Abstract
Background
Although gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) exhibit widely divergent behavior, limited biological information, apart from Ki67, is available to characterize malignancy. Identification of alternative biomarkers is therefore a key unmet need. Given the role of Internexin-alpha (INA)in neuronal development, we assessed its function in neuroendocrine cell systems and the clinical implications of its expression as a GEP-NEN biomarker.
Methods
Functional assays were undertaken to investigate the mechanistic role of INA in the pancreatic BON cell-line. Expression levels of INA were investigated in 50 pancreatic NENs (43 primaries,7 metastases), 43 small intestinal NENs (25 primaries, 18 metastases), normal pancreas (10)and SI-mucosa (16), normal EC cells (9), mouse xenografts (4) and NEN cell lines (6)by qPCR, western blot and immunostaining.
Results
In BON cells, decreased INA mRNA and protein were associated with inhibition of both proliferation and MAPK signaling. INA was not expressed in normal neuroendocrine cells but was over-expressed (2 to 42-fold) in NEN cell lines and murine xenografts. In pNENs, INA was over-expressed compared to pancreatic adenocarcinomas and normal pancreas (p=0.0001, 27-fold; p=0.02, 9-fold). INA transcripts positively correlated with Ki67 (r=0.5, p<0.0001) and CgA (r=0.59, p<0.0001). INA distinguished primaries and metastases (p=0.02). Expression was correlated with tumor size, infiltration and grade (p<0.05)
Conclusion
INA is a novel NEN biomarker whose expression associated with MAPK signaling and proliferation. In clinical samples, elevated INA correlated with Ki67and identified malignancy. INA may provide additional biological information relevant to delineation of both pNEN tumor phenotypes and clinical behavior.
Keywords: biomarker, BON, carcinoids, Internexin alpha, INA, Ki67, metastasis, neuroendocrine neoplasm, neurofilament, pancreatic neuroendocrine tumor
Introduction
Most gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) are sporadic lesions, although some, especially pancreatic neuroendocrine neoplasms (pNENs), may occur as part of familial tumor syndroms such as multiple endocrine neoplasia type 1 (MEN1 syndrome), von Hippel-Lindau disease (VHL), neurofibromatosis type 1 (NF-1), and tuberous sclerosis (TSC) 1. Overall, the incidence of GEP-NENs has increased exponentially during the last three decades 2. As such, they comprise 2% of all malignancies and, in terms of prevalence, GEP-NENs represent the second most common gastrointestinal malignancy after colorectal cancer 3. Diagnosis is late with metastases evident in 60–80% at presentation 4. Neuroendocrine carcinomas (NECs) often have the same poor prognosis as adenocarcinomas of the same organ, one example being colorectal neoplasms 5. The reasons for these poor outcomes reflect late diagnosis due to failure to identify non-specific symptoms as well as topographic investigations to identify tumors at an early stage and the lack of well-characterized biomarkers. At present, Ki-67 is the best characterized proliferation-associated marker and has been proposed as an important parameter in defining neuroendocrine neoplasia 6, 7. This cell-cycle associated protein has some utility as a prognostic predictor of 5-year survival in pNENs 8, but is less accurate in other NENs 9, 10. This might reflect both the different cells implicated in the genesis of this heterogeneous group of neoplasms(GEP-NENs)and the fact that the origin of the cells from which GEP-NENs arise is not well-understood (intestine and pancreas contain at least 17 different neuroendocrine cell types) 3.
The hybrid term “neuroendocrine” is a composite description of a cell type that exhibits mixed morphological and physiological attributes of both the neural and endocrine regulatory systems 3. These congruencies are also features of neoplasia; neuroblastomas can transdifferentiate into cells with a neuroendocrine features, highlighting the plasticity of their interchangeable phenotypes 11. Given these phenotypic commonalities 12, 13, we hypothesized that biomarkers relevant to a neural cell system might also be implicated in defining the phenotype of GEP-NENs.
Since internexin alpha (INA)and it’s family members (Figure 1)are components of a cytoskeletal protein class 14 (key to the neural system), we therefore investigated this group of class IV intermediate filaments or neurofilaments (NFs) as potential biomarkers. The basis for this analysis reflected our assessment using gene-interactome pathway analysis 15 of published GEP-NEN microarray data from our group. These studies identified INA to be over-expressed (3–10 fold) in both small intestinal NENs (SI-NENs) and pNENs 16. Both the level of expression and the observation that INA was co-expressed with a number of genes associated with neuronal development (Synapsin 1andChromogranin B), suggested that GEP-NENs and neuronal tumors express common biomarkers and that the INA protein family might play a role in neuroendocrine cell growth and development.
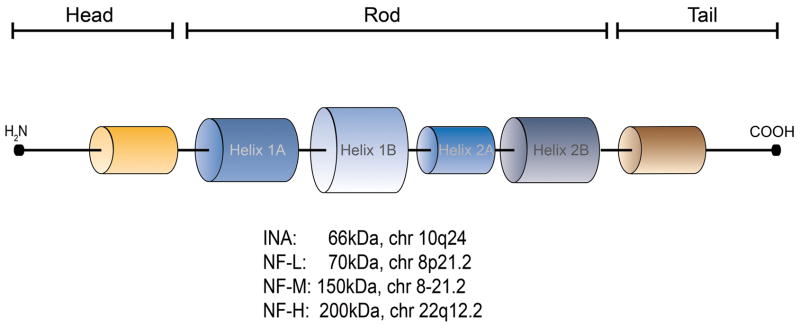
Figure 1.
Schematic structure of neurofilaments, chromosomal location and coding size. Each neurofilament (NF)has an amino-terminal and flexible phosphorylation target site (orange) at the head. The tail exhibits a carboxy-terminal flexible phosphorylation target site (brown). The long middle rod domain contains 4 alpha-helices, separated by short amino acid sequences (black). INA: Internexin alpha; NF-L: Neurofilament light; NF-M: Neurofilament medium; NF-H: Neurofilament heavy.
Class IV neurofilaments have previously been identified in post-mitotic neurons during the development of the central (CNS) and peripheral nervous system (PNS) 17, 18 and are expressed in enteric nerves 19. In adults, INA is found predominantly in the CNS and at low levels in the PNS where it is co-localized with NF-L (light), NF-M (medium) and NF-L (heavy) in most axons 14. In neoplasia of the nervous system, INA protein expression has been demonstrated in neuroblastomas 20, 21, medulloblastomas 22 and oligodendrogliomas 23 and elevated expression is considered of prognostic significance 24.
In the current study, we investigated whether neurofilaments were expressed in normal neuroendocrine cells, GEP-NEN cell lines and a xenograft model. In particular we assessed whether expression was related to proliferation. To assess whether this was representative of all NENs or specific to pancreatic NENs we thereafter identified if transcript and protein levels were differentially expressed in normal pancreatic tissue, pNENs and pancreatic adenocarcinoma. In order to evaluate the clinical relevance and specificity of our observations, we assessed the expression of INA in SI-NENs, Crohn’s disease and in normal small intestinal mucosa. While we particularly focused on INA, we also investigated expression levels of other members of this family (NF-L, NF-M and NF-H) to assess their utility as potential biomarkers of pNENs. Thereafter, in order to evaluate the question of whether differential expression provided information in respect of biological behavior, we examined whether transcripts or protein levels of these neurofilaments differed between GEP-NEN primaries and metastases. To evaluate the general utility of INA as a marker of NENs, we correlated expression with known neuroendocrine tumor biomarkers and progression parameters including Chromogranin A and B, Ki-67, tumor size, “differentiation” and metastatic spread 8, 25.
Materials and Methods
Cell lines: The SI-NEN cell lines KRJ-1, P-STS (both primary tumors), H-STS (liver metastasis), and L-STS (lymph node metastasis) were cultured as described 26. The human metastasized pancreatic NEN adherent cell line BON was cultivated in medium containing RPMI 1640, Hams’ F12 (1:1 ratio), 10% fetal calf serum (FCS) and penicillin and streptomycin(100 IU/ml) 27. In order to assess the role of INA in cell proliferation, we cultured BON cells (2.5×105/well) in 6-well plates and harvested them after 2, 3, 5 and 7 days for mRNA and protein isolation. In separate experiments, to evaluate the effect of growth-signaling on INA expression, BON cells (1×105 cells/ml) were seeded in 6-well plates (Falcon; BD, Franklin Lakes, NJ) and tested with PD98059 (a MAPK signaling pathway inhibitor -10−5 M) or Wortmannin (a PI3K/PKB inhibitor -10−8 M) for 1 hr prior to mRNA or protein collection. Cells were harvested by adding TRIZOL® (invitrogen, Carlsbad, CA) or lysis buffer (see below).
Murine xenograft model: The proliferation of the BON cell line was determined in vivo by subcutaneous injection into the flank of Nu/J mice (Jackson Laboratory, Bar Harbor, Maine). Animals were handled and stored according to IRB standards in the animal facility at the Yale University School of Medicine. Approximately 1×107 BON cells suspended in 0.2mL of saline were injected subcutaneously into the flank of four mice, and the mice were sacrificed after 60 days. Three pieces of tumor were harvested from each xenograft; qRT-PCR and Western blot was undertaken on each specimen to detect INA and qRT-PCR to detect the proliferation marker Ki67. We normalized in vivo data to INA levels obtained from day 2 (logarithmic growth phase in vitro)BON cells. This allowed us to directly compare xenograft expression to that of rapidly growing cells.
Human sample collection: The collection of pancreatic tissue from patients with pNENs (n=50), pancreatic adenocarcinoma (pAC, n=21) and normal pancreas (n=10) as well as small intestinal tissue from patients with SI-NENs (n=43) as well as normal SI-mucosa (n=16) was obtained according to the Ethics Committee requirements for Heidelberg and a standard IRB protocol for Yale University School of Medicine. Protocols included steps to minimize the time from resection to processing and freezing. Clinical sample details are provided in Table 1.
Table 1.
Sample details
| TISSUE TYPE | ORGAN | Number | Total |
|---|---|---|---|
| Neuroendocrine Neoplasms | Primary pNEN | 43 | 50 |
| Liver Metastasis of pNEN | 7 | ||
| Primary SI-NEN | 25 | 43 | |
| Liver Metastasis of SI-NEN | 18 | ||
| Control Cancer | Pancreatic Adenocarcinoma | 21 | |
| Non neoplastic tissue | Normal Pancreas | 10 | 35 |
| Normal Small bowel mucosa | 16 | ||
| Crohn’s Disease | 9 | ||
| NEN-cell lines | 2 BON, 3 STS, 1 KRJ-1 | 6 |
pNEN = Pancreatic Neuroendocrine Neoplasm; SI-NEN= Small Intestinal Neuroendocrine Neoplasm
Protein extraction and western blot analysis: Small pieces of tissue (1x2mm) or cell line lysates were manually homogenized (PYREX homogenizer) and prepared by adding 500μl of ice-cold cell lysis buffer (10xRIPA lysis buffer [Millipore, Temecula, CA], complete protease inhibitor [Roche, Indianapolis, IN], phosphatase inhibitor set 1&2 [Calbiochem, La Jolla, Ca], 100mM PMSF [Roche], 200mM Na3VO4 [Acros Organics, Geel, Belgium]and12.5mg/ml SDS [American Bioanalytical, Natick, MA]) 28. Tubes were centrifuged 20min at 12,000 g and supernatant protein quantified [BCA protein assay kit; Thermo Fisher Scientific, Rockford, IL]. Total protein lysates (15μg) were denaturated in SDS sample buffer, separated on an SDS-PAGE gel (10%) and transferred to a PVDF membrane (Bio-Rad, Hercules, CA, pore size 0.45 mm). After blocking (5% BSA,60min at room temperature) the membrane was incubated with primary antibodies (ABCAM, Cambridge, MA) in 5% BSA/PBS/Tween 20 overnight at 4°C. The membranes were incubated with HRP-conjugated secondary antibodies (Cell Signaling Technology, Beverly, MA) for 60min at room temperature and immunodetection was performed using Western Lightning™ Plus-ECL (PerkinElmer, Waltham, MA). Blots were exposed on X-OMAT-AR film 29. Cross-detection was avoided by stripping the membranes; in cell lines, protein expression was reported relative to that of β-actin (Sigma-Aldrich, St. Louis, MO). The optical density of the appropriately sized bands was measured using ImageJ software (NIH, USA). The ratio between neurofilament expression in neuroendocrine neoplasms or adenocarcinoma specimens and control protein was calculated 28.
RNA isolation and Reverse Transcription: Messenger RNA was extracted from each tissue sample using TRIZOL®(Invitrogen, Carlsbad, CA) and cleaned (RNeasy kit, Qiagen, Valencia, CA). After conversion to cDNA (High Capacity cDNA Archive Kit, Applied Biosystems, Carlsbad, CA) 28, RT-PCR analyses were performed using Assays-on Demand™ and the ABI 7900 Sequence Detection System 30. Primer sets were all obtained from Applied Biosystems and PCR mix on gels were performed to confirm presence of single bands for each primer set. PCR Data was normalized using the CT method; ALG9 was used as a housekeeping gene 31.
Immunostaining: An established immunohistochemical approach was used to identify target protein 32. Briefly, de-paraffinized sections were incubated with monoclonal antibodies (also used for western blot, INA [Abcam]; 1:500 dilution)overnight at 4 °C and then with FITC anti-rabbit IgG (1:250 dilution) for 60min at room temperature. Sections were rinsed with PBS[American Bioanalytical, Natick, MA]and nuclei stained with DAPI (1:100). Bound antibodies were visualized using immunofluorescent microscopy.
Statistical Evaluation: All statistical analyses were performed using Microsoft Excel, SPSS 14 and Prism 5(GraphPad Software, San Diego, CA). Comparison between NENs, adenocarcinoma and controls were performed using the Kruskal Wallis test, followed by the Dunn post-hoc test where appropriate. Binary comparisons were made using a 2-tailed Mann-Whitney Test. Correlations were undertaken using Spearman correlation. A p-value of <0.05 was designated assignificant. Data points >20standard deviations above the mean were excluded as outliers; a total of 3data points(pAC [data point >+28 std dev]; G1-pNET [data point >+103 std dev]; SI-NEN met [data point >+23 std dev]) were excluded.
Results
1. Neurofilament expression in normal neuroendocrine cells, NEN cell lines and murine xenografts
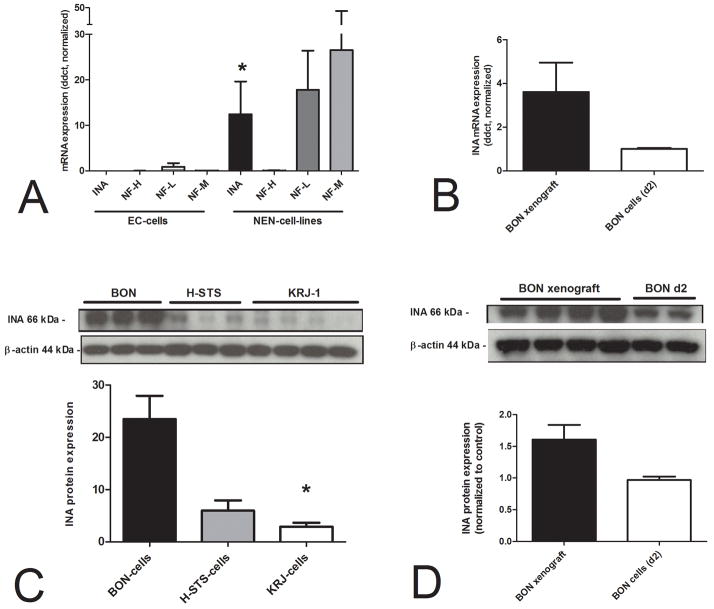
Prior to examining neurofilament expression in clinical samples, we screened a range of human normal and NEN cell lines for different neurofilament(INA, NF-L, NF-M and NF-H)expression. We used normal human EC cells (n=9 preparations), six different NEN cells lines and 4 murine xenografts. Using PCR, in normal EC cells we identified that most neurofilaments were either not expressedor were marginally (NF-L) evident (Figure 2A). INA, however, was significantly expressed in all six NEN cell-lines (p=0.0008), with the greatest expression in the pancreatic BON cell line (fold values 24–42). This was confirmed by western blot (Figure 2C), which demonstrated significant over-expression (p=0.03) of this 66kDa protein in BON cells in comparison to two SI-NEN cell lines, KRJ-1 and H-STS. NF-L and NF-M were both highly expressed in NEN cell-lines (but due to a high standard-deviation not significant versus normal)while NF-H was not identified. In BON xenografts, INA mRNA(Figure 2B)as well as protein (Figure 2D)were expressed at levels higher than in cultured cells demonstrating continued expression under in vivo conditions.
Figure 2.
RNA expression of neurofilaments in normal EC cells (n=9 preparations), 6 individual NEN cell lines (2 BON, 3 STS, 1 KRJ-1)and murine xenograft models. INA mRNA was expressed at a significantly higher level in the NEN cell lines in comparison to normal EC cells (2A,*p=0.0008). INA protein was expressed in all three different cell lines (3 samples BON-, 3 samples H-STS-, 4 samples KRJ-1-cells), but was significantly higher in the adherent BON cells (2C, *p=0.03). BON cell xenografts, grown subcutaneously in mice, exhibited higher INA both at an mRNA (2B) and protein level (2D) compared to BON cells(logarithmic growth phase day 2) in vitro.
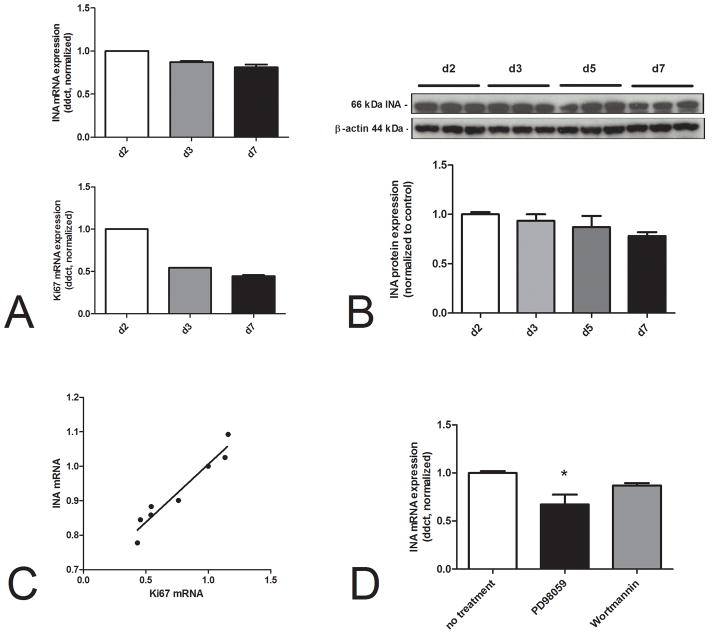
In order to assess a potential biological role for INAin GEP-NENs, we evaluated expression as a function of cell proliferation. Levels of INA(mRNA - Figure 3A, protein - Figure 3B) decreased after 3 up to7days of culture, and that correlated with decreasing expression ofKi67 (Spearman’s r=1.0, p<0.0001, Figure 3C)suggesting that INA expression may be a function of or related to proliferation.
Figure 3.
INA and BON cell proliferation. INA and Ki67 mRNA levels were progressively reduced in BON cells, harvested at day 2, 3and 7after sub-culture (3A). INA protein levels were similarly decreased (3B). Although the decreasing INA andKi67mRNA expression during seven days were not significant, both gene expressions were strongly correlated(Spearman’s r=1.0, p<0.0001)(3C). The MAPK-inhibitor, PD98059, significantly deceased INA mRNA in sub cultured BON-cells (3D, *p=0.03) indicating INA transcription was regulated by MAPK signaling.
Since both MAPK and PI3K/AKT(PKB) are key proliferative regulatory pathways in BON cells 34, we investigated the role of these signaling pathways in INA transcription. The MAP-kinase inhibitor, PD98059, inhibited INA mRNA levels(p=0.03), using a 1 hr assay, while Wortmannin had no effect (Figure 3D). This suggests that proliferation and signaling via MAPK was associated with regulation of INA transcription.
2. IN Aexpression in the normal and neoplastic pancreas
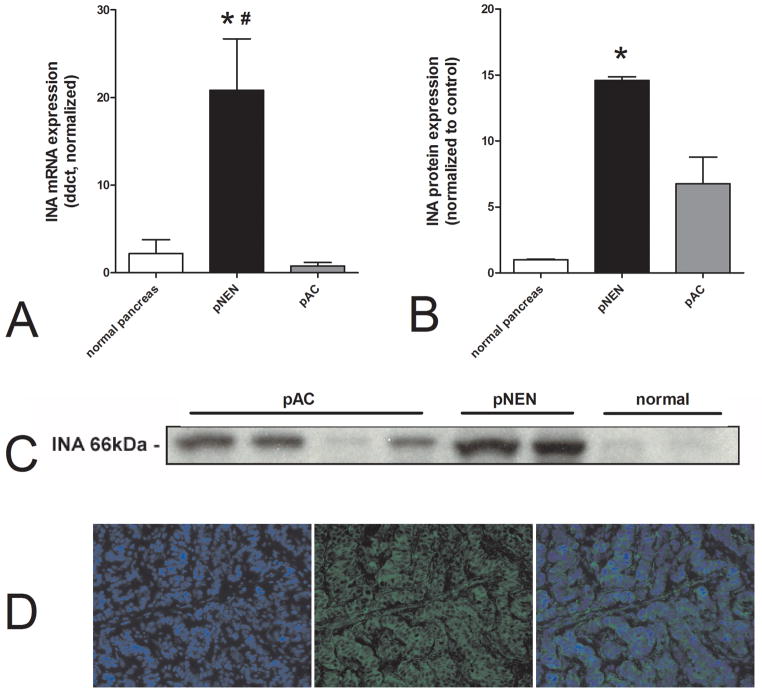
Having identified increased expression of INA in NEN cell lines and those levels correlated with the proliferation marker Ki67, we next examined whether mRNA and protein was expressed in human pNENs and was increased compared to normal pancreas. Figure 4A demonstrates the differences in mRNA expression in the normal pancreas, pancreatic neuroendocrine neoplasms and pancreatic adenocarcinomas. Levels distinguished between normal pancreas and pNENs (p=0.02) as well as between pACs and pNENs (p=0.0001). Protein levels(Figure 4B–C) followed a similar pattern; INA was significantly higher in pNENs than in normal pancreas (p=0.0072), and was higher than in pACs(ns). Immunohistochemical staining (Figure 4D), demonstrated that INA was cytoplasmically restricted in NENs suggesting it had no membrane receptor or nuclear role.
Figure 4.
Transcript levels of INA in normal pancreas (n=10), pancreatic adenocarcinomas (pACs, n=21) and pNENs (n=50)(4A). There was a significant difference in mRNA expression between normal pancreas and pNENs (*p=0.02) and between pNENs and pACs(#p=0.0001). Significant differences were also identified for protein levels of INA in pancreatic adenocarcinomas (n=4) compared to pNENs (n=2) and normal pancreas (n=2)(*p=0.0072)(4B,C). A post hoc test demonstrated a significant difference between pNENs and normal pancreas (4B). Immunohistochemical staining of INA expression in a pNEN; panel 1 DAPI nuclei (blue), panel 2 FITC-INA (green), panel 3 composite (4D). INA expression was cytoplasmically restricted in NENs suggesting it had no membrane receptor or nuclear role.
3. Examination of the relationship between INA and other pNENs biomarkers
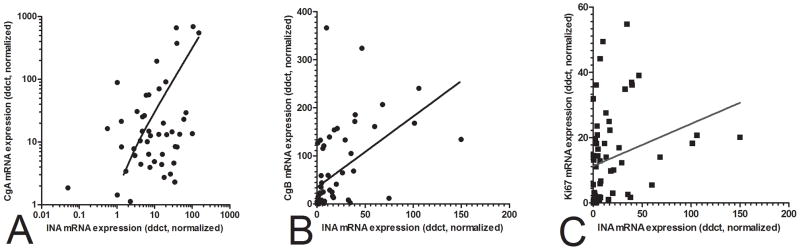
To further delineate a role for INA as a pNEN biomarker, we compared and correlated INA expression with common NEN markers including Chromogranin A, Band Ki67. There was a strong positive correlation(Spearman’s) between INA mRNA and each marker: Chromogranin A (r=0.59, p<0.0001), Chromogranin B (r=0.74, p<0.0001)and Ki67 (r=0.5, p<0.0001)(Figure 5A–C). These results indicate INA may be a neuroendocrine biomarker that positively correlates with proliferation.
Figure 5.
Utility of INA as a biomarker in pNENs correlation analysis of INA transcripts with Chromogranin and Ki67. Significant associations were identified for both CgA and CgB (5A,B) (Spearman’s r=0.59 and 0.74, p<0.0001, respectively)as well as for the proliferation marker, Ki67 (Spearman’s r=0.5, p<0.0001) (5C).
4. INA expression and tumor size, differentiation and pNEN metastasis
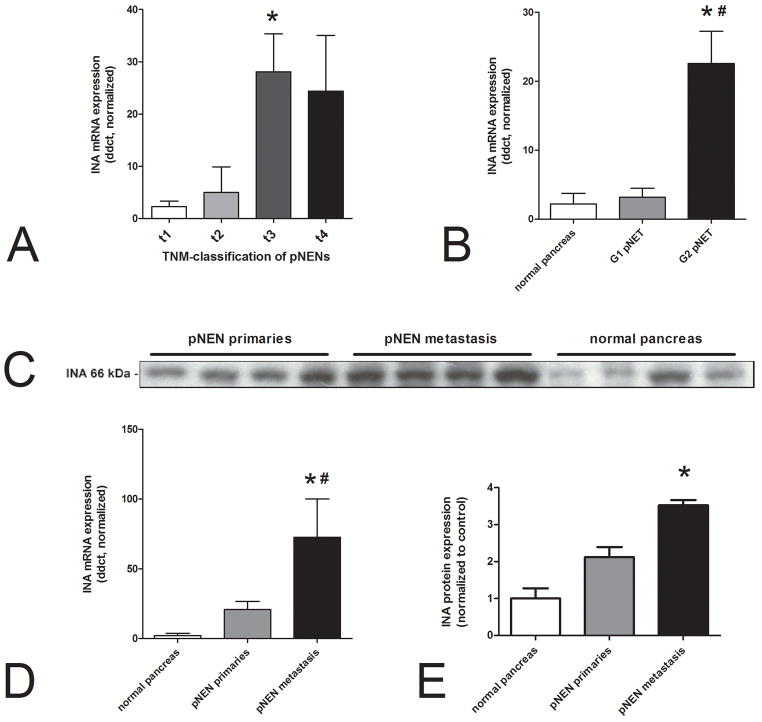
We next examined whether INA expression was associated with clinical features including stage and grade in pNENs. Using the TNM classification(Union for International Cancer Control (UICC)), larger tumors(T3)at resection were associated with higher INA levels (p=0.015) (Figure 6A). In addition, INA correlated with tumor size (diameter in cm, Spearman’s r=0.36, p=0.03, data not shown). By grading, INA was differentially expressed between G1-and G2-neoplasms. G2 pNENs especially expressed INA 7 fold higher than G1 pNETs (p<0.05)and normal pancreas (p=0.002) (Figure 6B). Comparisons of INA mRNA between pNEN primaries and metastasis demonstrated that levels were significantly elevated in metastases (p=0.007, 6-fold) (Figure 6D). No differences were noted for angioinvasion while tumors classified as “functioning” did not exhibit different INA expression compared to “non-functioning” tumors. With respect to outcome, only six of the 50 pNEN patients were deceased at follow-up; an analysis of INA expression identified this was increased (but not significantly data not shown) in the non-survivors. The western blot confirmed elevated expression in metastases(p<0.01) (Figure 6C,E).
Figure 6.
INA expression in pNENs as a function of tumor size, grade and metastasis. INA transcripts were significantly over-expressed in t3 pNENs compared to t1 (p=0.015) (6A) as well as in G2-pNETs compared to normal pancreas (*p=0.002)and to G1-pNETs(#p<0.05) (6B). INA mRNA was significantly elevated in pNEN metastases in comparison to normal pancreas (*p=0.003) and primaries (#p=0.02) (6D). At protein levels INA expression was higher in metastases (p<0.01) (6C,E).
5. INA in normal small intestinal mucosa and in small intestinal NENs
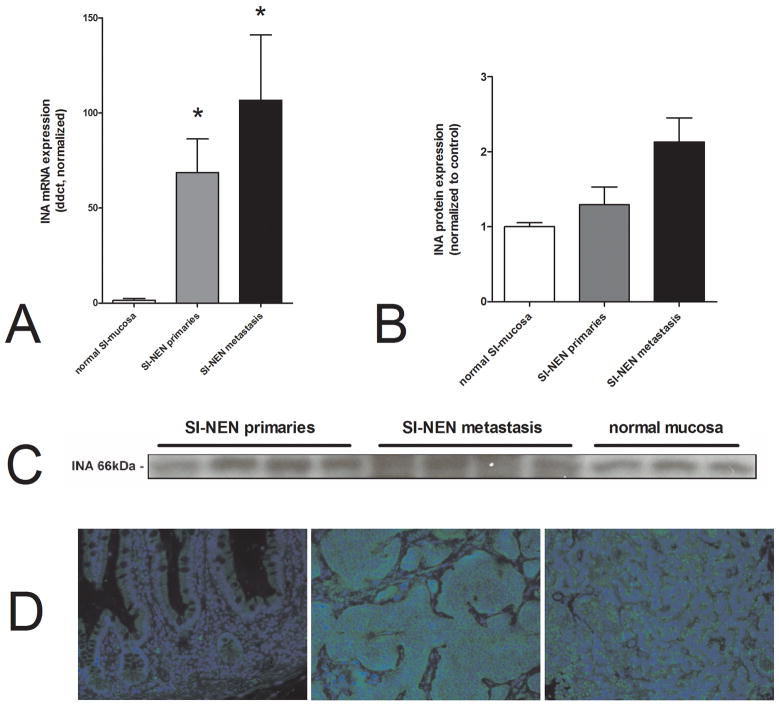
Analysis of primary SI-NENs and metastases demonstrated significantly increased INA mRNA (>10-fold, p=0.0002and p=0.0001, respectively) compared to normal mucosa (Figure 7A). These differences could be shown as a trend at protein level, particularly for the metastases(Figure 7B/C). Analysis by clinical feature identified that INA was differentially expressed between G1-and G2-neoplasms. G2 SI-NENs especially expressed INA 2 fold higher than G1 pNETs (p<0.05). An outcome analysis could not be performed as only two of the 43 SI-NEN patients were deceased at follow-up. Immunohistochemically, INA exhibited the same cytoplasmic expression (Figure 7D) as was noted in pNENs.
Figure 7.
Transcript expression of INA in normal small intestinal mucosa(n=16)and inSI-NENs (n=43). The mRNA expression was significantly higher in SI-NEN primaries and metastasis compared to normal mucosa (*p=0.0002 and p=0.0001, respectively)(7A). Protein levels of INA in SI-NEN primaries (n=4), SI-NEN metastasis (n=4) and normal mucosa (n=3)(7C). This western blot identified a trend for an increase in INA in metastases(p=0.057) (7B). Immunostaining of normal mucosa (7D, left) did not identify significant INA expression in the mucosa; expression was evident in the mesenteric plexus. Both SI-NEN primaries (7D, center) and liver metastases (7D, right) exhibit strong cytoplasmic stainingsuggesting it had no membrane receptor or nuclear role. Blue = nuclei (DAPI), green = INA (FITC)
6. Expression of NF-L, NF-M and NF-H in GEP-NENs
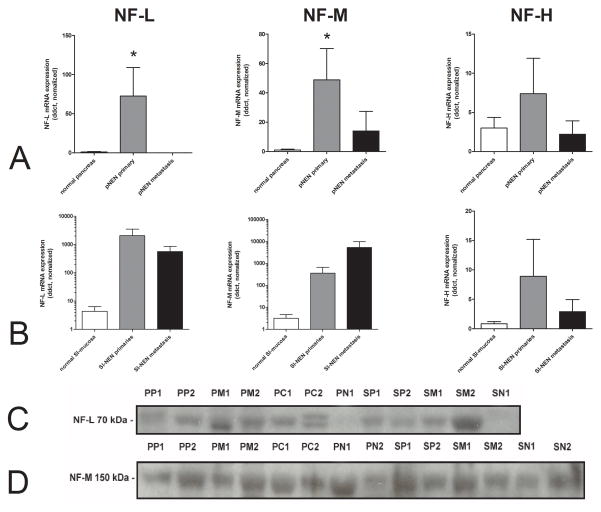
We next examined mRNA and protein expression of the other three class IV intermediate neurofilaments in pNENs and SI-NENs to evaluate whether their expression was similar to INA. Differences in transcript levels of NF-L and NF-M were noted between normal pancreas and pNENs (p<0.04)(Figure8A). NF-H mRNA levels were not different. In contrast, these neurofilaments were not differentially expressed in SI-NENs(Figure 8B). No significant differences between primaries, metastasis and normal tissue were noted at a protein level(Figure 8C and D).
Figure 8.
Expression of NF-L, NF-M and NF-H in pNENs (8A) and in SI-NENs (8B). NF-L and NF-M mRNA expression was significantly elevated in primary pNENs compared to normal pancreas (*p=0.0005 and p=0.0076, respectively). There was, however, no significant difference in expression between SI-NEN primaries or metastasis. At a protein level, NF-L (8C) was increased in pancreatic metastases (PM) in comparison to normal pancreas (PN) but due to the small amount of samples in each group it is not significant. NF-M showed similar expression in all tissue types(8D).
Note: The extremely high neurofilament levels in SI-NENs (log scale) were only detected in the more aggressive neuroendocrine carcinomas, particularly in metastasized neoplasms. PP = pNEN primary, PM= pNEN metastasis, PC= pancreatic adenocarcinoma, PN = normal pancreas; SP = SI-NEN primary, SM = SI-NEN metastasis, SN= normal small intestinal mucosa.
Discussion
Overall, GEP-NENs represent a conundrum since these tumors comprise adiverse group of lesions of different cell types that exhibit dissimilar biological and clinical behaviors. Despite this, they are largely treated as a uniform neoplastic entity. A key unmet need in the management of GEP-NENs is the identification of specific biomarkers that typify these lesions and hence provide appropriate clinical guidance for the optimal management strategy of individual tumors 35. Thus far, biomarker discovery in GEP-NENs has been based on analysis of proliferation, e.g. the contentious Ki67 36. As an alternative strategy, we sought to identify and examine the utility of neural tumor biomarkers since these lesions demonstrate overlapping phenotypes with NENs. INA, a class IV intermediate filament, is an example of a neural biomarker that has prognostic significance in oligodendroglial grade III tumors 24.
In these tumors, over-expression of INA is associated with both a longer progression free survival as well as a longer overall survival (50.4 month vs. 6.6. month and 16 month vs. median not reached) regardless of the treatment received 24. The identification of INA expression in oligodendroglial tumors might therefore be reflective of a “proneural” gene profile as described by Ducray et al. 37. This suggests INA playsa role both in neurogenesis as well as in neuro-survival in these lesions through protection or regeneration of the neuronal cells as its original tissue.
In the current study, in GEP-NENs, INA was over-expressed in the neoplastic phenotype (particularly in tumors of a pancreatic origin) as well as in proliferating NEN cell lines and murine xenograft tumors, compared to normal tissue or non-transformed neuroendocrine cells. This expression was more evident in metastases and was associated with both an increasing size of the lesion as well as higher grades of tumor. Transcript levels of INA correlated well with aggressive and advancing disease, with decreasing differentiation and with high levels of Ki67, markers commonly associated with a poor outcome 8, 25. It is important to acknowledge that normal tissue may be an imperfect comparator for tumor expression of a biomarker. However, given the absence of any other appropriate normal neuroendocrine controls as well as the current concept that neuroendocrine cells (as well as adenocarcinomas) are derived from the same multipotential stem cells 38–43, use of normal pancreatic or normal mucosal tissue provides the most appropriate base-line against which to assess INA values.
Our findings support the hypothesis that expression of INA may be reflective of a malignant phenotype and tumor aggressiveness in GEP-NENs. This raises the key biological question of what role INA plays in the regulation of GEP-NEN proliferation or metastasis. Evaluation of this hypothesis by functional assessment of INA in the BON cell line identified that expression levels(mRNA and protein) correlated with logarithmic growth (day 2) and Ki67 transcription, and that mRNA levels were regulated by MAP-kinase a key component of the proliferation-signaling pathway in this cell line 33. These results suggest INA is preferentially expressed in rapidly growing cells and expression may be a downstream response to proliferation signaling(MAPK)pathway activation. The elevated expression of this neurofilament in invasive GEP-NENs (and xenograft models) also suggests that INA may play a role inNEN metastasis. Since the protein is a cytoplasmically-restricted, cytoskeletal filament, we postulate that INA may play a role in regulating cell-cell-interactions, a fundamental component of tumor growth and invasion.
The heterogeneity of GEP-NENs, particularly their individual phenotypes, may provide further insight into the role of INA. Thus, in pNENs, which are relatively faster growing and more aggressive, INA expression correlated well with disease extent and aggression whereas in SI-NENs, which have a lower proliferation and low Ki67 expression, INA was less well correlated. It is well-known that pNENs represent a far more aggressive group of NENs with greater malignancy and substantially worse prognosis 44. The fact that INA was differentially expressed in pNEN primaries and metastasis in comparison to SI-NENs may reflect that it has a functional role in tumor growth and invasion in this group of more aggressive tumors. An assessment of outcomes identified that INA expression was elevated in deceased patients, but the small number (n=6) did not allow statistical significance. Longer-term follow-up will identify whether INA is a prognostic marker for pNENs.
In conclusion, INA and the other three class IV intermediate filaments are highly expressed at both mRNA and protein levels in GEP-NENs. Based on these observations, as well as the confirmation that they are also expressed in neuronal tissue 45, we propose that this class of proteins might better be considered not only as neural filament proteins but as neuroendocrine filaments (NEFs). While the precise functional role of these neurofilaments in the evolution of NEN genesis remains to be elucidated, we postulate that they may play a role in tumor growth and invasion, particularly in pNENs. If such were to be the case, demonstration of their expression in an individual neoplastic lesion might provide the opportunity to consider the class as representative of a target group for cytostatic therapies.
Acknowledgments
Funding support: SS is supported by the Deutsche Forschungsgemeinschaft: SCHI 1177/1-1. MK is supported by NIH: DK080871. BL is partially supported by the Murray Jackson Clinical Fellowship from the Genesis Oncology Trust Auckland, New Zealand.
Footnotes
The authors have nothing to disclose.
References
- 1.Calender A. Molecular genetics of neuroendocrine tumors. Digestion. 2000;62 (Suppl 1):3–18. doi: 10.1159/000051850. [DOI] [PubMed] [Google Scholar]
- 2.Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9(1):61–72. doi: 10.1016/S1470-2045(07)70410-2. [DOI] [PubMed] [Google Scholar]
- 3.Schimmack S, Svejda B, Lawrence B, Kidd M, Modlin IM. The diversity and commonalities of gastroenteropancreatic neuroendocrine tumors. Langenbecks Arch Surg. 2011 doi: 10.1007/s00423-011-0739-1. [DOI] [PubMed] [Google Scholar]
- 4.Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M. Chromogranin A-Biological Function and Clinical Utility in Neuro Endocrine Tumor Disease. Ann Surg Oncol. 2010 doi: 10.1245/s10434-010-1006-3. [DOI] [PubMed] [Google Scholar]
- 5.Vogelsang H, Siewert JR. Endocrine tumours of the hindgut. Best Pract Res Clin Gastroenterol. 2005;19(5):739–51. doi: 10.1016/j.bpg.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Rindi G, Kloppel G, Couvelard A, Komminoth P, Korner M, Lopes JM, et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2007;451(4):757–62. doi: 10.1007/s00428-007-0452-1. [DOI] [PubMed] [Google Scholar]
- 7.Kloppel G, Couvelard A, Perren A, Komminoth P, McNicol AM, Nilsson O, et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: towards a standardized approach to the diagnosis of gastroenteropancreatic neuroendocrine tumors and their prognostic stratification. Neuroendocrinology. 2009;90(2):162–6. doi: 10.1159/000182196. [DOI] [PubMed] [Google Scholar]
- 8.La Rosa S, Sessa F, Capella C, Riva C, Leone BE, Klersy C, et al. Prognostic criteria in nonfunctioning pancreatic endocrine tumours. Virchows Arch. 1996;429(6):323–33. doi: 10.1007/BF00198436. [DOI] [PubMed] [Google Scholar]
- 9.Van Eeden S, Quaedvlieg PF, Taal BG, Offerhaus GJ, Lamers CB, Van Velthuysen ML. Classification of low-grade neuroendocrine tumors of midgut and unknown origin. Hum Pathol. 2002;33 (11):1126–32. doi: 10.1053/hupa.2002.129204. [DOI] [PubMed] [Google Scholar]
- 10.Panzuto F, Nasoni S, Falconi M, Corleto VD, Capurso G, Cassetta S, et al. Prognostic factors and survival in endocrine tumor patients: comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer. 2005;12(4):1083–92. doi: 10.1677/erc.1.01017. [DOI] [PubMed] [Google Scholar]
- 11.Gestblom C, Hoehner JC, Hedborg F, Sandstedt B, Pahlman S. In vivo spontaneous neuronal to neuroendocrine lineage conversion in a subset of neuroblastomas. Am J Pathol. 1997;150(1):107–17. [PMC free article] [PubMed] [Google Scholar]
- 12.Tapia FJ, Polak JM, Barbosa AJ, Bloom SR, Marangos PJ, Dermody C, et al. Neuron-specific enolase is produced by neuroendocrine tumours. Lancet. 1981;1(8224):808–11. doi: 10.1016/s0140-6736(81)92682-9. [DOI] [PubMed] [Google Scholar]
- 13.Wiedenmann B, Waldherr R, Buhr H, Hille A, Rosa P, Huttner WB. Identification of gastroenteropancreatic neuroendocrine cells in normal and neoplastic human tissue with antibodies against synaptophysin, chromogranin A, secretogranin I (chromogranin B), and secretogranin II. Gastroenterology. 1988;95(5):1364–74. doi: 10.1016/0016-5085(88)90374-5. [DOI] [PubMed] [Google Scholar]
- 14.Paramio JM. Intermediate filaments: Landes Bioscience and Springer Science. Business Media, LLC; 2006. [Google Scholar]
- 15.Kidd M, Modlin IM, Mane SM, Camp RL, Eick G, Latich I. The role of genetic markers--NAP1L1, MAGE-D2, and MTA1--in defining small-intestinal carcinoid neoplasia. Ann Surg Oncol. 2006;13(2):253–62. doi: 10.1245/ASO.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Drozdov I, Tsoka S, Ouzounis CA, Shah AM. Genome-wide expression patterns in physiological cardiac hypertrophy. BMC Genomics. 2010;11:557. doi: 10.1186/1471-2164-11-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duprey P, Paulin D. What can be learned from intermediate filament gene regulation in the mouse embryo. Int J Dev Biol. 1995;39(3):443–57. [PubMed] [Google Scholar]
- 18.Kaplan MP, Chin SS, Fliegner KH, Liem RK. Alpha-internexin, a novel neuronal intermediate filament protein, precedes the low molecular weight neurofilament protein (NF-L) in the developing rat brain. J Neurosci. 1990;10(8):2735–48. doi: 10.1523/JNEUROSCI.10-08-02735.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rauch U, Klotz M, Maas-Omlor S, Wink E, Hansgen A, Hagl C, et al. Expression of intermediate filament proteins and neuronal markers in the human fetal gut. J Histochem Cytochem. 2006;54(1):39–46. doi: 10.1369/jhc.4A6495.2005. [DOI] [PubMed] [Google Scholar]
- 20.Foley J, Witte D, Chiu FC, Parysek LM. Expression of the neural intermediate filament proteins peripherin and neurofilament-66/alpha-internexin in neuroblastoma. Lab Invest. 1994;71(2):193–9. [PubMed] [Google Scholar]
- 21.Willoughby V, Sonawala A, Werlang-Perurena A, Donner LR. A comparative immunohistochemical analysis of small round cell tumors of childhood: utility of peripherin and alpha-internexin as markers for neuroblastomas. Appl Immunohistochem Mol Morphol. 2008;16(4):344–8. doi: 10.1097/PAI.0b013e318165fe78. [DOI] [PubMed] [Google Scholar]
- 22.Kaya B, Mena H, Miettinen M, Rushing EJ. Alpha-internexin expression in medulloblastomas and atypical teratoid-rhabdoid tumors. Clin Neuropathol. 2003;22(5):215–21. [PubMed] [Google Scholar]
- 23.Ducray F, Mokhtari K, Criniere E, Idbaih A, Marie Y, Dehais C, et al. Diagnostic and prognostic value of alpha internexin expression in a series of 409 gliomas. Eur J Cancer. 2011;47(5):802–8. doi: 10.1016/j.ejca.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 24.Mokhtari K, Ducray F, Kros JM, Gorlia T, Idbaih A, Taphoorn M, et al. Alpha-internexin expression predicts outcome in anaplastic oligodendroglial tumors and may positively impact the efficacy of chemotherapy: European organization for research and treatment of cancer trial 26951. Cancer. 2011 doi: 10.1002/cncr.25827. [DOI] [PubMed] [Google Scholar]
- 25.Arnold R, Rinke A, Klose KJ, Muller HH, Wied M, Zamzow K, et al. Octreotide versus octreotideplus interferon-alpha in endocrine gastroenteropancreatic tumors: a randomized trial. Clin Gastroenterol Hepatol. 2005;3(8):761–71. doi: 10.1016/s1542-3565(05)00481-7. [DOI] [PubMed] [Google Scholar]
- 26.Pfragner R, Behmel A, Hoger H, Beham A, Ingolic E, Stelzer I, et al. Establishment and characterization of three novel cell lines -P-STS, L-STS, H-STS -derived from a human metastatic midgut carcinoid. Anticancer Res. 2009;29(6):1951–61. [PubMed] [Google Scholar]
- 27.Siddique ZL, Drozdov I, Floch J, Gustafsson BI, Stunes K, Pfragner R, et al. KRJ-I and BON cell lines: defining an appropriate enterochromaffin cell neuroendocrine tumor model. Neuroendocrinology. 2009;89(4):458–70. doi: 10.1159/000209330. [DOI] [PubMed] [Google Scholar]
- 28.Svejda B, Kidd M, Kazberouk A, Lawrence B, Pfragner R, Modlin IM. Limitations in small intestinal neuroendocrine tumor therapy by mTor kinase inhibition reflect growth factor-mediated PI3K feedback loop activation via ERK1/2 and AKT. Cancer. 2011 doi: 10.1002/cncr.26011. [DOI] [PubMed] [Google Scholar]
- 29.Kidd M, Modlin IM, Pfragner R, Eick GN, Champaneria MC, Chan AK, et al. Small bowel carcinoid (enterochromaffin cell) neoplasia exhibits transforming growth factor-beta1-mediated regulatory abnormalities including up-regulation of C-Myc and MTA1. Cancer. 2007;109(12):2420–31. doi: 10.1002/cncr.22725. [DOI] [PubMed] [Google Scholar]
- 30.Kidd M, Eick G, Shapiro MD, Camp RL, Mane SM, Modlin IM. Microsatellite instability and gene mutations in transforming growth factor-beta type II receptor are absent in small bowel carcinoid tumors. Cancer. 2005;103(2):229–36. doi: 10.1002/cncr.20750. [DOI] [PubMed] [Google Scholar]
- 31.Kidd M, Nadler B, Mane S, Eick G, Malfertheiner M, Champaneria M, et al. GeneChip, geNorm, and gastrointestinal tumors: novel reference genes for real-time PCR. Physiol Genomics. 2007;30(3):363–70. doi: 10.1152/physiolgenomics.00251.2006. [DOI] [PubMed] [Google Scholar]
- 32.Kidd M, Modlin IM, Mane SM, Camp RL, Shapiro MD. Q RT-PCR detection of chromogranin A: a new standard in the identification of neuroendocrine tumor disease. Ann Surg. 2006;243(2):273–80. doi: 10.1097/01.sla.0000197734.28551.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karhoff D, Sauer S, Schrader J, Arnold R, Fendrich V, Bartsch DK, et al. Rap1/B-Raf signaling is activated in neuroendocrine tumors of the digestive tract and Raf kinase inhibition constitutes a putative therapeutic target. Neuroendocrinology. 2007;85(1):45–53. doi: 10.1159/000100508. [DOI] [PubMed] [Google Scholar]
- 34.Pitt SC, Chen H, Kunnimalaiyaan M. Inhibition of phosphatidylinositol 3-kinase/Akt signaling suppresses tumor cell proliferation and neuroendocrine marker expression in GI carcinoid tumors. Ann Surg Oncol. 2009;16(10):2936–42. doi: 10.1245/s10434-009-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Modlin IM, Moss SF, Chung DC, Jensen RT, Snyderwine E. Priorities for improving the management of gastroenteropancreatic neuroendocrine tumors. J Natl Cancer Inst. 2008;100(18):1282–9. doi: 10.1093/jnci/djn275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Z, Tang LH, Klimstra DS. Effect of Tumor Heterogeneity on the Assessment of Ki67 Labeling Index in Well-differentiated Neuroendocrine Tumors Metastatic to the Liver: Implications for Prognostic Stratification. Am J Surg Pathol. 2011;35(6):853–60. doi: 10.1097/PAS.0b013e31821a0696. [DOI] [PubMed] [Google Scholar]
- 37.Ducray F, Idbaih A, de Reynies A, Bieche I, Thillet J, Mokhtari K, et al. Anaplastic oligodendrogliomas with 1p19q codeletion have a proneural gene expression profile. Mol Cancer. 2008;7:41. doi: 10.1186/1476-4598-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novelli MR, Williamson JA, Tomlinson IP, Elia G, Hodgson SV, Talbot IC, et al. Polyclonal origin of colonic adenomas in an XO/XY patient with FAP. Science. 1996;272(5265):1187–90. doi: 10.1126/science.272.5265.1187. [DOI] [PubMed] [Google Scholar]
- 39.Taylor RW, Barron MJ, Borthwick GM, Gospel A, Chinnery PF, Samuels DC, et al. Mitochondrial DNA mutations in human colonic crypt stem cells. J Clin Invest. 2003;112(9):1351–60. doi: 10.1172/JCI19435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greaves LC, Preston SL, Tadrous PJ, Taylor RW, Barron MJ, Oukrif D, et al. Mitochondrial DNA mutations are established in human colonic stem cells, and mutated clones expand by crypt fission. Proc Natl Acad Sci U S A. 2006;103(3):714–9. doi: 10.1073/pnas.0505903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDonald SA, Greaves LC, Gutierrez-Gonzalez L, Rodriguez-Justo M, Deheragoda M, Leedham SJ, et al. Mechanisms of field cancerization in the human stomach: the expansion and spread of mutated gastric stem cells. Gastroenterology. 2008;134(2):500–10. doi: 10.1053/j.gastro.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 42.Bouwens L, Kloppel G. Islet cell neogenesis in the pancreas. Virchows Arch. 1996;427(6):553–60. doi: 10.1007/BF00202885. [DOI] [PubMed] [Google Scholar]
- 43.Paris M, Tourrel-Cuzin C, Plachot C, Ktorza A. Review: pancreatic beta-cell neogenesis revisited. Exp Diabesity Res. 2004;5(2):111–21. doi: 10.1080/15438600490455079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM. Theepidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40(1):1–18. vii. doi: 10.1016/j.ecl.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Liem RK. Molecular biology of neuronal intermediate filaments. Curr Opin Cell Biol. 1993;5 (1):12–6. doi: 10.1016/s0955-0674(05)80003-1. [DOI] [PubMed] [Google Scholar]