Abstract
Introduction
Chylothorax is a very rare complication of chronic lymphocytic leukemia.
Presentation of case
We describe the case of an 83-year old woman with chronic lymphocytic leukemia, complicated by recurrent chylothorax and ultimately treated by pleurodesis with bleomycin.
Discussion
There are several options for management of patients presenting with chylothorax due to chronic lymphocytic leukemia.
Conclusion
Pleurodesis is a reasonable and effective treatment modality for patients with refractory chylothorax.
Keywords: Chylothorax, Chronic lymphocytic leukemia, Pleurodesis
1. Introduction
Chylothorax or the presence of chyle in the pleural cavity is most commonly the result of neoplastic diseases, with lymphoma being the leading cause, followed by lung cancer, and is also encountered as a postoperative complication in operations involving the aorta or esophagus.1 Chylothorax is only rarely reported as the result of chronic lymphocytic leukemia (CLL), with only a few cases reported in the literature.2,3 We describe the case of a 83-year old woman with chronic lymphocytic leukemia complicated by recurrent chylothorax and ultimately treated by pleurodesis.
2. Case presentation
The patient presented to the emergency department with gradually worsening dyspnea of few days duration. She had been diagnosed with chronic lymphocytic leukemia 2 years ago. The chest X-ray revealed a large effusion into the right pleural cavity (Fig. 1). Chest CT scan showed no mediastinal adenopathy (Fig. 2). She had no fever and she mentioned no night sweats, chest pain, cough and pleuritic pain.
Fig. 1.
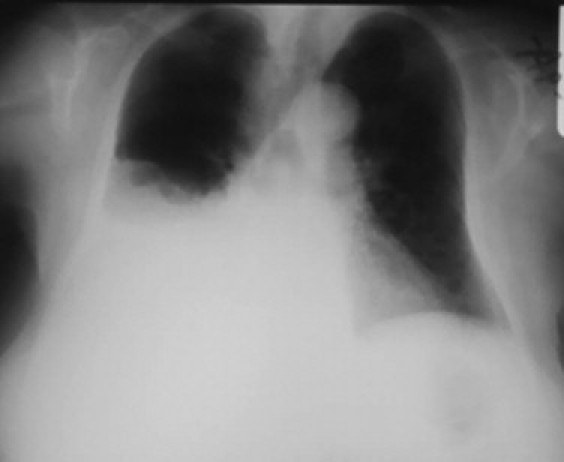
A large right pleural effusion is obvious in chest X-ray.
Fig. 2.
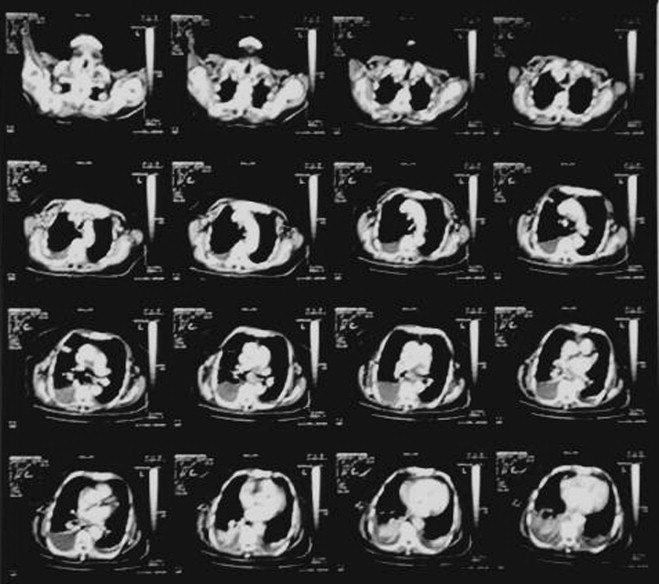
Chest CT scan. No mediastinal adenopathy is noted.
Tuberculin test was negative and she did not mention travelling abroad recently. On thoracentesis the fluid was milky with triglyceride and cholesterol levels at 1168 and 143 mg/dl respectively and the diagnosis of chylothorax was made. Lymphocytes were predominant at the pleural fluid.
A chest tube was inserted and about 1500 ml of milky fluid was drained. Consequently the patient was given nothing by mouth and started on total parenteral nutrition. Chemotherapy or mediastinal irradiation options were not offered to the patient, because she had no mediastinal adenopathy and also she was very debilitated. Conservative treatment with nothing per os and TPN was really effective, as chyle production gradually diminished from about 1000 ml daily for the first 3 days of treatment to about 100 ml/day after ten days of starting treatment. Thereafter we started oral feeding of the patient first on a medium chain triglyceride diet for few days with subsequent switch to a full diet. There was no increase in the amount of chyle after several days of feeding, at which point the chest tube was removed and the patient was discharged home. Unfortunately, the chylothorax recurred after about two weeks. A chest tube was inserted and a trial of conservative treatment was tried again, but with no results at this time. Daily production of chyle was over 500 ml per day. After about ten days of unsuccessful treatment, the patient was discharged home with a Heimlich valve connected to the chest tube (we connected the Heimlich valve to a urinary bag, to which we made a small opening to its upper corner by scissors, so that to avoid potential air entrapment) with instructions for a medium chain triglyceride diet and weekly clinical and laboratory examinations on an outpatient basis, as well as daily measurement and recording of chyle production. The patient and her relatives were also instructed to contact us, whenever they noticed abrupt discontinuation of fluid production or shortness of breath, because these facts might be related to chest tube blockage or fever as evidence of infectious complications related to the chest tube. The patient had a daily production of chyle between 500 and 1000 ml per day at home. One month later on telephone conversation we were told that the previous 24-h chyle production was only 100 ml. The patient was instructed to come immediately to the hospital for examination. Chest X-ray showed a very small pleural effusion, so we decided to perform pleurodesis with bleomycin, by instilling four ampoules of bleomycin through the chest tube into the right pleural cavity and then clamping the tube for several hours. We removed the chest tube the following day. There were no local complications related to the use of bleomycin. On routine follow-up with chest X-ray every 15 days for the following six months there was no recurrence of chylothorax (Fig. 3) (note there are bilateral pleural effusions in chest X-ray performed about 4 months later. The patient was subjected to thoracentesis, which revealed clear, serous fluid).
Fig. 3.
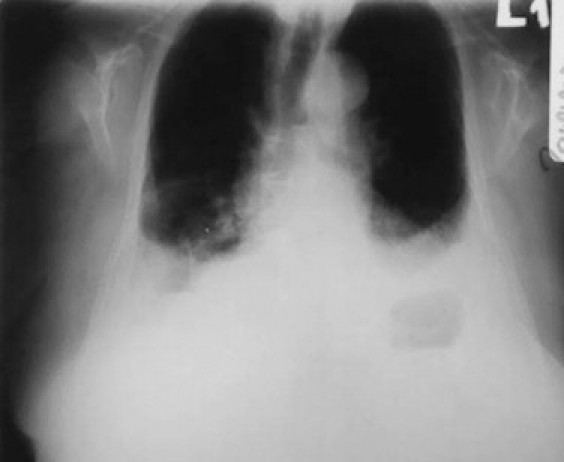
Chest X-ray performed at routine follow-up. Note bilateral pleural effusions, but no recurrence of chylothorax. The patient was subjected to thoracentesis, which revealed clear, serous fluid.
3. Discussion
Although CLL is often associated with pleural effusion,4 chylothorax from CLL is rare, with only few cases identified in the literature. Malignancies cause chylothorax through disruption or compression of the thoracic duct from mediastinal adenopathy or mass effect of the tumor itself. CLL causes diffuse adenopathy but rarely causes mediastinal adenopathy significant enough to obstruct the thoracic duct,2 which probably explains the rarity of chylothorax as a manifestation of that disease entity. The mechanism of how CLL causes chylothorax is not fully elucidated. There exist a few theories to explain how CLL may result in chylothorax. The most common mode is the classic way in which a malignancy in general causes chylothorax, that is through mediastinal adenopathy, as we mentioned above. Another model, probably most suitable and specific for CLL-induced chylothorax, involves the flow of leukemic lymphocytes through the lymphatic system. The presence of an extremely large number of abnormal lymphocytes in CLL may cause cell clustering in the lymphatic system. This sludging may result in obstruction of either the thoracic duct or lymphatics draining the pleura, resulting in a chylothorax.3 That model suits perfectly in our case, because no enlarged mediastinal lymph nodes were evident on chest CT scan.
Initial management of chylothorax due to CLL may be generally conservative, as is true with most cases of chylothorax due to various causes, with patients being given nothing by mouth and started on total parenteral nutrition. If the chylothorax does not resolve after about two weeks of conservative treatment, and depending on the amount of chyle produced, more aggressive measures have to be taken. If enlarged mediastinal lymph nodes are present, either chemotherapy or radiotherapy,5 or both can be offered as a treatment option.
In addition medical thoracoscopic pleurodesis of the pleural space can be tried as a therapeutic measure to resolve the chylothorax.6 In cases not responding to the measures mentioned above, surgery can be performed to ligate the thoracic duct,7 via a right thoracotomy incision or thoracoscopically.
In the case we present, treatment options were quite limited, because of the poor general condition of the patient. We resorted in the solution of Heimlich valve, after the recurrence of chylothorax and the unsuccessful second course of conservative treatment. That is not an ideal treatment strategy, because the patient loses albumin, proteins and other nutrients through the chyle, resulting in an impaired immune and nutritional status. It seems that our instructions to the patient for medium-chain triglyceride diet leaded to a marked diminution of daily chyle production, rendering us able to perform pleurodesis at a favorable time point, with the hope that creation of adhesions into the right pleural cavity might close the “fistulous” connection of the thoracic duct with the right pleural cavity. We judged that favorable conditions for performing pleurodesis existed, that is the lung to be fully expanded and daily production of pleural fluid to be negligible, so we decided to proceed to pleurodesis.
We are not aware of any other case of refractory CLL-induced chylothorax treated successfully by simple pleurodesis.
4. Conclusion
CLL-induced chylothorax is quite rare, so little clinical evidence exists to guide the clinician in determining which treatment modality is best for these patients. Mediastinal irradiation (even in the absence of mediastinal adenopathy), mediastinal irradiation followed by talc pleurodesis, and thoracic duct ligation with or without pleurodesis may all be reasonable options for the treatment of CLL-induced chylothorax.2,3,7 In the case we present pleurodesis was successful without use of other modalities. There seemed to be no other definite solution to the problem of that debilitated patient than to try a pleurodesis when circumstances allowed.
Conflict of interest
None.
Funding
None.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Author's contribution
G. Philippakis and M. Moustardas involved in study design, data analysis and writing the manuscript and G. Philippakis helped in data collections.
References
- 1.Light R.W. Pleural diseases. 4th ed. Lippincott Williams & Wilkins; Philadelphia: 2001. Chylothorax and pseudochylothorax. p. 327–43. [Google Scholar]
- 2.Zimhony O., Davidovitch Y., Shtalrid M. Chronic lymphocytic leukaemia complicated by chylothorax. J Intern Med. 1994;235:375–377. doi: 10.1111/j.1365-2796.1994.tb01090.x. [DOI] [PubMed] [Google Scholar]
- 3.Rice T.W., Milstone A.P. Chylothorax as a result of chronic lymphocytic leukemia: case report and review of the literature. South Med J. 2004;97(March (3)):291–294. doi: 10.1097/01.SMJ.0000072363.19239.FE. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins P.F., Ward M.J., Davies P., Fletcher J. Non-Hodgkin's lymphoma, chronic lymphatic leukaemia and the lung. Br J Dis Chest. 1981;75:22–30. doi: 10.1016/s0007-0971(81)80004-6. [DOI] [PubMed] [Google Scholar]
- 5.Bruneau R., Rubin P. The management of pleural effusions and chylothorax in lymphoma. Radiology. 1965;85:1085–1092. doi: 10.1148/85.6.1085. [DOI] [PubMed] [Google Scholar]
- 6.Mares D.C., Mathur P.N. Medical thoracoscopic talc pleurodesis for chylothorax due to lymphoma: a case series. Chest. 1998;114:731–735. doi: 10.1378/chest.114.3.731. [DOI] [PubMed] [Google Scholar]
- 7.Fahimi H., Casselman F.P., Mariani M.A., van Boven W.J., Knaepen P.J., van Swieten H.A. Current management of postoperative chylothorax. Ann Thorac Surg. 2001;71:448–451. doi: 10.1016/s0003-4975(00)02033-6. [DOI] [PubMed] [Google Scholar]


