5) conditions that were optimized for cysteinyl cathepsins (fluorescent activities in the adventitia region, Figure 5C, top left panel). Such elastinolytic cathepsin activities can be largely inhibited by 20 μmol/L of E64d (Figure 5C, top right panel). In contrast, AAA lesions from Ctsl–/– mice had greatly reduced adventitia fluorescence (Figure 5C, bottom left panel), and E64d further reduced adventitia elastinolytic cathepsin activities (Figure 5C, bottom right panel).
Reduced elastin degradation in the media (Figure 5A) in AAA lesions from Ctsl–/– mice, impaired expression of cathepsins in aortic SMC from Ctsl–/– mice (Figure 5B), and decreased elastinolytic cathepsin activities in the adventitia (Figure 5C) and possibly in the media from Ctsl–/– mouse AAA lesions all suggest that the absence of CatL affected medial elastinolytic cathepsin expression. To examine this hypothesis, we immunostained AAA lesions from both Ctsl+/+ and Ctsl–/– mice from the 14-day time point for CatS and CatK. Medial expressions of both tested cathepsins were much lower in AAA lesions from Ctsl–/– mice than in those from Ctsl+/+ mice (Figure 5D). Reduced medial cathepsin expression therefore may account in part for better preservation of elastin fragmentation (Figure 5A), and for impaired aorta expansion (Figure 1B), in Ctsl–/– mice compared with Ctsl+/+ mice.
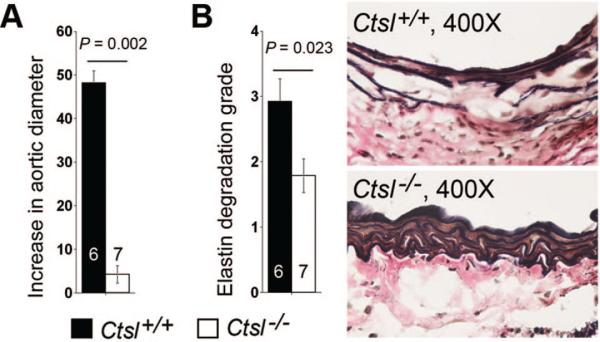
Intranasal exposure of porcine pancreatic elastase develops secondary immune responses via infiltration of inflammatory cells (eg, macrophages, neutrophils, CD4+ and CD8+ T cells) and production of proinflammatory cytokines (eg, IL1β, IL6, TNF-α) in mouse lungs.25 The same elastase perfusion in aortas also may have developed these immune responses that were independent from CatL activities, and therefore may have obscured the effect of CatL in AAA formation and progression. To exclude these possibilities and further confirm a role of CatL in AAA, we applied both Ctsl+/+ and Ctsl–/– mice to a periaortic CaCl2 injury-induced experimental AAA model. One month after the injury, aortic diameters in Ctsl+/+ mice increased by nearly 50%, whereas those in Ctsl–/– mice showed negligible changes (≈4%) (Figure 6A). Medial elastin degradation also was reduced significantly in Ctsl–/– mice compared with Ctsl+/+ mice (Figure 6B), supporting a direct role of CatL in AAA formation.
Figure 6.
Periaortic CaCl2 injury-induced abdominal aortic aneurysms (AAA). A, Aortic diameter increase in percentage at 4 weeks after injury. B, Medial elastin fragmentation grades, as determined according to previously published keys (Ref. 19). Representative figures are shown to the right. Data are mean±SE. P<0.05 is considered statistically significant, Mann-Whitney U test. The number of mice for each group is indicated in the bar.
Discussion
This study demonstrated an important role of elastinolytic CatL in AAA. Although other mechanisms require further investigation, we found that CatL contributed to AAA formation by regulating monocyte and T-cell recruitment, vascular wall matrix protein degradation, lesion cell proliferation, protease expression, and angiogenesis, but it had no effect on SMC or total lesion cell apoptosis. This study also revealed functional differences between CatL and CatK: In human AAA lesions, CatL is expressed mainly in macrophages and weakly in SMC.10 CatK may have different cellular expression profiles in AAA lesions. For example, CatK is highly expressed in both macrophages and SMC in human atheroma.27 CatK in human AAA lesions may have similar expression patterns to those in human atherosclerotic lesions. Further, both CatL and CatK are cysteine proteases with potent elastinolytic, collagenolytic, and fibrinogenolytic activities,6,28,29 and even share >50% cDNA sequence homologies,30 but they may have different activities on other untested substrates. In a separate study, we showed that mice lacking CatK also failed to develop AAA in the same experimental model as the current study, but with different mechanisms.14 First, although the T-cell number, but not the macrophage content, was significantly lower in AAA lesions from Ctsk–/– mice than in those from Ctsk+/+ mice, T cells or monocytes from Ctsk–/– mice showed no differences from those of Ctsk+/+ mice in transmigration through collagen-coated transwells. The absence of CatK affected T-cell proliferation but not monocytes. In contrast, deficiency of CatL did not affect T-cell or monocyte proliferation (data not shown) but significantly reduced their transmigration through collagen-coated or collagen and EC monolayer double-coated transwells,15 which may explain reduced T cells and macrophages in AAA lesions from Ctsl–/– mice (Figure 2A/2B). Further, CatL, but not CatK is involved in antigen presentation. Deficiency of CatL reduces circulating CD4+ T cells,18 which may also contribute to low CD4+ T cells in AAA lesions from Ctsl–/– mice (Figure 2B). Thus, CatK and CatL contributed to AAA lesion inflammatory cell accumulation with different mechanisms. Second, both in vitro aortic ring assay and anti-mouse CD31 monoclonal antibody immunostaining of AAA lesion sections showed that the absence of CatK did not affect microvessel sprouting from aortic rings or CD31+ microvessel numbers in AAA lesions. In contrast, we hardly detected any microvessel sprouting in aortic rings from Ctsl–/– mice with or without bFGF (Figure 3C). Furthermore, CD31+ or laminin-5 γ2+ microvessel numbers were significantly fewer in Ctsl–/– mouse AAA lesions than in Ctsl+/+ mouse AAA lesions (Figure 3A/3B). Our studies therefore demonstrated that CatL, but not CatK, affected angiogenesis. We currently do not know the molecular explanation for these observations. Different cathepsins may produce different proangiogenic or antiangiogenic fragments from the matrix; for example, we demonstrated that CatS produces proangiogenic γ2 fragments from laminin-5.24 In contrast, CatV—but not CatL, CatB, or CatK—produces antiangiogenic angiostatin-like fragments from plasminogen.31 CatL and CatK may produce different bioactive molecules from the matrix, a hypothesis that merits further investigation. Third, CatL played negligible roles in SMC apoptosis. PDTC-induced apoptosis of aortic SMC in vitro from Ctsl–/– mice showed no difference from those of Ctsl+/+ mice. AAA lesion apoptotic cell numbers from Ctsl–/– mice also were not different from those in Ctsl+/+ mice (Figure 4B). In contrast, CatK is essential to SMC apoptosis and to AAA lesion cell apoptosis. Aortic SMC from Ctsk–/– mice were very well protected from PDTC-induced apoptosis. TUNEL-positive cells in AAA lesions from Ctsk–/– mice were much fewer than in those from Ctsk+/+ mice. Therefore, CatK, but not CatL, plays an important role in cell apoptosis. Similar findings have been demonstrated in osteoclasts; the absence of CatK leads to overgrowth of osteoclasts in vitro and in vivo.32 The role of CatL in cell apoptosis, however, seems much more complicated. Much of the data from CatL genetically deficient or antisense cDNA inactivated cells suggested a protective role of CatL in cell apoptosis.33 In contrast, CatL inhibition or depletion promoted cell apoptosis in different cell types.34–36 Therefore, the role of CatL in apoptosis remains controversial and may be cell type-dependent.
In contrast to our unpublished data regarding CatK in mouse experimental AAA, Bai et al recently reported different observations in angiotensin-II (Ang-II) infusion-induced AAA from apolipoprotein E-deficient (Apoe–/–) mice. In these mice, the presence or absence of CatK did not affect AAA formation.37 Through unknown mechanisms, Ang-II infusion enhanced peripheral active CD4+CD25+ T cells and Lg6G+ leukocytes in Apoe–/–Ctsk–/– mice and increased AAA lesional CD45+ leukocytes and Mac-3+ macrophages in these mice.37 Ang-II–induced increase of inflammatory cells in Apoe–/–Ctsk–/– mice in both peripheral and AAA lesions may have compensated the effect of CatK deficiency, thereby obscuring the difference in AAA formation between Apoe–/–Ctsk+/+ and Apoe–/–Ctsk–/– mice. We examined this hypothesis by infusing Ang-II (1000 ng/min/kg) to Ctsk+/+ and Ctsk–/– mice. Ang-II infusion did not produce AAA in mice that were wild-type for ApoE, but it increased blood Ly6G+ neutrophils and Ly6G+CD11b+ activated neutrophils in Ctsk+/+ mice and minimized the differences in blood CD4+, CD4+CD8+, and CD4+CD25+ T cells between Ctsk+/+ and Ctsk–/– mice. Different experimental models therefore may yield different conclusions, although we have not tested whether CatL deficiency also will reduce Ang-II–induced AAA as it did aortic elastase perfusion-induced AAA (Figure 1B) or periaortic CaCl2 injury-induced AAA (Figure 6A).
CatL is a lysosomal cysteine protease that presumably resides in the late endosomes and lysosomes, which provide an acidic environment for optimal CatL activities.6 Our longstanding interest has been in how this intracellular protease degrades extracellular elastin in the aortic wall and leads to AAA expansion. Although we did not examine it in this study, seminal studies from Stephen J. Weiss and colleagues demonstrated by culturing human monocyte-derived macrophages with water-insoluble elastin fibers, that macrophages bind tightly onto extracellular elastin, followed by increased expression of macrophage vacuolar-type H+-ATPase and rapid acidification in the cell–elastin interface—thus forming sequestered and acidic environments into which CatL and other cathepsins (eg, CatS and CatK) are released from macrophages and deposited into this milieu.38,39 Aortic SMC may act in the same fashion as macrophages do during elastin lamina fragmentation, and this hypothesis merits further investigation. Another unsolved puzzle is that deficiency of one protease may affect the expression of others. Although absence of CatS did not affect cathepsin and MMP expression or activities in EC,40 CatL deficiency greatly reduced the expressions of most cathepsins and MMPs in MHEC (Figure 3D) and SMC (Figure 5B). We reported similar observations in chymase- and tryptase-deficient mast cells and monocytes.4,41 Absence of these serine proteases reduced significantly most cysteinyl cathepsins or MMPs in these inflammatory cells, although the exact mechanisms involved in these protease expression regulation remain unknown.
Together, our data from experimental AAA establish a selective and important role of CatL in mouse AAA formation. We hope that selective inhibition of CatL using pharmacological inhibitors will benefit patients with AAA, atherosclerosis, and other cardiovascular diseases. Validated biomarkers associated with these potential therapies are essential to assessing the efficacy of selective cathepsin inhibitors. The recent development of potent, highly selective, irreversible or reversible CatL inhibitors42–46 has allowed examination of the potential efficacy of these small molecule compounds in patients with AAA, or in experimental AAA models. Several serum biomarker candidates, such as peroxiredoxin-1,46 elastin peptide and plasmin-antiplasmin complex,47 D-dimer,48 and mast cell tryptase,41 have been examined in AAA patients and have correlated with AAA lesion sizes or growth rate. Selective inhibition of CatL may associate with changes in these biomarker candidates. Combination of these biomarker studies and efficacy tests of selective CatL inhibitors in humans and in experimental models, therefore, will be of common interest to the fields of cysteine protease cathepsins and cardiovascular diseases.
Acknowledgments
The authors thank Mrs Eugenia Shvartz for technical assistance and Ms Sara Karwacki for editorial assistance.
Sources of Funding
This study is supported by National Institutes of Health grants HL60942, HL81090, HL88547 (G.P.S.), HL56985 (P.L.), and by EIA award 0840118N from the American Heart Association (G.P.S.).
Footnotes
Jiusong Sun and Galina K. Sukhova contributed equally to the study.
Disclosures
None.
References
- 1.Hellenthal FA, Buurman WA, Wodzig WK, Schurink GW. Biomarkers of AAA progression. Part 1: extracellular matrix degeneration. Nat Rev Cardiol. 2009;6:464–474. doi: 10.1038/nrcardio.2009.80. [DOI] [PubMed] [Google Scholar]
- 2.Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, Ennis TL, Shapiro SD, Senior RM, Thompson RW. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105:1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun J, Zhang J, Lindholt JS, Sukhova GK, Liu J, He A, Abrink M, Pejler G, Stevens RL, Thompson RW, Ennis TL, Gurish MF, Libby P, Shi GP. Critical role of mast cell chymase in mouse abdominal aortic aneurysm formation. Circulation. 2009;120:973–982. doi: 10.1161/CIRCULATIONAHA.109.849679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng GG, Martin-McNulty B, Sukovich DA, Freay A, Halks-Miller M, Thinnes T, Loskutoff DJ, Carmeliet P, Dole WP, Wang YX. Urokinase-type plasminogen activator plays a critical role in angiotensin II-induced abdominal aortic aneurysm. Circ Res. 2003;92:510–517. doi: 10.1161/01.RES.0000061571.49375.E1. [DOI] [PubMed] [Google Scholar]
- 6.Chapman HA, Riese RJ, Shi GP. Emerging roles for cysteine proteases in human biology. Annu Rev Physiol. 1997;59:63–88. doi: 10.1146/annurev.physiol.59.1.63. [DOI] [PubMed] [Google Scholar]
- 7.Rizas KD, Ippagunta N, Tilson MD., III Immune cells and molecular mediators in the pathogenesis of the abdominal aortic aneurysm. Cardiol Rev. 2009;17:201–210. doi: 10.1097/CRD.0b013e3181b04698. [DOI] [PubMed] [Google Scholar]
- 8.Mäyränpää MI, Trosien JA, Fontaine V, Folkesson M, Kazi M, Eriksson P, Swedenborg J, Hedin U. Mast cells associate with neovessels in the media and adventitia of abdominal aortic aneurysms. Vasc Surg. 2009;50:388–395. doi: 10.1016/j.jvs.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 9.Sun J, Sukhova GK, Wolters PJ, Yang M, Kitamoto S, Libby P, MacFarlane LA, Mallen-St Clair J, Shi GP. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–724. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Sukhova GK, Yang JT, Sun J, Ma L, Ren A, Xu WH, Fu H, Dolganov GM, Hu C, Libby P, Shi GP. Cathepsin L expression and regulation in human abdominal aortic aneurysm, atherosclerosis, and vascular cells. Atherosclerosis. 2006;184:302–311. doi: 10.1016/j.atherosclerosis.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Shi GP, Sukhova GK, Grubb A, Ducharme A, Rhode LH, Lee RT, Ridker PM, Libby P, Chapman HA. Cystatin C deficiency in human atherosclerosis and aortic aneurysms. J Clin Invest. 1999;104:1191–1197. doi: 10.1172/JCI7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdul-Hussien H, Soekhoe RG, Weber E, von der Thüsen JH, Kleemann R, Mulder A, van Bockel JH, Hanemaaijer R, Lindeman JH. Collagen degradation in the abdominal aneurysm: a conspiracy of matrix metalloproteinase and cysteine collagenases. Am J Pathol. 2007;170:809–817. doi: 10.2353/ajpath.2007.060522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pagano MB, Bartoli MA, Ennis TL, Mao D, Simmons PM, Thompson RW, Pham CT. Critical role of dipeptidyl peptidase I in neutrophil recruitment during the development of experimental abdominal aortic aneurysms. Proc Natl Acad Sci U S A. 2007;104:2855–2860. doi: 10.1073/pnas.0606091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun J, Sukhova GK, Zhang J, Chen H, Sjöberg S, Libby P, Xia M, Xiong N, Gelb BD, Shi GP. Cathepsin K deficiency reduces elastase perfusion-induced abdominal aortin aneurysms in mice. Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.111.235002. epub ahead of print August 4, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitamoto S, Sukhova GK, Sun J, Yang M, Libby P, Love V, Duramad P, Sun C, Zhang Y, Yang X, Peters C, Shi GP. Cathepsin L deficiency reduces diet-induced atherosclerosis in low-density lipoprotein receptor-knockout mice. Circulation. 2007;115:2065–2075. doi: 10.1161/CIRCULATIONAHA.107.688523. [DOI] [PubMed] [Google Scholar]
- 16.Liapis CD, Paraskevas KI. The pivotal role of matrix metalloproteinases in the development of human abdominal aortic aneurysms. Vasc Med. 2003;8:267–271. doi: 10.1191/1358863x03vm504ra. [DOI] [PubMed] [Google Scholar]
- 17.Thompson RW, Liao S, Curci JA. Vascular smooth muscle cell apoptosis in abdominal aortic aneurysms. Coron Artery Dis. 1997;8:623–631. doi: 10.1097/00019501-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa T, Roth W, Wong P, Nelson A, Farr A, Deussing J, Villadangos JA, Ploegh H, Peters C, Rudensky AY. Cathepsin L: critical role in Ii degradation and CD4 T cell selection in the thymus. Science. 1998;280:450–453. doi: 10.1126/science.280.5362.450. [DOI] [PubMed] [Google Scholar]
- 19.Sukhova GK, Zhang Y, Pan JH, Wada Y, Yamamoto T, Naito M, Kodama T, Tsimikas S, Witztum JL, Lu ML, Sakara Y, Chin MT, Libby P, Shi GP. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;111:897–906. doi: 10.1172/JCI14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun J, Sukhova GK, Yang M, Wolters PJ, MacFarlane LA, Libby P, Sun C, Zhang Y, Liu J, Ennis TL, Knispel R, Xiong W, Thompson RW, Baxter BT, Shi GP. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J Clin Invest. 2007;117:3359–3368. doi: 10.1172/JCI31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim YC, Garcia-Cardena G, Allport JR, Zervoglos M, Connolly AJ, Gimbrone MA, Jr, Luscinskas FW. Heterogeneity of endothelial cells from different organ sites in T-cell subset recruitment. Am J Pathol. 2003;162:1591–1601. doi: 10.1016/S0002-9440(10)64293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang KC, Raymond WW, Lazarus SC, Caughey GH. Dog mastocytoma cells secrete a 92-kD gelatinase activated extracellularly by mast cell chymase. J Clin Invest. 1996;97:1589–1596. doi: 10.1172/JCI118583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abraham M, Karni A, Dembinsky A, Miller A, Gandhi R, Anderson D, Weiner HL. In vitro induction of regulatory T cells by anti-CD3 antibody in humans. J Autoimmun. 2008;30:21–28. doi: 10.1016/j.jaut.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang B, Sun J, Kitamoto S, Yang M, Grubb A, Chapman HA, Kalluri R, Shi GP. Cathepsin S controls angiogenesis and tumor growth via matrix-derived angiogenic factors. J Biol Chem. 2006;281:6020–6029. doi: 10.1074/jbc.M509134200. [DOI] [PubMed] [Google Scholar]
- 25.Tsai JC, Jain M, Hsieh CM, Lee WS, Yoshizumi M, Patterson C, Perrella MA, Cooke C, Wang H, Haber E, Schlegel R, Lee ME. Induction of apoptosis by pyrrolidinedithiocarbamate and N-acetylcysteine in vascular smooth muscle cells. J Biol Chem. 1996;271:3667–3670. [PubMed] [Google Scholar]
- 26.Sajjan U, Ganesan S, Comstock AT, Shim J, Wang Q, Nagarkar DR, Zhao Y, Goldsmith AM, Sonstein J, Linn MJ, Curtis JL, Hershenson MB. Elastase- and LPS-exposed mice display altered responses to rhinovirus infection. Am J Physiol Lung Cell Mol Physiol. 2009;297:L931–L944. doi: 10.1152/ajplung.00150.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sukhova GK, Shi GP, Simon DI, Chapman HA, Libby P. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest. 1998;102:576–583. doi: 10.1172/JCI181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang M, Zhang Y, Pan J, Sun J, Liu J, Libby P, Sukhova GK, Doria A, Katunuma N, Peroni OD, Guerre-Millo M, Kahn BB, Clement K, Shi GP. Cathepsin L activity controls adipogenesis and glucose tolerance. Nat Cell Biol. 2007;9:970–977. doi: 10.1038/ncb1623. [DOI] [PubMed] [Google Scholar]
- 29.Yang M, Sun J, Zhang T, Liu J, Zhang J, Shi MA, Darakhshan F, Guerre-Millo M, Clement K, Gelb BD, Dolgnov G, Shi GP. Deficiency and inhibition of cathepsin K reduce body weight gain and increase glucose metabolism in mice. Arterioscler Thromb Vasc Biol. 2008;28:2202–2208. doi: 10.1161/ATVBAHA.108.172320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi GP, Chapman HA, Bhairi SM, DeLeeuw C, Reddy VY, Weiss SJ. Molecular cloning of human cathepsin O, a novel endoproteinase and homologue of rabbit OC2. FEBS Lett. 1995;357:129–134. doi: 10.1016/0014-5793(94)01349-6. [DOI] [PubMed] [Google Scholar]
- 31.Puzer L, Barros NM, Paschoalin T, Hirata IY, Tanaka AS, Oliveira MC, Brömme D, Carmona AK. Cathepsin V, but not cathepsins L, B and K, may release angiostatin-like fragments from plasminogen. Biol Chem. 2008;389:195–200. doi: 10.1515/BC.2008.020. [DOI] [PubMed] [Google Scholar]
- 32.Chen W, Yang S, Abe Y, Li M, Wang Y, Shao J, Li E, Li YP. Novel pycnodysostosis mouse model uncovers cathepsin K function as a potential regulator of osteoclast apoptosis and senescence. Hum Mol Genet. 2007;16:410–423. doi: 10.1093/hmg/ddl474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu KF, Wu CL, Huang SC, Wu CM, Hsiao JR, Yo YT, Chen YH, Shiau AL, Chou CY. Cathepsin L mediates resveratrol-induced autophagy and apoptotic cell death in cervical cancer cells. Autophagy. 2009;5:451–460. doi: 10.4161/auto.5.4.7666. [DOI] [PubMed] [Google Scholar]
- 34.Wille A, Gerber A, Heimburg A, Reisenauer A, Peters C, Saftig P, Reinheckel T, Welte T, Bühling F. Cathepsin L is involved in cathepsin D processing and regulation of apoptosis in A549 human lung epithelial cells. Biol Chem. 2004;385:665–670. doi: 10.1515/BC.2004.082. [DOI] [PubMed] [Google Scholar]
- 35.Levicar N, Dewey RA, Daley E, Bates TE, Davies D, Kos J, Pilkington GJ, Lah TT. Selective suppression of cathepsin L by antisense cDNA impairs human brain tumor cell invasion in vitro and promotes apoptosis. Cancer Gene Ther. 2003;10:141–151. doi: 10.1038/sj.cgt.7700546. [DOI] [PubMed] [Google Scholar]
- 36.Zajc I, Hreljac I, Lah T. Cathepsin L affects apoptosis of glioblastoma cells: a potential implication in the design of cancer therapeutics. Anticancer Res. 2006;26:3357–3364. [PubMed] [Google Scholar]
- 37.Bai L, Beckers L, Wijnands E, Lutgens SP, Herías MV, Saftig P, Daemen MJ, Cleutjens K, Lutgens E, Biessen EA, Heeneman S. Cathepsin K gene disruption does not affect murine aneurysm formation. Atherosclerosis. 2010;209:96–103. doi: 10.1016/j.atherosclerosis.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Reddy VY, Zhang QY, Weiss SJ. Pericellular mobilization of the tissue-destructive cysteine proteinases, cathepsins B, L, and S, by human monocyte-derived macrophages. Proc Natl Acad Sci U S A. 1995;92:3849–3853. doi: 10.1073/pnas.92.9.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Punturieri A, Filippov S, Allen E, Caras I, Murray R, Reddy V, Weiss SJ. Regulation of elastinolytic cysteine proteinase activity in normal and cathepsin K-deficient human macrophages. J Exp Med. 2000;192:789–799. doi: 10.1084/jem.192.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi GP, Sukhova GK, Kuzuya M, Ye Q, Du J, Zhang Y, Pan JH, Lu ML, Cheng XW, Iguchi A, Perrey S, Lee AM, Chapman HA, Libby P. Deficiency of the cysteine protease cathepsin S impairs microvessel growth. Circ Res. 2003;92:493–500. doi: 10.1161/01.RES.0000060485.20318.96. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Sun J, Lindholt JS, Sukhova GK, Sinnamon M, Stevens RL, Adachi R, Libby P, Thompson RW, Shi GP. Mast cell tryptase deficiency attenuates mouse abdominal aortic aneurysm formation. Circ Res. 2011;108:1316–1327. doi: 10.1161/CIRCRESAHA.111.243758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.James IE, Marquis RW, Blake SM, Hwang SM, Gress CJ, Ru Y, Zembryki D, Yamashita DS, McQueney MS, Tomaszek TA, Oh HJ, Gowen M, Veber DF, Lark MW. Potent and selective cathepsin L inhibitors do not inhibit human osteoclast resorption in vitro. J Biol Chem. 2001;276:11507–11511. doi: 10.1074/jbc.M010684200. [DOI] [PubMed] [Google Scholar]
- 43.Chowdhury SF, Sivaraman J, Wang J, Devanathan G, Lachance P, Qi H, Ménard R, Lefebvre J, Konishi Y, Cygler M, Sulea T, Purisima EO. Design of noncovalent inhibitors of human cathepsin L. From the 96-residue proregion to optimized tripeptides. J Med Chem. 2002;45:5321–5329. doi: 10.1021/jm020238t. [DOI] [PubMed] [Google Scholar]
- 44.Shenoy RT, Chowdhury SF, Kumar S, Joseph L, Purisima EO, Sivaraman J. A combined crystallographic and molecular dynamics study of cathepsin L retrobinding inhibitors. J Med Chem. 2009;52:6335–6346. doi: 10.1021/jm900596y. [DOI] [PubMed] [Google Scholar]
- 45.Shah PP, Myers MC, Beavers MP, Purvis JE, Jing H, Grieser HJ, Sharlow ER, Napper AD, Huryn DM, Cooperman BS, Smith AB, III, Diamond SL. Kinetic characterization and molecular docking of a novel, potent, and selective slow-binding inhibitor of human cathepsin L. Mol Pharmacol. 2008;74:34–41. doi: 10.1124/mol.108.046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez-Pinna R, Ramos-Mozo P, Madrigal-Matute J, Blanco-Colio LM, Lopez JA, Calvo E, Camafeita E, Lindholt JS, Meilhac O, Delbosc S, Michel JB, de Ceniga MV, Egido J, Martin-Ventura JL. Identification of peroxiredoxin-1 as a novel biomarker of abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2011;31:935–943. doi: 10.1161/ATVBAHA.110.214429. [DOI] [PubMed] [Google Scholar]
- 47.Urbonavicius S, Urbonaviciene G, Honoré B, Henneberg EW, Vorum H, Lindholt JS. Potential circulating biomarkers for abdominal aortic aneurysm expansion and rupture—a systematic review. Eur J Vasc Endovasc Surg. 2008;36:273–280. doi: 10.1016/j.ejvs.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 48.Takagi H, Manabe H, Kawai N, Goto S, Umemoto T. Plasma fibrinogen and D-dimer concentrations are associated with the presence of abdominal aortic aneurysm: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2009;38:273–277. doi: 10.1016/j.ejvs.2009.05.013. [DOI] [PubMed] [Google Scholar]