Abstract
Objective
This study examined the use of a Video Doctor plus provider cueing to promote provider advice and smoking cessation outcomes in pregnancy.
Design
A randomized clinical trial was conducted from 2006 to 2008.
Setting
Five, community, prenatal clinics in the San Francisco Bay Area of the United States.
Participants
410 pregnant patients completed screening for behavioral risks including tobacco use in the past 30 days. Pregnant smokers (N = 42) were randomized regardless of their intention to quit smoking.
Methods
Participants were assigned to either usual care or intervention. Intervention participants received 15-minute Video Doctor sessions plus provider cueing, at baseline and 1 month, prior to their routine prenatal visit. The Video Doctor delivered interactive tailored messages, an educational worksheet for participants and a cueing sheet for providers.
Main outcome measures
Receipt of advice from the provider and 30-day smoking abstinence, both by self report.
Results
Intervention participants were more likely to receive provider advice on tobacco use at both prenatal visits during the intervention period (61% vs 16%, p = 0.003). The intervention yielded a significantly greater decrease in the number of days smoked and in cigarettes smoked per day. The 30-day abstinence rate at 2 months post baseline was 2.5 times greater in the intervention group; the difference was not statistically significant (26.1% vs 10.5%, p = 0.12).
Conclusions
The Video Doctor plus provider cueing is an efficacious adjunct to routine prenatal care by promoting provider advice and smoking reduction among pregnant smokers.
Keywords: smoking cessation, pregnancy, prenatal care, patient education, randomized controlled trial
Introduction
Health burdens attributed to cigarette smoking and exposure to tobacco smoke during pregnancy are well documented (1–3). The Centers for Disease Control and Prevention (CDC) estimated 776 infants died annually from 2000 to 2004 due to smoking during pregnancy (4). The Pregnancy Risk Assessment Monitoring System (PRAMS) collected annual data from 31 states and found a significant decline in smoking prevalence among pregnant women from 15.2% in 2000 to 13.8% in 2005). Despite the decline, the CDC concluded that current tobacco-control efforts targeting pregnant women are ineffective in reaching the Healthy People 2010 national health objective of reducing smoking prevalence to 1% among pregnant women (5).
According to PRAMS and the California Maternal and Infant Health Assessment (MIHA) Survey, women’s smoking prevalence rates by state in 2006 ranged from a low of 7.7% in California (6) to a high of 35% in West Virginia (7). It was estimated that 23% of pregnant smokers spontaneously quit smoking before the first prenatal care visit (8). Many pregnant smokers quit smoking during pregnancy, ranging from a high of 62.3% in New Jersey to a low of 29.9% in West Virginia (7). Prenatal care represents an opportune time to assist pregnant smokers to quit smoking.
The 2008 Clinical Practice Guidelines for treating tobacco use and dependence recommends that clinicians should offer effective tobacco dependence interventions to pregnant smokers throughout the course of pregnancy (9). Currently, it is not known how many pregnant smokers are being offered the recommended smoking cessation interventions during prenatal care (5). Research has reported approximately 30% to 66% of prenatal care providers provided counseling or referrals to smoking cessation programs (10–13). In the general population of non-pregnant smokers, it is also well documented that only a portion of all smokers receive advice to quit from their physicians at their medical visits, ranging from 12% from clinical records (14) to 61% from smokers’ self-report (15), to 75% from providers’ self-report (16). The rates of providing smoking cessation counseling in the prenatal and general medical care settings appear to be similar.
There has been a call for systematic smoking cessation interventions in prenatal care settings given that only a portion of patients receive advice to quit smoking. We previously reported partial baseline data collected for a randomized trial of a prenatal Video Doctor intervention (13) that supported and simplified prenatal providers’ efforts to screen and counsel their patients about behavioral risks. The intervention increased provider-patient discussion of tobacco use and intimate partner violence. Based on a sample of 34 pregnant smokers, the trial showed that 100% of patients randomized to the intervention group (in which prenatal providers received a cueing sheet) were advised by the provider to quit smoking compared with 60% of patients in the usual care group. The purpose of this study was to examine the efficacy of the Video Doctor plus provider cueing intervention in promoting smoking cessation in prenatal care settings by a) increasing receipt of provider advice on smoking cessation during a 1-month intervention period; and b) increasing smoking cessation outcomes.
Methods
This study is part of the Health in Pregnancy (HIP) Study, a randomized, controlled trial to determine if a brief, interactive, multimedia intervention for pregnant women can reduce their risks related to smoking, alcohol, illicit drug use, and intimate partner violence (IPV). The current report focuses on the smoking cessation intervention and outcomes among 42 participants enrolled in the HIP program who reported smoking cigarettes in the past 30 days. Our prior report included the baseline data of the first 34 of these participants who self-reported tobacco use on baseline risk assessment (13).
Procedures
Details of the HIP study and recruitment were previously reported (13). HIP was launched in five prenatal clinics in the San Francisco Bay Area in June 2006. Participant recruitment was completed in December 2007. Participants were English-speaking women 18 years or older, less than 26-weeks pregnant, and receiving prenatal care at one of the participating clinics. Prior to a regularly scheduled prenatal appointment, participants completed a risk assessment using a laptop computer via a low-literacy computerized interview with audio voiceover. The computer program collected socio-demographic information and pregnancy history; it screened for tobacco, alcohol, drug use, and IPV. Smoking within the previous 30 days was assessed by five questions adapted from the California Adult Tobacco Survey (17). These included; i) smoking status, ii) number of days smoked cigarettes in the past 30 days, iii) number of cigarettes smoked on a normal day when smoking occurred, iv) the greatest number of cigarettes smoked during a single day, and v) intention to quit smoking. Women reporting risks were stratified by risk combination and randomly assigned by the computer to intervention or usual care groups. All participants were reassessed using a similar computerized interview at 1 and 2 months following the initial (baseline) assessment. At both baseline and 1-month assessments, in addition to completing a computerized assessment prior to the prenatal appointment, participants also completed a brief post-visit interview to report whether tobacco use or other reported behavioral risks had been discussed with their provider. All participants received a gift card to a grocery or department store as compensation for completing an assessment in the amounts of $30, $40, and $50 at baseline, 1-, and 2-month follow-ups, respectively. Study procedures were approved by the University of California San Francisco’s Committee on Human Research and the institutional review boards responsible for each site.
Intervention group
Participants assigned to the intervention group received the Video Doctor plus provider cueing intervention. The Video Doctor model was selected by the CDC in 2008 as one of the best evidence-based practices in reducing HIV risks (18). Video Doctor is a multimedia interactive intervention delivered on a laptop computer via a secure internet connection. An actor-portrayed Video Doctor delivered interactive risk-reduction messages, designed to simulate an ideal discussion with a prenatal health care provider who provided non-judgmental counseling following several key principles of Motivational Interviewing (19, 20). Using a library of digital video clips, extensive branching logic, and participant input, the program tailored messages to the participant’s risk profile and intention to change. At the conclusion of each intervention session, the program automatically printed two documents: 1) a cueing sheet for providers, which offered a summary of the patient’s risk profile and suggested risk-reduction counseling statements, and 2) an educational worksheet for participants with questions for self-reflection, harm reduction tips, and local resources. The cueing sheet was placed in the patient’s medical record for the provider’s use during the prenatal appointment. All providers received a brief orientation to the use of the cueing sheets. Figure 1 shows a graphical illustration of the intervention components.
Figure 1.
Intervention components of the Video Doctor plus provider cueing
Usual care group
Participants in the usual care group did not interact with the Video Doctor and the program did not produce a cueing sheet or educational worksheet. Following completion of the assessment, participants assigned to the usual care group proceeded to their prenatal appointment and received the clinic’s usual care. Behavioral counseling for the usual care group was determined by the clinician.
Outcome Measures
Receipt of provider advice was assessed during a post-visit interview immediately after the prenatal visit at baseline and 1-month follow-up. Smoking cessation outcomes were assessed at 2-month follow-up by the following: a) self-reported 30-day abstinence, b) decrease from baseline in number of days smoked in the past 30 days, and c) decrease from baseline in the number of cigarettes smoked on typical day when smoking occurred.
Data analyses
Data analyses were conducted in August 2009. We compared intervention and usual care groups using the chi-square test for dichotomous variables such as smoking abstinence, and the Wilcoxon rank-sum test for discrete count variables such as receipt of provider advice, days smoked and cigarettes per day. We assessed sensitivity of the results to losses to follow-up by assuming missing responses on receipt of advice were negative for receiving advice and missing responses on abstinence were negative for abstinence (i.e. the patient continued smoking). To examine the effect of provider advice on smoking abstinence, we performed a separate logistic regression analysis of the relationship between the “dose” of provider advice (at 0, 1, or 2 visits) and smoking abstinence.
Results
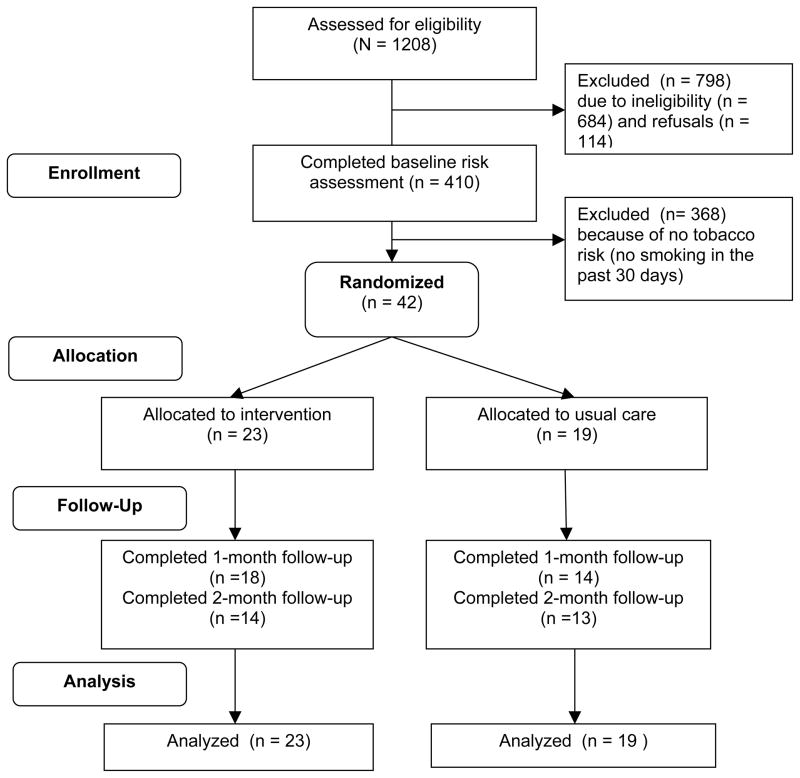
Out of 410 eligible pregnant women who completed baseline risk assessments, 42 (10.2%) reported smoking cigarettes in the past 30 days and were randomized (Figure 2). Participants assigned to the intervention group (N=23) and the usual care group (N=19) were similar in demographics, pregnancy history, risk profiles, and baseline smoking behaviors (Table 1). A majority (64.3%) of the participants in the study indicated they had reduced smoking since they became pregnant; 66.7% were thinking of quitting smoking in the next 30 days at the baseline assessment. About half (54.8%) smoked daily, and 24% smoked at least 10 cigarettes a day on a typical day when they smoked. Complete data about receipt of provider advice were obtained on 32 (76.2%) participants, and complete data on abstinence were obtained on 27 (64.3%) participants. There was no statistical difference in data completion rates between the two treatment groups (p = 0.43).
Figure 2.
Flowchart of study participants
Table 1.
Baseline characteristics of study participants
| Intervention (n = 23) | Usual Care (n = 19) | |
|---|---|---|
| Age, mean, y (SD) | 27.5 (6.7) | 26.8 (5.3) |
| Race/ethnicity, n (%) | ||
| Hispanic/Latino | 6 (26.1) | 3 (15.8) |
| Black or African-American | 8 (34.8) | 7 (36.8) |
| White | 4 (17.4) | 6 (31.6) |
| Other or multiple races | 5 (21.7) | 3 (15.8) |
| Educational attainment, n (%) | ||
| Less than high school diploma | 6 (26.1) | 4 (21.1) |
| High school diploma or GED | 8 (34.8) | 10 (52.6) |
| Some College | 9 (39.1) | 4 (21.1) |
| College Degree | 0 (0.0) | 1 (5.3) |
| Marital status, n (%) | ||
| Never married | 6 (26.1) | 11 (57.9) |
| Currently married | 11 (47.8) | 5 (26.3) |
| Formerly married | 6 (26.1) | 3 (15.8) |
| Pregnancy history, n (%) | ||
| Previously pregnant | 20 (87.0) | 16 (84.2) |
| Gestational weeks, mean (SD) | 18.2 (5.6) | 20.6 (5.1) |
| Risk profile,a n (%) | ||
| Tobacco use only with no other risks | 13 (56.5) | 12 (63.2) |
| Alcohol use | 2 (8.7) | 0 (0.0) |
| Illicit drug use | 3 (13.0) | 1 (5.3) |
| Intimate partner violence | 7 (30.4) | 7 (36.8) |
| Smoking status | ||
| Smoked about the same amount as before pregnancy | 4 (17.4) | 0 (0.0) |
| Have cut down since pregnancy | 12 (52.2) | 15 (78.9) |
| Smoked cigarette once in a while | 7 (30.4) | 4 (21.1) |
| Smoking behaviors in the past 30 days, mean (range) | ||
| Days Smoked | 23.5 (5–30) | 21.6 (4–30) |
| Cigarettes smoked per day | 6.8 (1–20) | 6.7 (1–20) |
| Maximum cigarettes smoked per day | 10.1 (1–20) | 10.4 (1–20) |
| Intention to quit tobacco | ||
| within the next 30 days | 17 (73.9) | 11 (57.9) |
| within the next 6 months | 2 (8.7) | 4 (21.1) |
| no intention to quit | 4 (17.4) | 4 (21.1) |
Not mutually exclusive
GED, general educational development test; SD, standard deviation
% may not add up to 100% because of round up errors
Receipt of provider advice
For participants who completed both post-visit interviews and reported receipt of provider advice on tobacco use, intervention participants were significantly more likely to be advised by their prenatal providers at both visits than those in usual care (77.8% vs 21.4%, p = 0.002). Similar results were obtained in a sensitivity analysis that imputed missing data as indicative of failure to receive advice at the 1-month follow-up (Table 2).
Table 2.
Outcomes by treatment group
| Observed a | Sensitivity Analysis b | |||||
|---|---|---|---|---|---|---|
| Intervention | Usual Care | p-value | Intervention | Usual Care | p-value | |
| Receipt of provider advice | (n = 18) | (n = 14) | 0.001c | (n = 23) | (n = 19) | 0.003 c |
| None (no advice at both visits) | 0 (0.0%) | 3 (21.4%) | 1 (4.6%) | 4 (21.0%) | ||
| Once (advice at one of the visits) | 4 (22.2%) | 8 (57.2%) | 8 (34.8%) | 12 (63.2%) | ||
| Twice (advice at both visits) | 14 (77.8%) | 3 (21.4%) | 14 (60.9%) | 3 (15.8%) | ||
| Self-reported 30-day abstinence | (n = 14) 6 (42.5%) |
(n = 13) 2 (15.4%) |
0.118 d | (n = 23) 6 (26.1%) |
(n = 19) 2 (10.5%) |
0.201d |
| Mean decrease in days smoked | (n = 14) 14.3 |
(n = 13) 1.1 |
0.004c | -- | -- | |
| Mean decrease in cigarettes smoked per day | (n = 13) 3.9 |
(n = 13) -0.1 |
0.050 c | -- | -- | |
Observed rates were computed based on participants who provided data.
Rates calculated with missing data imputed as no advice or no abstinence correspondingly.
Wilcoxon rank-sum test
Pearson chi-square test
Smoking cessation outcomes
For participants who completed the 2-month follow-up assessing smoking behaviors, 6 out of 14 (42.5%) in the intervention group reported 30-day abstinence compared with 2 out of 13 (15.5%) in the usual care group. This relative abstinence rate of 2.8 (95% CI 0.7–11.4) was not statistically significant (Table 2). Imputing missing abstinence data as indicative of smoking, the relative abstinence rate was 2.5 (95% CI 0.6 – 10.9). The Video Doctor plus provider cueing group showed statistically significant decreases in number of days smoked and number of cigarettes per day from baseline to 2-month follow up (Table 2). We conducted a separate logistic regression analysis of the effect of receiving provider advice on 30-day smoking abstinence. Including all participants (N = 42) in the analysis, we imputed missing abstinence data as indicative of smoking and missing data on receipt of advice as failure to receive advice. The abstinence rates for receiving provider advice at zero, one, and two prenatal visits were 0.0%, 10.0% and 35.3%, respectively. Results suggested a significant association between the number of times provider advice was received and abstinence (odds ratio = 5.6; 95% CI 1.1 – 28.9; p = 0.041).
Discussion
Given the serious consequences caused by smoking during pregnancy to both maternal health and to the health of the unborn fetus, smoking cessation interventions should be implemented in all prenatal care settings (9, 21). Advice from prenatal providers is regarded as a key element for effective interventions with pregnant smokers (9). Current findings show that the implementation of two 15-minute automated Video Doctor sessions plus provider cueing prior to routine prenatal visits yielded a significant increase in patients’ report of provider advice on tobacco use and a significant reduction in smoking among pregnant smokers at two months. Findings from this randomized trial support the use of the Video Doctor plus provider cueing as a promising solution to overcome some of the common barriers to consistently and effectively providing tobacco intervention to pregnant smokers.
Research evidence supports numerous benefits of stopping smoking during pregnancy (9, 21). A recent Cochrane review of 56 randomized trials with over 20,000 pregnant women demonstrated a nearly 15% reduction in preterm birth and low birth-weight observed among women who received smoking interventions (21). Despite research evidence and clinical guideline recommendations supporting the implementation of smoking cessation interventions as a routine part of prenatal care (8, 21), findings from the current study based on observed data that sampled two prenatal visits approximately one month apart, show that under usual care 79% of pregnant smokers did not receive advice from their providers at every routine prenatal visit. One in five (21%) pregnant smokers received no advice at any of their visits.
The most commonly described barriers to providing smoking cessation intervention during prenatal care include competing demands, time constraints, insufficient personnel support, insufficient training or skills, and clinicians’ perception of limited efficacy of existing intervention for pregnant smokers (10, 11, 21–23). This study showed that provider cueing, in the form of a simple cueing sheet containing a reminder and suggested counseling statements being placed in the medical record prior to a prenatal appointment, was effective in increasing provider advice on tobacco use during prenatal visits. The cueing sheet directly addresses some of the common barriers experienced at the provider level, such as time constraints, training background, or perceived efficacy. More importantly, the Video Doctor plus provider cueing is effective in yielding provider advice to be delivered routinely at each prenatal visit, resulting in 78% of the patients receiving advice from their providers at both visits as compared with only 21% of patients advised on their tobacco use at both visits in usual care. Research has suggested that providers were more likely to offer smoking cessation advice if they perceived their patients as more ready to quit smoking or more receptive of their assistance (24); therefore provider’s perception of patients’ low readiness is another barrier that may lead to failure to provide advice on a routine basis. Almost one in five participants in the current study indicated no intention to quit smoking at the time of enrollment; yet the Video Doctor plus provider cueing approach tested in the study has also demonstrated an advantage of screening, cueing provider, and delivering direct risk-reduction messages in a consistent and routine manner to all pregnant smokers including those smokers who were less ready to quit smoking. Our observed data also showed that 100% of the participants receiving the Video Doctor plus provider cueing intervention reported receipt of provider advice in at least one of the two prenatal visits.
In addition to effectively increasing routine provider advice in prenatal care settings, the brief Video Doctor plus provider cueing intervention showed a significant reduction in both the amount and the number of days smoked at 2 months after initiating the intervention. Meta-analyses of smoking cessation trials that used more intensive approaches showed an approximately 6% increase in smoking abstinence rates during pregnancy (8, 21). Using a conservative estimation of the treatment effect by coding missing data as smoking (or non-abstinent), we have observed a net increase of nearly 16% in 30-day self-reported abstinence in the intervention group. The difference, however, was not statistically significant. In comparison to the abstinence rate estimated at 13.3% (95% CI = 9.0 – 19.4) from psychosocial interventions with pregnant smokers based on meta-analysis of 8 selected randomized trials published between 1993 to 2006 (9), the abstinence rate at 26% with missing data imputed as continued smoking in the Video Doctor plus provider cueing intervention is promising and warrants further research.
Our analysis of the relationship between provider advice and smoking cessation showed an association between the “dose” of provider advice and abstinence. Taken together, these observations supported the use of the Video Doctor plus Provider Cueing in prenatal care setting for both increasing provider advice as well as in promoting smoking cessation outcomes.
Researchers have suggested that women who continue to smoke throughout pregnancy under the current tobacco control environment might reflect a subgroup of women who are more socially disadvantaged, have less access to resources, are more addicted to tobacco, and have multiple comorbidities or risk factors (5, 12, 25–27). For example, one study found the experience of socioeconomical stress was significantly associated with continued smoking in pregnancy (25). Research also has suggested depression was associated with continued smoking during pregnancy (28, 29). Our study did not assess mental health comorbidities such as depression; however, half of the study sample was screened positive for multiple behavioral risks, and one in three reported intimate partner violence. The Video Doctor plus provider cueing intervention tested in this study screened and delivered risk-reduction messages for one to multiple behavioral risks; the observed findings based on two brief intervention sessions are encouraging especially in the context of working with women who have multiple risk factors. The results demonstrate the feasibility of simultaneously addressing these risks in prenatal care settings using this brief intervention approach.
There are several limitations of the study that should be taken into consideration. First, tobacco use data were based on self-report without biochemical verification. Previous research has supported the use of computerized assessment, as employed by the current study, in eliciting greater disclosure of sensitive health-risk information than traditional assessment methods (30). In addition, this study used multiple-choice question format to assess smoking status, which was found to promote self-disclosure rates in tobacco use among pregnant women (9). Second, the intervention tested was brief, having been delivered prior to two routine prenatal visits approximately 1 month apart. Similarly, outcomes were observed in a short window at 2 months after baseline. The short study timeline did not allow measurements of longer term outcomes throughout pregnancy and may not allow a sufficient time interval post treatment to observe changes, particularly among participants who were less ready to quit smoking at the time of enrollment. Third, the losses to follow-up were substantial. The study retained about two thirds (64%) of the participants at 2 months, although there was no differential attrition between treatment groups and the results obtained were similar with sensitivity analyses imputing missing data as negative outcomes. Lastly, the findings were based on a small sample of pregnant smokers. Replication of the findings using a larger sample will be needed.
Despite these limitations, this study employed a proactive recruitment approach which involved screening for multiple behavioral risk factors of more than 400 eligible pregnant women, across five prenatal care clinics and readiness to quit smoking was not a requirement to be included in the study. The smoking prevalence observed in the current study (10.2%) was comparable to that reported by the State-wide MIHA data of San Francisco (7.8%; 95% CI: 5.7 – 9.8) (6), which supported the representativeness of the study sample in closely resembling the low-income pregnant patient populations that present at community clinics in metropolitan areas. Prenatal care is an opportune moment to effectively address tobacco use among pregnant smokers (23, 24). The current study demonstrated the feasibility and efficacy of implementing a brief, multi-media Video Doctor plus provider cueing intervention in prenatal care settings. The intervention supports and simplifies prenatal providers’ efforts to screen and counsel their patients about tobacco use risks, and resulted in increased provider advice and improved smoking reduction among pregnant smokers.
Responding to calls for smoking cessation intervention for pregnant women, the Health in Pregnancy program was designed to support prenatal providers’ smoking cessation efforts with minimal additional burden. The Health in Pregnancy Video Doctor plus provider cueing achieved important increases in the provision of smoking cessation advice among prenatal providers and reductions in smoking among pregnant women. Given the challenges of changing behaviors, and the health risks of prenatal smoking to mother and child, our results are notable. Video Doctor plus provider cueing is an efficacious adjunct to routine prenatal care with the capacity to have important clinical and public health impact.
Acknowledgments
Michelle Derman-Berger, Angelina Elliot, Alice Graham, and Huy Ngo served as Research Assistants, recruiting participants and conducting data collection at the clinic sites. Paul Gilbert and Rebecca Jackson, MD helped with development and implementation of the study in the field. The Health in Pregnancy study was funded by a grant from the U.S. DHHS National Institute on Drug Abuse (R01 DA 15597). The preparation of this manuscript was in part supported by a NIDA center grant (P50 DA 009253).
Footnotes
Disclosure of interests
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Center for Disease Control and Prevention. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services; 2004. [PubMed] [Google Scholar]
- 2.Center for Disease Control and Prevention. The Health Cconsequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services; 2006. [PubMed] [Google Scholar]
- 3.Roelands J, Jamison MG, Lyerly AD, James AH. Consequences of smoking during pregnancy on maternal health. J Womens Health. 2009;18:867–72. doi: 10.1089/jwh.2008.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Center for Disease Control and Prevention. Smoking-attributable mortality, years of potential life lost, and productivity losses - United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226–8. [PubMed] [Google Scholar]
- 5.Tong VT, Jones JR, Dietz PM, D'Angelo D, Bombard JM. Trends in smoking before, during, and after pregnancy - Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 31 sites, 2000–2005. MMWR Surveill Summ. 2009;58:1–29. [PubMed] [Google Scholar]
- 6.California Department of Public Health. Statewide Tables from the 2006 Maternal and Infant Health Assessment (MIHA) Survey: Table B1. Smoking and Alcohol Use, 2006 (Updated July 2008) 2008 [Excel] Available from: http://www.cdph.ca.gov/data/surveys/Pages/StatewideTablesfromthe2006MaternalandInfantHealthAssessment(MIHA)survey.aspx.
- 7.Pregnancy Risk Assessment Monitoring System (PRAMS) CPONDER - CDC's PRMAS On-line Data for Epidemiologic Research, Data for all states for - 2006 Tobacco Use. [database on the Internet]2008 [cited 08/05/2009] Available from: http://apps.nccd.cdc.gov/cPONDER/
- 8.Kim SY, England LJ, Kendrick JS, Dietz PM, Callaghan WM. The contribution of clinic-based interventions to reduce prenatal smoking prevalence among US women. Am J Public Health. 2009;99:893–8. doi: 10.2105/AJPH.2008.144485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiore MC, Jaen CR, Baker T, Bailey WC, Benowitz NL, Curry SJ, et al. Clinical Practice Guideline. Vol. 2008. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; May, 2008. Treating Tobacco Use and Dependence: 2008 Update. [Google Scholar]
- 10.Jordan TR, Dake JR, Price JH. Best practices for smoking cessation in pregnancy: do obstetrician/gynecologists use them in practice? J Womens Health. 2006;15:400–41. doi: 10.1089/jwh.2006.15.400. [DOI] [PubMed] [Google Scholar]
- 11.Hartmann KE, Wechter ME, Payne P, Salisbury K, Jackson RD, Melvin CL. Best practice smoking cessation intervention and resource needs of prenatal care providers. Obstet Gynecol. 2007;110:765–70. doi: 10.1097/01.AOG.0000280572.18234.96. [DOI] [PubMed] [Google Scholar]
- 12.Tong VT, England LJ, Dietz PM, Asare LA. Smoking patterns and use of cessation interventions during pregnancy. Am J Prev Med. 2008;35:327–33. doi: 10.1016/j.amepre.2008.06.033. [DOI] [PubMed] [Google Scholar]
- 13.Calderon SH, Gilbert P, Jackson R, Kohn MA, Gerbert B. Cueing prenatal providers effects on discussions of intimate partner violence. Am J Prev Med. 2008;34:134–7. doi: 10.1016/j.amepre.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bentz CJ, Bayley KB, Bonin KE, Fleming L, Hollis JF, Hunt JS, et al. Provider feedback to improve 5A's tobacco cessation in primary care: A cluster randomized clinical trial. Nicotine Tob Res. 2007;9:341–9. doi: 10.1080/14622200701188828. [DOI] [PubMed] [Google Scholar]
- 15.Cokkinides VE, Halpern MT, Barbeau EM, Ward E, Thun MJ. Racial and ethnic disparities in smoking-cessation interventions: Analysis of the 2005 National Health Interview Survey. Am J Prev Med. 2008;34:404–12. doi: 10.1016/j.amepre.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Schnoll RA, Rukstalis M, Wileyto EP, Shields AE. Smoking cessation treatment by primary care physicians: An update and call for training. Am J Prev Med. 2006;31:233–9. doi: 10.1016/j.amepre.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Pierce JP, White MM, Gilpin EA, Berry CC, Maklan DM, Cross J, et al. California Tobacco Survey (CTS) California Department of Health Services; UC San Diego: 1999. [Google Scholar]
- 18.Kuehn BM. Reducing HIV Risks. JAMA. 2008;299:2847. [Google Scholar]
- 19.Rollnick S, Miller W. What is motivational interviewing? Behav Cognitive Psychother. 1995;23:325–34. doi: 10.1017/S1352465809005128. [DOI] [PubMed] [Google Scholar]
- 20.Rollnick S, Miller WR, Butler CC. Motivational Interviewing in Health Care. New York: Guilford Press; 2008. [Google Scholar]
- 21.Lumley J, Chamberlain C, Dowswell T, Oliver S, Oakley L, Watson L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2009:CD001055. doi: 10.1002/14651858.CD001055.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang JC, Dado D, Frankel RM, Rodriguez KL, Zickmund S, Ling BS, et al. When pregnant patients disclose substance use: Missed opportunities for behavioral change counseling. Patient Educ Couns. 2008;72:394–401. doi: 10.1016/j.pec.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herzig K, Danley D, Jackson R, Petersen R, Chamberlain L, Gerbert B. Seizing the 9-month moment: addressing behavioral risks in prenatal patients. Patient Educ Couns. 2006;61:228–35. doi: 10.1016/j.pec.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Flocke SA, Kelly R, Highland J. Initiation of health behavior discussions during primary care outpatient visits. Patient Educ Couns. 2009;75:214–9. doi: 10.1016/j.pec.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weaver K, Campbell R, Mermelstein R, Wakschlag L. Pregnancy smoking in context: The influence of multiple levels of stress. Nicotine Tob Res. 2008;10:1065–73. doi: 10.1080/14622200802087564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hannover W, Thyrian JR, Ebner A, Roske K, Grempler J, Kuhl R, et al. Smoking during pregnancy and postpartum: Smoking rates and intention to quit smoking or resume after pregnancy. J Womens Health. 2008;17:631–40. doi: 10.1089/jwh.2007.0419. [DOI] [PubMed] [Google Scholar]
- 27.Ebert LM, Fahy K. Why do women continue to smoke in pregnancy? Women Birth. 2007;20:161–8. doi: 10.1016/j.wombi.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Blalock JA, Robinson JD, Wetter DW, Cinciripini PM. Relationship of DSM-IV-based depressive disorders to smoking cessation and smoking reduction in pregnant smokers. Am J Addict. 2006;15:268–77. doi: 10.1080/10550490600754309. [DOI] [PubMed] [Google Scholar]
- 29.Linares Scott TJ, Heil SH, Higgins ST, Badger GJ, Bernstein IM. Depressive symptoms predict smoking status among pregnant women. Addict Behav. 2009;34:705–8. doi: 10.1016/j.addbeh.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerbert B, Bronstone A, Pantilat S, McPhee S, Allerton M, Moe J. When asked, patients tell: Disclosure of sensitive health-risk behaviors. Med Care. 1999;37:104–11. doi: 10.1097/00005650-199901000-00014. [DOI] [PubMed] [Google Scholar]