Abstract
B-cell depletion therapy can be effective for treating B-cell lymphomas as well as many human and murine autoimmune diseases. B-cell-deficient mice are normally resistant to spontaneous autoimmune thyroiditis (SAT), but they develop SAT if regulatory T cells are transiently depleted during the first 3–6 weeks after birth. This was also a critical time when B-cell depletion effectively inhibited development of SAT in adult mice. The current study was undertaken to test the hypothesis that transient depletion of B cells using anti-CD20 would be sufficient to suppress SAT if B cells were depleted early in life and that inhibition of SAT would be due to the activity of Treg that functioned most effectively when B cells were absent or low. The results presented here support this hypothesis and indicate that development of autoimmune disease in adults is effectively inhibited when anti-CD20 is administered 1–3 weeks after birth. After 3 weeks, transient B-cell depletion is no longer effective, and B-cell depletion must be maintained to effectively suppress autoimmune disease. B-cell depletion in 1- to 3-week-old mice depletes all B-cell subsets, whereas B-cell depletion initiated in adults spares many marginal zone B cells. Following early B-cell depletion, splenic Treg increase in number, and depletion of Treg reverses the inhibitory effect of anti-CD20 on disease development. Early transient depletion of B cells could be useful for preventing autoimmune disease in individuals at high risk for developing autoimmune diseases as adults.
Keywords: autoimmunity, anti-CD20, B cells, Treg
Introduction
Spontaneous autoimmune thyroiditis (SAT) develops in all wild-type (WT) NOD.H-2h4 mice given 0.05% NaI in their drinking water (1–4). CD4+ T cells and B cells form clusters in the thyroid infiltrates, and B cells are required for development of SAT (1). Although B-cell-deficient (B−/−) and WT NOD.H-2h4 mice depleted of B cells by treatment from birth with anti-IgM are resistant to SAT, T cells from B−/− mice can function as effector cells for SAT if B cells are provided during the maturation of T cells from bone marrow precursors (5). Transient B-cell depletion in WT mice for the first 4–6 weeks after birth prevents development of SAT in adult mice, but if B-cell-deficient mice are given B cells as adults, they produce antithyroglobulin antibodies but do not develop SAT (5). These results suggest that the role of B cells in SAT is not simply to produce autoantibodies and that B cells might function as important antigen-presenting cells (APC) for initial activation of CD4+ effector cells. Effector T cells able to induce SAT could be activated in B−/− mice if CD25+ regulatory T cells (Treg) were transiently depleted (6), suggesting that if CD4+ T cells initially encounter autoantigen when B cells are low or absent, Treg are preferentially activated and SAT does not develop.
B cells are required for development of most spontaneous autoimmune diseases in mice, including diabetes, Sjogren’s syndrome, scleroderma, systemic lupus erythematosus (SLE) and SAT (5–12). B cells are also important for the development of some experimentally induced autoimmune diseases, such as arthritis (13, 14). B cells produce autoantibodies and contribute to the pathogenesis of autoimmune diseases through multiple mechanisms including functioning as APC for activation of CD4+ T cells (7, 8, 10, 13, 15, 16). B cells can also function as regulatory cells to inhibit immune responses and autoimmune diseases (17–19).
Depletion of B cells using antibodies directed against CD20 expressed by all mature B cells (Rituxan) is effective for treating B-cell lymphomas as well as several autoimmune diseases, including rheumatoid arthritis and SLE in humans (20–22). Anti-CD20 also suppresses development of many murine autoimmune diseases (11–13, 23–32), although in some models, B-cell depletion has opposite effects and can result in more severe disease (17–19, 28–30, 33).
We recently showed that administration of anti-CD20 to adult NOD.H-2h4 mice inhibited development of SAT and production of anti-mouse thyroglobulin (MTg) autoantibodies (24). In those experiments, B-cell depletion in peripheral blood was maintained throughout the 8-week period of SAT development by reinjection of anti-CD20 every 3 weeks (24). Before anti-CD20 became available for depleting B cells in adult mice, we showed that SAT could be inhibited by depletion of B cells using anti-IgM beginning at birth (5). In those studies, B-cell depletion had to be initiated on the day of birth to be effective but had to be maintained for only 4–6 weeks to effectively inhibit SAT development (5). We therefore hypothesized that continued administration of anti-CD20 might not be required for effective suppression of SAT if B cells were depleted early in life before pathogenic T cells were activated. The current study was undertaken to test this hypothesis. The results indicate that transient B-cell depletion beginning 1–3 weeks after birth is sufficient to inhibit development of SAT and antithyroglobulin antibodies in adult mice. Transient depletion of CD25+ Treg reversed the inihibitory effect of anti-CD20 on development of SAT and autoantibodies, suggesting that Treg may effectively suppress development of autoimmunity in adults if pathogenic T cells initially encounter antigen when B cells are absent.
Methods
Mice
NOD.H-2h4 mice express H-2Kk, I-Ak and Dd on the NOD background (34). Mice were bred and maintained in the animal facility at the University of Missouri as previously described (1, 2, 24). Both male and female mice were used in these studies, but all mice in a given experiment were the same sex. For some experiments, TCRα−/− NOD.H-2h4 mice were used as a source of mice lacking T cells. All animal protocols were approved by the University of Missouri Animal Care and Use Committee.
Experimental design
Anti-CD20 IgG2a mAb 18B12 was described previously (24). Anti-CD20 or isotype control was administered intraperitoneally (i.p.) or subcutaneously (s.c.) to WT NOD.H-2h4 mice beginning at various times after birth. For transient B-cell depletion, mice were given two injections of anti-CD20 with a 5- to 7-day interval between injections (100–150 μg per injection). For depletion of Treg, anti-CD20-treated or untreated mice were given two or three weekly injections of 0.3 mg anti-CD25 mAb PC61 or rat Ig (6) at various times relative to anti-CD20 treatment. This resulted in essentially complete depletion of CD25+ cells in the spleen and blood and depletion of all CD25+Foxp3+ T cells in the spleen and blood of Foxp3 GFP mice. CD25+ T cells gradually returned and reached pre-injection levels 3–4 weeks later (data not shown). Because CD25 depletion was not maintained for the duration of the experiment, it is referred to as transient Treg depletion in the text. Anti-CD25 was generated in our laboratory as previously described (6) or purchased from BioXcell (Lebanon, NH, USA). At 7–8 weeks of age, mice were given 0.05% NaI in their drinking water, and thyroids were removed 7–9 weeks later for evaluation of SAT severity (6, 24).
Adoptive transfer of T cells
To determine the effect of B-cell depletion and/or subsequent Treg depletion on the activity of CD4+ T cells that function as effector T cells for SAT, mice were given anti-CD20 or isotype control at 12 and 18 days of age as indicated above. Some mice were given two weekly injections of anti-CD25 to deplete Treg beginning 1 week after the second injection of anti-CD20. At 8 weeks of age, all mice were given NaI in their drinking water, and thyroids were removed 7 weeks later to evaluate SAT severity. Splenocytes from three mice in each group were pooled, and splenocytes from three naive NOD.H-2h4 mice were passed through nylon wool to deplete most B cells and enrich T cells. The resulting T-cell populations were determined to be >85% T cells and contained both CD4+ and CD8+ T cells. T cell enriched cells were transferred i.v. to irradiated (300 rad) TCRα−/−NOD.H-2h4 mice. Recipients were given NaI water, and thyroids were removed 8 weeks later.
Assessment of SAT
After 7–9 weeks on NaI water, thyroids were removed and one thyroid lobe from each mouse was fixed in formalin, sectioned and stained with hematoxylin and eosin (H&E) as previously described (6, 24). The other thyroid lobe was snap frozen in liquid nitrogen and stored at −70C for later use. All slides were scored by two individuals, and differences in interpretation were very rare. Thyroid histopathology scores were based on the percentage of thyroid follicles replaced by infiltrating lymphocytes as previously described in detail (2, 6). Briefly, a score of 0 indicates a normal thyroid or a few inflammatory cells infiltrating the thyroids. A 1+ severity score is defined as an infiltrate of at least 125 inflammatory cells in one or several foci, a 2+ score represents 10–20 foci of cellular infiltration, each the size of several follicles, with replacement or destruction of up to 1/4 of the gland. A 3+ score indicates that 1/4 to 1/2 of the thyroid follicles are replaced by infiltrating inflammatory cells, and 4+ indicates that >1/2 of the thyroid follicles are replaced or destroyed by inflammatory cells.
Autoantibody determination
MTg-specific autoantibodies were determined by ELISA using serum from individual mice diluted 1/50 or 1/100 as previously described (24).
Flow cytometry
Peripheral blood was obtained several times during all experiments to monitor depletion of CD19+ B cells. Spleens of some experimental mice were analyzed for expression of CD4, CD8, CD25, Foxp3, CD19, B220, CD21 and CD23 by flow cytometry (FACScan and FACSCalibur) as previously described (24). Antibodies were obtained from eBioscience (SanDiego, CA, USA).
Statistical analysis
A two-tailed Student’s t-test was used to determine the significance of differences in SAT severity and anti-MTg autoantibodies in experiments with two experimental groups. The significance of differences in experiments with three or more experimental groups was determined by the kruskal–wallis non-parametric test using GraphPad Prism software (GraphPad Software, Inc., LaJolla, CA, USA). The P values are provided in the footnotes to the tables; P values <0.05 were considered significant.
Results
Transient depletion of B cells 1–3 weeks after birth is sufficient to inhibit SAT development
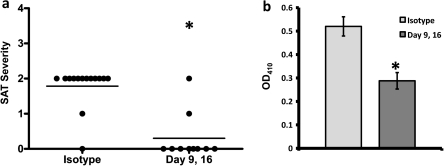
To test the hypothesis that transient B-cell depletion early in life should be sufficient to inhibit development of SAT in adults, WT NOD.H-2h4 mice were given anti-CD20 at 9 and 16 days of age. Analysis of B cells in peripheral blood by flow cytometry indicated that most (>90%) circulating CD19+ B cells were depleted for 2–3 weeks after the second injection of anti-CD20. B cells gradually returned over the next 3–4 weeks and when mice were given NaI water at 8 weeks of age, levels of circulating B cells were only slightly lower than those in mice given isotype control antibody (data not shown). Groups of anti-CD20 and isotype-treated control mice were given NaI water at 8 weeks of age and thyroids were removed 8 weeks later (Fig. 1). SAT severity and anti-MTg autoantibody responses were significantly reduced in anti-CD20-treated mice compared with isotype controls (P < 0.01). At the time thyroids were removed, anti-CD20-treated and isotype control mice had comparable numbers of B cells and there were no differences in the relative proportions of MZ, FO or T2 B cells in the two groups (data not shown).
Fig. 1.
Early transient depletion of B cells is sufficient to inhibit development of SAT in NOD.H-2h4 mice. NOD.H-2h4 mice were given 100 μg anti-CD20 or isotype control i.p. and s.c. 9 and 16 days after birth. All mice were given NaI in their water at 8 weeks of age and thyroids were removed 2 months later. (a) Symbols represent SAT severity scores of individual mice 8 weeks after NaI water. The mean severity score for the group is indicated by the line. (b) Anti-MTg autoantibodies diluted 1/50 in serum from individual mice 8 weeks after NaI water. Results are expressed as mean OD410 ± SEM. Significant differences between isotype control and anti-CD20-treated mice are indicated by the asterisk (P < 0.01).
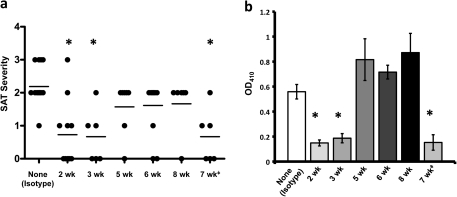
To determine if suppression of SAT following transient depletion of B cells was dependent on the age when B-cell depletion was initiated, groups of mice were given two weekly injections of anti-CD20 beginning at 2, 3, 5, 6 or 8 weeks of age. At 8 weeks, all mice and a group of mice given isotype control were given NaI water and thyroids were removed 8 weeks later (Fig. 2). Most mice given anti-CD20 beginning at 2 or 3 weeks of age developed minimal SAT, whereas two injections of the same amount of anti-CD20 beginning at 5 weeks of age or later had little effect on SAT development. Effects of administering anti-CD20 at 4 weeks of age were variable and are not shown. Anti-MTg autoantibody responses were reduced in mice given anti-CD20 at 2–3 weeks of age, but antibody responses were comparable to those of isotype-treated controls when B-cell depletion was delayed until 5–8 weeks of age (Fig. 2). As shown previously (24), when anti-CD20 was given to adult mice beginning at 7 weeks of age, SAT and autoantibody responses were suppressed when B-cell depletion was maintained throughout the 8 weeks of SAT development (Fig. 2; 7 weeks), but SAT was not suppressed if B-cell depletion was initiated in adults but was not maintained (Fig. 2). As noted above, >90% of CD19+ B cells in blood were depleted by anti-CD20 regardless of when treatment began. Recovery of B cells in blood began 2–3 weeks after injection of anti-CD20 and was essentially completed 6–7 weeks after the last anti-CD20 injection (data not shown). As shown below and in our previous study (24), B-cell depletion in the spleen was less complete and recovery began more quickly. These results indicate that if B-cell depletion is initiated early in life, transient depletion of B cells is sufficient to effectively suppress development of autoimmunity and autoantibody production in adults. By contrast, when B-cell depletion is initiated in adults, continued B-cell depletion is required to inhibit SAT and autoantibody production.
Fig. 2.
Suppression of SAT after transient B-cell depletion is dependent on the age when B cells are depleted. NOD.H-2h4 mice were given 150 μg anti-CD20 beginning at the indicated ages and an additional 100 μg anti-CD20 3–7 days after the first injection. Mice were given NaI water at 8 weeks of age and thyroids were removed 8 weeks later to assess SAT severity. Results are pooled from two separate experiments and are representative of four different experiments. The 7 weeks group (‡) represents mice that were given anti-CD20 every 3 weeks beginning at 7 weeks of age to maintain B-cell depletion as previously described (24). (a) SAT severity scores of individual mice 8 weeks after NaI water; the mean severity score for each group is indicated by the line. (b) Anti-MTg autoantibodies expressed as mean OD410 ± SEM of individual sera diluted 1/50. Groups that differ significantly from the isotype control are indicated by the asterisk (P < 0.05).
B-cell subsets and their susceptibility to depletion by anti-CD20 differ in young versus adult NOD.H-2h4 mice
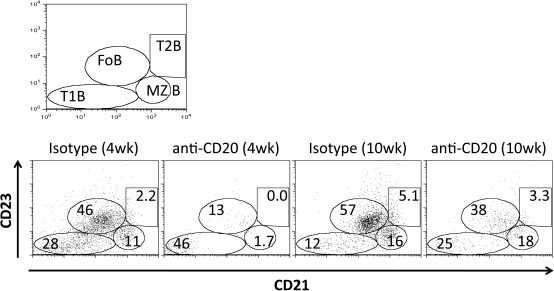
We previously showed that splenic marginal zone (MZ) B cells were relatively resistant to depletion by anti-CD20 when anti-CD20 was given to adult NOD.H-2h4 mice (24). Because MZ B cells develop later than other B-cell subsets in most strains of mice (35), the ability of transient early B-cell depletion to effectively inhibit development of SAT might be explained, at least in part, if B-cell depletion was more complete when initiated before the appearance of most MZ B cells. To address this question, B-cell subsets in spleens of NOD.H-2h4 mice of various ages were examined by flow cytometry, and the effect of anti-CD20 on depletion of different B-cell subsets was determined (Table 1). Spleens of 2-week-old NOD.H-2h4 mice had very few MZ or transitional (T2) B cells. By 3 weeks of age, the percentage of MZ B cells had nearly doubled, and T2 B cells were unchanged. By 4–5 weeks of age, the percentages of MZ and T2 cells were nearly as high as in adults (Table 1). Administration of anti-CD20 at 10 and 16 days of age resulted in depletion of essentially all splenic MZ and T2 B cells, as well as most follicular (FO) B cells 12–14 days later (Fig. 3, Table 2). Although anti-CD20-treated mice had significant numbers of splenic CD19+ B cells 2 weeks after anti-CD20, most B cells in the younger mice belonged to the so-called newly formed (CD23lo CD21lo) subset, and they had very low numbers of other B-cell subsets. Of particular interest, MZ B cells in mice given anti-CD20 at 2 and 3 weeks of age were almost completely depleted (Table 2). In contrast, B-cell depletion was less complete when anti-CD20 was given to adult mice. In particular, MZ B cells were relatively resistant to depletion as previously reported (24), with MZ B cells being reduced by ∼80% (Fig. 3, Table 2). These results suggest that more complete B-cell depletion might, at least in part, explain why anti-CD20 was more effective at inhibiting SAT development when given to mice <3 weeks of age.
Table 1.
Splenic CD4+ T cells and B-cell subsets in NOD.H-2h4 mice vary with age
| Age (weeks) | CD4 | CD19 | FO | MZ | T2 | T1 |
| 2 | 5.0 ± 0.8 | 61.0 ± 4.6 | 19.1 ± 4.3 | 2.6 ± 0.5 | 2.6 ± 1.6 | 41.3 ± 6.4 |
| 3 | 7.4 ± 1.0 | 50.4 ± 4.1 | 33.0 ± 1.8 | 5.1 ± 1.8 | 2.7 ± 0.9 | 29.3 ± 5.1 |
| 4–5 | 11.6 ± 1.9 | 55.8 ± 3.7 | 32.2 ± 6.7 | 9.8 ± 2.2 | 4.9 ± 2.0 | 24.5 ± 7.0 |
| 7–8 | 22.0 ± 1.6 | 47.1 ± 2.5 | 37.2 ± 1.9 | 16.6 ± 2.7 | 6.0 ± 0.5 | 11.1 ± 1.5 |
Mean percentages of positive cells ± SEM for six to eight individual mice. The percentage of each B-cell subset is expressed as a percentage of B220+ cells (see Methods).
Fig. 3.
Effectiveness of B-cell depletion by anti-CD20 varies depending on the age when B cells are depleted. Flow cytometric analysis of B220+ B cells in the spleens of NOD.H-2h4 mice given isotype control or anti-CD20 10 and 16 days after birth (4 weeks groups) or at 8 weeks of age (10 weeks groups). Spleens were removed 14 days after injection of antibody and analyzed by flow cytometry. The results are representative of the groups shown in Table 2 and include six to eight individual mice assayed for each group.
Table 2.
Early administration of anti-CD20 depletes most MZ and T2 B cells
| Agea | CD19 | FO | MZ | T2 | T1 |
| Iso (4 weeks) | 35.8 ± 1.9 (56.3)b | 19.2 ± 2.7 (53.8) | 4.1 ± 0.5 (11.3) | 0.64 ± 0.5 (1.8) | 9.5 ± 1.9 (26.5) |
| αCD20 (4 weeks) | 2.9 ± 0.5 (6.6) | 0.29 ± 0.1 (10.2) | 0.04 ± 0.04 (1.5) | <1 ± 0 | 1.2 ± 2 (41.5) |
| Iso (10 weeks) | 33.5 ± 2.4 (46.5) | 22.8 ± 1.9 (49.1) | 6.2 ± 0.8 (18.4) | 1.3 ± 0.5 (3.9) | 6.8 ± 1.4 (20.3) |
| αCD20 (10 weeks) | 8.3 ± 1.0 (11.7) | 2.4 ± 2 (29.3) | 1.2 ± 3 (15) | 0.2 ± 2 (2.2) | 2.4 ± 7 (29.6) |
In lines 1 and 2, mice were given anti-CD20 or isotype control at 10 and 16 days of age, and splenic B cells were evaluated by flow cytometry 12 days later when mice were 4 weeks of age. Mice in lines 3 and 4 were given anti-CD20 or isotype control at 6 and 7 weeks of age and B cells were evaluated 12 days later when mice were 9 weeks of age.
Results are expressed as the mean number of cells per spleen (×106) ± SEM for seven to eight individual mice for each cell population. Values in parentheses are the percentages of each cell type. CD19 cells are expressed as the number/percentage of CD19 ± SEM of total spleen cells. Numbers and percentages of the B-cell subsets are expressed as the number or percentage of each subset relative to the total percentage of CD19+ B cells. Mean splenocyte numbers for each group were as follows: line 1, 63.6 × 106; line 2, 66.4 × 106; line 3, 72.1 × 106 and line 4, 70.6 × 106.
Splenic CD4+Foxp3+ T cells are increased after B-cell depletion
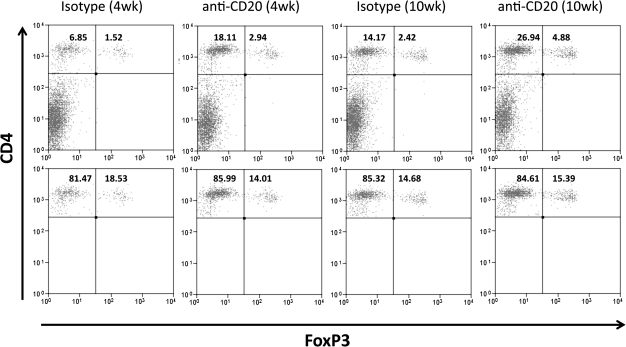
B−/− NOD.H-2h4 mice are resistant to SAT, but they develop SAT if Treg are transiently depleted in the first month after birth (6). These results suggest that Treg might be increased when B cells are low or absent. To determine if splenic Treg increased after B-cell depletion, Foxp3 GFP NOD.H-2h4 mice were given anti-CD20 at 10 and 16 days of age or at 6 weeks of age, and splenic CD4+Foxp3+ cells were examined 12 days later. Compared with age-matched mice given isotype control, mice given anti-CD20 had more splenic Foxp3+ CD4+ T cells (Table 3, Fig. 4). This was most evident when mice were given anti-CD20 at 2 weeks of age (line 2), primarily because 2-week-old mice have many fewer CD4+ T cells than adults. After B-cell depletion, total spleen cell numbers are relatively unchanged, but the percentage of CD4+ T cells in spleens of young mice is greatly increased. Therefore, young mice have more Foxp3+ T cells than age-matched mice given isotype control. Because adult mice have more CD4+ T cells and B-cell depletion is less complete, the differences in Foxp3+ CD4+ T-cell numbers in anti-CD20-treated and isotype controls are less evident and not statistically significant.CD8+ T cell numbers in spleens and lymph nodes of NOD.H-2h4 mice only slightly lower than the numbers of CD4+ T cells, and percentages of splenic CD8+ T cells were also greater in both groups of mice given anti-CD20. There were no detectable CD8+Foxp3+ T cells, and the CD4:CD8 ratio remained constant after B-cell depletion (data not shown). Taken together, the results in Tables 1–3 suggest that the ability of early B-cell depletion to effectively inhibit autoimmunity in adults could be due to increased numbers of Treg after B-cell depletion and also to the fact that B-cell depletion is more complete in young mice than in adults.
Table 3.
Increased splenic Foxp3+ Treg after depletion of B cells in NOD.H-2h4 mice
| Treatmenta | Splenocytes (×106)b | CD4+ (%) | CD4+ (×106) | CD4+Foxp3+ (%) | Treg/spleen (×106) |
| Isotype | 46.3 ± 0.8 | 9.6 ± 1.1 | 4.5 ± 0.7 | 14.1 ± 0.4 | 0.62 ± 0.1 |
| Anti-CD20 | 47.4 ± 0.2 | 23.2 ± 0.8 | 10.9 ± 0.6 | 12.3 ± 0.4 | 1.36 ± 0.1 |
| Isotype | 57.6 ± 0.2 | 17 ± 0.5 | 9.8 ± 0.6 | 10.9 ± 0.8 | 1.10 ± 0.1 |
| Anti-CD20 | 52.7 ± 0.2 | 26.4 ± 1.9 | 14.1 ± 1.4 | 11.3 ± 0.7 | 1.53 ± 0.2 |
Foxp3GFP NOD.H-2h4 mice were given two injections of 125 μg anti-CD20 IgG2a or isotype control at 10 and 16 days of age (lines 1 and 2) or at 6 weeks of age (lines 3 and 4). Foxp3+ Treg in spleens were determined 12 days later, i.e. mice in lines 1 and 2 were 4 weeks old and mice in lines 3 and 4 were almost 8 weeks old. At the time of assay, isotype controls in line 1 had 60.8 ± 1.2% CD19+ B cells compared with 10.5 ± 1.5 in anti-CD20-treated mice and mice in line 3 had 59.4 ± 0.9% CD19+ B cells compared with 18.4 ± 3.1 in age-matched anti-CD20-treated mice (line 4).
Mean ± SEM of six to seven mice per group. P < 0.01 for anti-CD20-treated compared with control mice in lines 1 and 2 and P > 0.05 for anti-CD20 compared with controls in lines 3 and 4.
Fig. 4.
Flow cytometric analysis of Foxp3+ Treg in anti-CD20-treated Foxp3 GFP NOD.H-2h4 mice. The top panels show representative results for Foxp3+ cells as a percentage of the total splenocytes. The percentage of CD4+ T cells for each group is the sum of the upper left and upper right quadrants. In the bottom panels, splenocytes are gated on CD4+ T cells and the percentage of Foxp3+ Treg expressed as a percentage of CD4+ T cells is indicated in the upper right quadrant. Results are representative of the groups shown in Table 3.
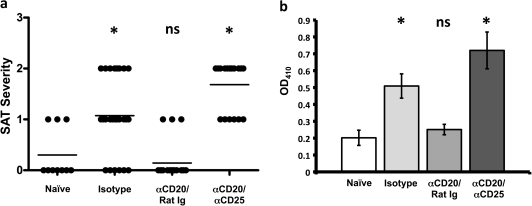
Transient depletion of Treg reverses the inhibitory effect of anti-CD20 on SAT development
To determine if suppression of SAT in adult WT mice after transient depletion of B cells early in life might be explained by the activity of Treg, mice were given two injections of anti-CD20 or isotype control at 10 and 16 days of age. One week later, half of the mice were given three weekly injections of anti-CD25 or rat Ig (e.g. at 3, 4 and 5 weeks of age), as previously described (6). At 8 weeks of age, all mice were given NaI water and thyroids were removed 8 weeks later (Fig. 5). As expected, most mice that were given anti-CD20 at 2 and 3 weeks of age developed no or mild (1+) SAT, and they also had lower anti-MTg autoantibody responses compared with mice given isotype control. However, when anti-CD20-treated mice were given anti-CD25, SAT severity scores and autoantibody responses were comparable to those of isotype controls (Fig. 5). As shown previously (6), anti-CD25 had little effect on SAT severity or anti-MTg autoantibody responses in mice given isotype control antibody. The results indicate that suppression of SAT in adult mice transiently depleted of B cells early in life is due, at least in part, to the activity of Treg. If Treg are transiently depleted, B-cell depletion has little or no effect on development of SAT and anti-MTg autoantibody responses in adults.
Fig. 5.
Suppression of SAT in mice given anti-CD20 is abrogated following transient depletion of Treg. Mice were given anti-CD20 or isotype control at 10 (100 μg) and 16 (150 μg) days of age. Some mice were given three weekly injections of anti-CD25 (PC 61) or rat Ig as a control beginning 1 week after the second injection of anti-CD20 or isotype control. All mice were given NaI water at 8 weeks of age, and thyroids were removed 8 weeks later. Results are pooled from two separate experiments and are representative of five similar experiments. (a) SAT severity scores of individual mice 8 weeks after NaI water; the mean severity score for each group is indicated by the line. (b) Anti-MTg autoantibody responses expressed as mean OD410 ± SEM of individual sera diluted 1/50. Groups that differ significantly from the isotype control are indicated by the asterisk (P < 0.05).
Based on our earlier results (6), we hypothesized that the effects of Treg would be greatest during the time B cells were depleted because mice with few B cells have increased percentages of CD4+ T cells and more Foxp3+ cells (Table 3). If so, delaying Treg depletion until after B-cell repopulation should result in more effective suppression of effector T cells and development of SAT should be impaired. To test this hypothesis, mice were given anti-CD20 at 9 and 15 days of age. Three days later, one group was given anti-CD25 as in Fig. 5. B-cell repopulaton in peripheral blood was monitored by flow cytometry, and after 6 weeks, circulating B cells in anti-CD20-treated mice had returned to >50% of normal levels (data not shown). At that time, half of the remaining mice were given 3 weekly injections of anti-CD25 (e.g. at 6, 7 and 8 weeks of age) and the rest were given rat Ig. One week later, mice were given NaI water and thyroids were removed 8 weeks later (Table 4). As in previous experiments, when anti-CD25 was given to anti-CD20-treated mice, while B cells were depleted, they developed SAT comparable to that of isotype controls (line 1 versus line 3). However, when Treg depletion was delayed until most B cells had repopulated the mice, there was less effect on development of SAT (line 2 versus line 4). Anti-CD25 had no significant effects on SAT or anti-MTg autoantibody responses in mice given isotype control (Table 4, line 1 versus line 5).
Table 4.
Depletion of Treg when B cells are low or absent is most effective at reversing the inhibitory effects of anti-CD20
| Treatmenta | SAT severityb |
Anti-MTgc | |||
| 0 | 1+ | 2+ | 3+ | 1/50 | |
| Isotype | 2 | 2 | 10 | 0 | 0.417 ± 0.077 |
| αCD20 | 10 | 3 | 2 | 0 | 0.196 ± 0.033 |
| αCD20/αCD25 | 0 | 2 | 7 | 3 | 0.695 ± 0.114 |
| αCD20/αCD25 | 5 | 1 | 3 | 1 | 0.302 ± 0.078 |
| Isotype/αCD25 | 3 | 3 | 4 | 0 | 0.531 ± 0.116 |
Mice were given anti-CD20 or isotype control at 9 (100 μg) and 15 (150 μg) days of age. Mice in line 3 were given three weekly injections of anti-CD25 (PC 61) beginning 1 week after the second injection of anti-CD20 when B-cell depletion was maximal. Mice in lines 4 and 5 were given three weekly injections of anti-CD25 beginning 4 weeks after the last injection of anti-CD20 when B cells in peripheral blood were at >50% of levels in isotype-treated controls. All mice were given NaI in their water beginning 1 week after the last injection of anti-CD25 in the mice in lines 4 and 5, and thyroids were removed 8 weeks later. Results are pooled from two separate experiments and are representative of three separate and similar experiments.
Numbers of mice with various SAT severity scores 8 weeks after NaI water (P < 0.05, line 2 versus lines 1 and 3; P > 0.05, line 2 versus line 4).
Anti-MTg autoantibodies expressed as mean OD410 ± SEM of 1/50 dilutions of serum.
Anti-CD20 inhibits activation of effector T cells, and Treg depletion reverses this inhibition
To begin to address the mechanism by which early B-cell depletion inhibits SAT, mice were given anti-CD20 or isotype control at 2 weeks of age. Half of the mice were given anti-CD25 1 and 2 weeks later, and mice were given NaI water at 8 weeks of age. After 8 weeks, splenic T cells were isolated from these donors as well as from a group of naive mice and transferred to lightly irradiated (300 Rad) TCRα−/− mice (see Methods). Histology of donor thyroids confirmed that donors given isotype control or anti-CD20 plus anti-CD25 had SAT (2–3+ severity), whereas donors given anti-CD20 had reduced (0-1+) SAT. Recipients were given NaI water and thyroids were removed 8 weeks later (Fig. 6). Mice that were not given T cells did not develop SAT (data not shown), and mice given T cells from naive mice developed minimal SAT. Although SAT severity was relatively low in these adoptive transfer experiments, recipients of T cells from donor mice with SAT (isotype control group) developed more severe SAT compared with mice given naive T cells, indicating that effector T cells had been activated in the former group. Mice given T cells from anti-CD20-treated donors developed SAT comparable to that seen in recipients of naive T cells, indicating that B-cell depletion inhibited activation of effector T cells. In contrast, anti-CD20-treated donors that were given anti-CD25 to deplete Treg had activated effector T cells since T cells from Treg-depleted anti-CD20-treated donors induced SAT comparable to that of isotype control-treated mice (Fig. 6). These results suggest that B-cell depletion inhibits development of SAT by inhibiting activation of effector T cells, and this is due, at least in part, to the activity of Treg in spleens of anti-CD20-treated mice. As in other experiments, anti-MTg autoantibody responses were highest in groups with higher SAT severity scores and lower in groups with lower severity scores.
Fig. 6.
T cells from isotype control-treated donors with SAT provide help to B cells from TCRα−/− mice, whereas T cells from anti-CD20-treated donors have minimal activity unless Treg are depleted. TCR-α−/− mice were irradiated (300 rad) and given 5 × 106 splenic T cells (purified using nylon wool as described in Methods). T-cell donors were either naive NOD.H-2h4 mice or mice that were given the indicated treatment before receiving NaI water for 8 weeks (SAT donors). Recipients were given NaI in their water, and thyroids were removed 8 weeks later. Results are pooled from three separate experiments. Mice not given T cells did not develop SAT and their anti-MTg autoantibody responses were <0.100 OD units (data not shown). (a) SAT severity scores of individual mice 8 weeks after NaI water; the mean severity score for each group is indicated by the line. (b) Anti-MTg autoantibody responses expressed as mean OD410 ± SEM of individual sera diluted 1/50. Groups differing significantly from the isotype control are indicated by the asterisk (P < 0.05).
Discussion
The results of this study describe a novel mechanism by which transient B-cell depletion early in life can inhibit development of autoimmune disease in adults. Depletion of B cells in both mice and humans using antibody directed against the CD20 molecule expressed on mature B cells is very effective for inhibiting autoimmune diseases (11–13, 20–33). In most murine studies, autoimmunity was prevented when B-cell depletion was initiated prior to disease onset and maintained throughout the period of autoimmune disease development (13, 24–30). The results of this study are the first to show that transient depletion of B cells is sufficient to suppress autoantibody production and development of autoimmune disease if B-cell depletion takes place early in life, presumably before autoreactive T cells become activated (Figs. 1 and 2). However, when B cells are depleted in adults, B-cell depletion has to be maintained over a longer period of time to effectively inhibit autoimmune disease and autoantibody production (Fig. 2).
Our previous studies demonstrated that SAT did not develop in B−/− mice unless Treg were transiently depleted in the first month of life (6). Based on those results, we hypothesized that if B cells were depleted in young WT mice, Treg might be expanded and it should be possible to suppress SAT development without sustained B-cell depletion. As shown in Table 3, B-cell depletion resulted in increased numbers of splenic Foxp3+ Treg. This was most obvious in young mice because they have low numbers of CD4+ T cells compared with adults, and when B cells are effectively depleted, both the percentages and the absolute numbers of CD4+ T cells are greatly increased. Although the proportion of CD4+ T cells that are Foxp3+ is unchanged after B-cell depletion, the absolute number of Tregs is increased. In adults, CD4+ T-cell numbers and percentages are higher, and B-cell depletion is less complete, so the increase in Treg numbers is less evident. The results of this study indicate that the increased numbers of Treg in mice given anti-CD20 early in life are likely to be physiologically relevant because Treg depletion completely reversed the inhibitory effect of early transient B-cell depletion on development of SAT and anti-MTg autoantibody responses (Fig. 5). Treg depletion was most effective when Treg were depleted, while B cells were depleted and had relatively little effect after B-cell repopulation (Table 4). There are several possible explanations that might explain why Treg depletion was less effective after B-cell reconstitution. For example, Treg were present over a longer period of time so that their ability to inhibit activation of effector T cells which is optimal when B cells are absent (6, 36) is prolonged. It is also possible that the repopulating B cells might function as regulatory cells to inhibit disease. Regulatory B cells clearly can inhibit other autoimmune diseases (17–19), and experiments are in progress to address this question. We are also generating Foxp3-DTR NOD.H-2h4 mice which will be useful in further experiments to allow us to specifically deplete Foxp3+ Treg rather than all CD25+ T cells which include some non-Treg.
In addition to the fact that Treg numbers were increased after B-cell depletion, Treg activated in the relative absence of B cells may be more effective at suppressing activation of effector CD4+ T cells that are beginning to expand in response to autoantigen, as suggested in our earlier results with B cell-deficient mice and by results of others (6, 36). Because it is not known when effector CD4+ T cells first respond to autoantigen in SAT or any other spontaneous autoimmune disease, it is difficult to directly address this issue. However, our results suggest that Treg may have their major effects on potential effector cells when B cells are absent (Table 4), and depletion of Treg in adult WT mice had relatively little effect on development of SAT (Table 4 and Fig. 5) (6). It should be noted that whereas depleton of Treg had little or no effect on development of SAT in WT NOD.H-2h4 mice in our studies, Nagayama et al. (37) showed that SAT severity was increased in WT NOD.H-2h4 mice following depletion of Treg by anti-CD25. Using the same injection schedule of anti-CD25 in our WT NOD.H-2h4 mice, we do not see more severe SAT in most experiments. There may also be differences in different colonies of NOD.H-2h4 mice that could account for these results. For example, Nagayama et al. (37) reported that their mice were lymphopenic, whereas our NOD.H-2h4 mice are not.
Another reason anti-CD20 more effectively suppressed development of autoimmunity when administered to young mice as compared with adults may be due to the fact that depletion of MZ B cells by anti-CD20 was more complete in young mice (Table 2, Fig. 3). Because MZ B cells develop later than other B-cell subsets (35), administration of anti-CD20 before most MZ B cells develop (1–2 weeks after birth in NOD.H-2h4 mice) resulted in almost complete depletion of this B-cell subset. MZ B cells have been shown to be more effective at activating naive CD4+ T cells than follicular B cells (38), and MZ B cells in NOD mice were shown to be important APC for activation of T cells in pancreatic lymph nodes (39). Although the role of MZ versus follicular B-cell subsets in SAT is not known, the fact that early B-cell depletion delayed the appearance of MZ B cells and suppressed SAT development would be consistent with the results mentioned above.
The fact that Treg suppress development of autoimmune diseases in B−/− mice has been demonstrated for several spontaneous organ-specific autoimmune diseases, including SAT, diabetes and Sjogren’s syndrome (6, 36, 40). Indeed, we believe that this could be a general mechanism that explains, at least in part, why B−/− mice are resistant to most spontaneous autoimmune diseases. A possible role for Treg in suppression of autoimmune disease in mice given anti-CD20 to deplete B cells and in humans treated with Rituxan has been suggested previously (23, 41, 42). To our knowledge, this report is the first to show that the age at which B-cell depletion occurs influences the effectiveness of suppression of autoimmunity and that the inhibitory effects of B-cell depletion can be reversed by transient Treg depletion. While this paper was being reviewed, two other reports using different autoimmune disease models showed that Tregs were expanded following B-cell depletion by anti-CD20 (43, 44). Thus, it is likely that activation of Treg in the relative absence of B cells provides one explanation for the efficacy of B cell targeted therapy in autoimmune disease. However, it is not the only mechanism because B cells can also function to suppress some autoimmune diseases, such as experimental autoimmune encephalomyelitis, colitis and biliary cirrhosis (17–20, 30, 33). Indeed, the partial B-cell depletion in adult NOD.H-2h4 mice treated with anti-CD20 could lead to activation of regulatory B cells that could act directly or indirectly through Treg (18) to suppress SAT. Continued analysis of the mechanisms underlying suppression of autoimmune diseases in animal models after B-cell depletion will lead to a better understanding of the benefits and potential hazards for using B-cell depletion for treating autoimmune diseases in man. The results presented here may provide a rationale for testing the effectiveness of early transient depletion of B cells as a potential means of preventing autoimmunity in certain high-risk individuals.
Funding
NIH Grant (AI 076395-01); Arthritis National Research Foundation; Children's Miracle Network Telethon.
References
- 1.Braley-Mullen H, Sharp GC, Medling B, Tang H. Spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J. Autoimmun. 1999;12:157. doi: 10.1006/jaut.1999.0272. [DOI] [PubMed] [Google Scholar]
- 2.Yu S, Medling B, Yagita H, Braley-Mullen H. Characteristics of inflammatory cells in spontaneous autoimmune thyroiditis of NOD.H-2h4 mice. J. Autoimmun. 2001;16:37. doi: 10.1006/jaut.2000.0458. [DOI] [PubMed] [Google Scholar]
- 3.Rasooly L, Burek CL, Rose NR. Iodine-induced autoimmune thyroiditis in NOD.H-2h4 mice. Clin. Immunol. Immunopathol. 1996;81:287. doi: 10.1006/clin.1996.0191. [DOI] [PubMed] [Google Scholar]
- 4.Verma S, Hutchings P, Guo J, Mclachlan S, Rapoport B, Cooke A. Role of MHC class I expression and CD8+ T cells in the evolution of iodine-induced thyroiditis in NOD-H2(h4) and NOD mice. Eur. J. Immunol. 2000;30:1191. doi: 10.1002/(SICI)1521-4141(200004)30:4<1191::AID-IMMU1191>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 5.Braley-Mullen H, Yu S. Early requirements for B cells for development of spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J. Immunol. 2000;165:7262. doi: 10.4049/jimmunol.165.12.7262. [DOI] [PubMed] [Google Scholar]
- 6.Yu S, Maiti PK, Dyson M, Jain R, Braley-Mullen H. B cell-deficient NOD-H2h4 mice have CD4+CD25+ T regulatory cells that inhibit the development of spontaneous autoimmune thyroiditis. J. Exp. Med. 2006;203:349. doi: 10.1084/jem.20051438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J. Immunol. 1998;161:3912. [PubMed] [Google Scholar]
- 8.Noorchasm H, Lieu YK, Noorchasm N, et al. I-Ag7-mediated antigen presentation by B lymphocytes is critical in overcoming a checkpoint in T cell tolerance to islet β cells of nonobese diabetic mice. J. Immunol. 1999;163:743. [PubMed] [Google Scholar]
- 9.Wong FS, Wen L, Tang M, et al. Investigation of the role of B cells in type I diabetes in the NOD mouse. Diabetes. 2005;53:2581. doi: 10.2337/diabetes.53.10.2581. [DOI] [PubMed] [Google Scholar]
- 10.Chan O, Hannum LG, Haberman AH, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J. Exp. Med. 1999;189:1639. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasegawa M, Hamaguchi Y, Yanaba K, et al. B-lymphocyte depletion reduces skin fibrosis and autoimmunity in the tight-skin mouse model for systemic sclerosis. Am. J. Pathol. 2006;169:954. doi: 10.2353/ajpath.2006.060205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayakawa H, Tedder TF, Zhuang Y. B-lymphocyte depletion ameliorates Sjogren's syndrome in Id3 knockout mice. Immunology. 2007;122:1. doi: 10.1111/j.1365-2567.2007.02614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamel K, Doodes P, Cao Y, et al. Suppression of proteogycan-induced arthritis by anti-CD20 B cell depletion therapy is mediated by reduction in autoantibodies and CD4+ T cell reactivity. J. Immunol. 2008;180:4994. doi: 10.4049/jimmunol.180.7.4994. [DOI] [PubMed] [Google Scholar]
- 14.O’Neill SK, Shlomchik MK, Giant T, et al. Antigen-specific B cells are required as APC’s and autoantibody-producing cells for induction of severe autoimmune arthritis. J. Immunol. 2005;174:3781. doi: 10.4049/jimmunol.174.6.3781. [DOI] [PubMed] [Google Scholar]
- 15.Harvey B, Gee R, Haberman A, Shlomchik MJ, Mamula M. Antigen presentation and transfer between B cells and macrophages. Eur. J. Immunol. 2007;37:1739. doi: 10.1002/eji.200636452. [DOI] [PubMed] [Google Scholar]
- 16.Yan J, Harvey B, Gee R, Shlomchik MJ, Mamula MJ. B cells drive early T cell autoimmunity in vivo prior to autoantigen presentation. J. Immunol. 2006;178:3447. doi: 10.4049/jimmunol.177.7.4481. [DOI] [PubMed] [Google Scholar]
- 17.Mauri C, Ehrenstein MR. The ‘short’ history of regulatory B cells. Trends Immunol. 2007;29:34. doi: 10.1016/j.it.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Mann ME, Maresz K, Shriver LP, Tan Y, Dittel BN. B cell regulation of CD4+CD25+ T regulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. J. Immunol. 2007;178:3447. doi: 10.4049/jimmunol.178.6.3447. [DOI] [PubMed] [Google Scholar]
- 19.Matsushita T, Yanaga K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J. Clin. Invest. 2008;118:3420. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thatayatikom A, White AJ. Rituximab: a promising therapy in systemic lupus erythematosus. Autoimmun. Rev. 2006;5:18. doi: 10.1016/j.autrev.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Sanz I, Anolik J, Looney RJ. B cell depletion therapy in autoimmune diseases. Front. Biosci. 2007;12:2546. doi: 10.2741/2254. [DOI] [PubMed] [Google Scholar]
- 22.Leandro MJ, de la Torre I. Translational mini-review series on B cell-directed therapies: the pathogenic role of B cells in autoantibody-associated autoimmune diseases-lessons from B cell-depletion therapy. Clin. Exp. Immunol. 2009;157:191. doi: 10.1111/j.1365-2249.2009.03978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu C, Rodriguez-Pinto D, Du W, et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J. Clin. Invest. 2008;117:3857. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu S, Dunn R, Kehry MR, Braley-Mullen H. B cell depletion inhibits spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J. Immunol. 2008;180:7706. doi: 10.4049/jimmunol.180.11.7706. [DOI] [PubMed] [Google Scholar]
- 25.Yanaba K, Hamaguchi Y, Venture G, Streeter D, St Clair EW, Tedder TF. B cell depletion delays collagen-induced arthritis in mice: arthritis induction requires synergy between humoral and cell-mediated immunity. J. Immunol. 2007;179:1369. doi: 10.4049/jimmunol.179.2.1369. [DOI] [PubMed] [Google Scholar]
- 26.Ahuja A, Shope J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B cells in murine lupus: efficacy and resistance. J. Immunol. 2007;179:3551. doi: 10.4049/jimmunol.179.5.3351. [DOI] [PubMed] [Google Scholar]
- 27.Bekar KW, Owen T, Dunn R, et al. Prolonged effects of short-term anti-CD20 B cell depletion therapy in murine systemic lupus erythematosus. Arthritis Rheum. 2010;62:2443. doi: 10.1002/art.27515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haas K, Watanabe MR, Matsushika T, et al. Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/W F1 mice. J. Immunol. 2010;184:4789. doi: 10.4049/jimmunol.0902391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kyaw T, Tay C, Khan A, et al. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J. Immunol. 2010;185:4410. doi: 10.4049/jimmunol.1000033. [DOI] [PubMed] [Google Scholar]
- 30.Moritoki Y, Lian ZX, Lindor K, et al. B-cell depletion with anti-CD20 ameliorates autoimmune cholangitis but exacerbates colitis in transforming growth factor-beta receptor II dominant negative mice. Hepatology. 2009;50:1893. doi: 10.1002/hep.23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang A-H, Skupsky J, Scott DW. Effect of B cell depletion using anti-CD20 therapy on inhibitory antibody formation to human FVIII in hemophilia A mice. Blood. 2011;117:2223. doi: 10.1182/blood-2010-06-293324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ueki I, Abiru N, Kobayashi M, et al. B cell-targeted therapy with anti-CD20 monoclonal antibody in a mouse model of Graves’ hyperthyroidism. Clin. Exp. Immunol. 2011;163:309. doi: 10.1111/j.1365-2249.2010.04301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhirapong A, Lieo A, Yang GX, et al. B cell depletion therapy exacerbates murine primary biliary cirrhosis. Hepatology. 2011;53:527. doi: 10.1002/hep.24044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Podolin PL, Pressey A, DeLarato NH, Fischer PA, Peterson LB, Wicker LS. I-E+ nonobese diabetic mice develop insulitis and diabetes. J. Exp. Med. 1993;178:793. doi: 10.1084/jem.178.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pillai S, Cariappa A, Moran ST. Marginal zone B cells. Ann. Rev. Immunol. 2005;23:1161. doi: 10.1146/annurev.immunol.23.021704.115728. [DOI] [PubMed] [Google Scholar]
- 36.Marino E, Batten M, Groom J, et al. Marginal zone B cells of non-obese diabetic mice expand with diabetes onset, invade the pancreatic lymph nodes and present autoantigen to diabetogenic T cells. Diabetes. 2007;58:1568. doi: 10.2337/db07-0589. [DOI] [PubMed] [Google Scholar]
- 37.Nagayama Y, Horie I, Saitoh O, Nakahara M, Abiru N. CD4+CD25+ naturally occurring regulatory T cells and not lymphopenia play a role in the pathogenesis of iodide-induced autoimmune thyroiditis in NOD.H-2h4 mice. J. Autoimmun. 2007;29:195. doi: 10.1016/j.jaut.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Attanavanich K, Kearney JF. Marginal zone, but not follicular, B cells are potent activators of naïve B cells. J. Immunol. 2004;172:803. doi: 10.4049/jimmunol.172.2.803. [DOI] [PubMed] [Google Scholar]
- 39.Marino E, Villanueva J, Walters S, Liuwantara D, Mackay F, Grey ST. CD4+CD25+ T cells control autoimmunity in the absence of B-cells. Diabetes. 2009;58:1568. doi: 10.2337/db08-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mellanby RJ, Thomas D, Phillips JM, Cooke A. Diabetes in non-obese diabetic mice is not associated with quantitative changes in CD4+CD25+ Foxp3+ regulatory T cells. Immunology. 2007;121:15. doi: 10.1111/j.1365-2567.2007.02546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sfikakis PP, Souliotis VL, Fragiakaki KG, Moutsopoulos HM, Boletis JN, Theofilopoulos AN. Increased expression of the Foxp3 functional marker of regulatory T cells following B cell depletion with rituximab in patients with lupus nephritis. Clin. Immunol. 2007;123:66. doi: 10.1016/j.clim.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Vallerskog T, Gunnarsson I, Widhe M, et al. Treatment with rituximab affects both the cellular arm and the humoral arm of the immune system in patients with SLE. Clin. Immunol. 2007;122:62. doi: 10.1016/j.clim.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 43.Hamel K, Cao Y, Ashaye S, et al. B cell depletion enhances T regulatory cell activity essential in the suppression of arthritis. J. Immunol. 2011;187:4900. doi: 10.4049/jimmunol.1101844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serreze DV, Chapman HD, Niens M, et al. Loss of intra-islet CD20 expression may complicate efficacy of B cell-directed type 1 diabetes therapies. Diabetes. 2011;60:2914. doi: 10.2337/db11-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]