Summary
A non-exothermic material that demonstrates clinical pain relief comparable to polymethylacrylate (PMMA) for vertebroplasty and promotes bone healing is desirable. The purpose of this investigation is to demonstrate clinical pain score improvement and bone healing following vertebroplasty with a novel bi-phasic ceramic cement.
Twenty patients were prospectively treated for compression fractures in a single center in the USA with the injectable bi-phasic ceramic bone substitute. Statistical comparison of pain scores was made during a 12 month follow-up retrospectively against a matched cohort of patients treated with PMMA vertebroplasty by the same neuroradiologist (HPH) in the same setting. The bone remodeling material was also evaluated with histology in a New Zealand white rabbit model.
The bi-phasic material demonstrated a pre-operative mean VAS score of 8.5 (± 1.6) with a significant post-operative pain relief mean VAS score of 1.8 (± 2.5) after one week, which was maintained throughout the 12 month follow-up period. These data are in line with the pain scores for the PMMA treated cohort. CT scans six and 12 months after surgery with the bi-phasic cement showed healing of the osteoporotic fractures. In the rabbit model, histology with the study material showed evidence of incorporation, new bone growth and bone healing in a cancellous bone defect.
Both the clinical results and the histologic evidence of bone healing and new bone growth support the application of this new bioinjectable material as an alternative to the use of PMMA for vertebroplasty.
Key words: bio-ceramic, bone healing, compressed fracture, pain, vertebroplasty
Introduction
Vertebral fracture is the most common complication of osteoporosis 1 and compression fractures may account for as much as 33% to 46 % of all osteoporotic fractures 2, with one to 1.5 million presenting clinically worldwide each year 3. They cause severe morbidity, leaving patients with incapacitating back pain for several months or longer 4. Historically, treatment of VCFs consisted of rest and relief of pain through analgesics. The advent of percutaneous material delivery, introduced in 1984 with polymethylmethacrylate 5 has provided a viable treatment option.
Injection of PMMA stabilizes the fracture thus preventing micro-motion at the fracture site which helps to alleviate pain 6,7 and quickly improve the quality of life 3,7 and this is now a well-recognized and accepted method for treatment. However, the PMMA exothermic curing process creates thermal necrosis and fibrous tissue surrounding the polymer 8 and this can potentially restrain fusion of the fractured bone interfaces. Also the risk of adjacent segmental fractures has been described as high as 20% within the first year leading to further intervention 9.
A non-exothermic material that more closely represents the mechanical properties of trabecular bone, eliminates fracture micro-motion, promotes bone healing and reduces the risk of adjacent fractures 10, could represent an attractive material.
The objective of this study was to investigate a novel injectable bi-phasic ceramic (Cerament™) bone substitute for vertebroplasty because of its potential to avoid thermal damage while providing stabilization and opportunity for new bone growth. Specifically, this investigation was to demonstrate the time course of radiographic healing and pain relief after human vertebral compression fractures treated with the bio-ceramic bone substitute. Our report describes the clinical outcome of the initial 20 patients in a prospective pilot study on vertebroplasty. Comparison was made retrospectively to the most recent matched population of patients who had PMMA vertebroplasty by the same neuroradiologist (HPH) in the same setting. Additionally, histological confirmation of incorporation of the resorbable study material, bone healing, and new bone formation were studied using a rabbit model with a distal femoral cancellous bone defect.
Materials and Methods
Materials
The bi-phasic ceramic bone substitute (CERAMENT™, Bone Support A.B., Lund, Sweden) is composed of a mixture of synthetic calcium sulfate cement (60% by weight) and hydroxyapatite (40% by weight). These two components are mixed with a water-soluble radio-contrast agent, iohexol, to make the material radiopaque. The combination produces a flowable and injectable paste that is easily introduced into the vertebral bodies through the pedicles at the time of vertebroplasty. The material then sets within 15 minutes and fully hardens within 45 minutes. This material has an immediate compressive strength similar to that of healthy cancellous bone 11.
The CSC component gradually reabsorbs during the first months being replaced by in-growing bone that remodels to form trabecular bone, whereas the HA component has a slower reabsorbing rate and is incorporated into the newly formed trabecular bone 12.
The bio-ceramic bone substitute used in this study has CE mark approval in the European Union for vertebroplasty, and is FDA cleared in the USA as bone void filler. Patients included into this study signed an informed consent for the use of Cerament™ in vertebroplasty.
Human Clinical Series
Beginning in May 2008, 20 patients with vertebral compression fractures were treated for compressed fractures ranging from AO classifications A1.1 to A1.3. All procedures were performed by a single neuroradiologist (HPH) at a free-standing ambulatory surgery center (Table 1). Patients were treated either bi- or uni-pedicularly by percutaneous injection of the fracture site. A retrospective analysis was made of the most recent matched population of patients who had PMMA vertebroplasty by the same neuroradiologist (HPH) in the same setting, to statistically compare the primary outcome of pain.
Table 1.
Patient characteristics.
| Treatment | Sex | Av. age (years) |
Av. vol. injected (ml./level) |
|---|---|---|---|
| Cerament™ | 10M/10F | 75 | 2.5 |
| PMMA | 8M/12F | 78 | 4.2 |
Enrollment criteria included patients who had sustained low energy trauma vertebral compression fractures in the region from T5 to L5. Vertebral height compression of the affected vertebral bodies was limited to 0% to 60% compared to the posterior height. Fractures were verified by magnetic resonance imaging, and vertebral compression history confirmed by the patient to be within 8 weeks. A maximum of two level vertebroplasties was allowed.
Exclusion criteria were age below 50 years, retropulsion of fracture fragment, and concomitant diseases which might be worsened by invasive treatment of the fracture such as local tumor. Patients were also excluded if they had a history of anaphylactic reaction towards iodine-based radio contrast agents, known hyperthyreosis and/or thyroid adenoma, known bleeding disorders, had a local infection at site of implantation, or were treated with anticoagulant medication. When patients meeting the enrollment criteria were identified, they were offered the vertebroplasty procedure and informed consent was obtained. The “practice of medicine” clinical protocol has been approved by the Clinical Protocol Committee of Indian River Radiology, Vero Beach, Florida, USA.
Prior to vertebroplasty, visual analog scale pain scores (0-10 scale) were recorded. A trans-pedicular approach was used with either 11 gauge or 13 gauge Jamshidi needles. A C-arm with a 23 inch image intensifier was used for fluoroscopy. The injection of the bi-phasic material was constantly monitored by fluoroscopy to identify its location within the vertebral bodies. The tip of the needle was gently retracted with bio-ceramic bone substitute delivery to seal the fractured bone fragments while minimizing the potential for leakage. When appropriate, the process was repeated for the contra-lateral pedicle. Typically a total of 2-5 ml of the material is deployed per level. The total volume of bio-ceramic material was recorded during each procedure.
Outcome Measures
Follow-up VAS pain scores were obtained after one week, three, six and 12 months. Post-vertebroplasty CT scans were targeted to treated levels to limit exposure to radiation. This allowed examination of the time course of bone resorption and healing, and assessment against the animal CT scans. An immediate post-procedure CT scan was obtained and repeated at six and 12 months. Follow-up x-rays were obtained as needed.
Animal Study
Animal studies were conducted solely on the investigational material to examine the resorption of the material and the concomitant incorporation of new bone. Six six-month-old female New Zealand white rabbits had bilateral distal femoral lateral condyle cancellous defects (drill-hole 8 mm length × 5 mm diameter) randomly filled with the bio-ceramic bone substitute or left empty. The rabbits received a combination of ketamine (11 mg/kg intramuscularly) and xylazine (0.22 mg/kg intramuscularly) for pre-anesthesia followed by isoflurane inhalation anesthesia (1.5%) for the duration of the surgery.
Rabbits had in vivo μCT scans (voxel resolution of 28 μm) at three, seven and 12 weeks to observe volumetric defect occupancy with mineralized material and were sacrificed after twelve weeks. Decalcified sections through the healing bone defect were stained with H and E, and undecalcified sections were evaluated using calcein fluorochrome labeling of new bone growth (injected three days before sacrifice).
All procedures for the rabbit study were approved by the Institutional Animal Care and Use Committee of the University of Louisville, Louisville, Kentucky, USA.
Outcome Measure
Bone healing was quantified by calculating the percentage of new bone growth in each section.
Statistical Methodology
Data are presented as mean ± standard deviation. Single factor ANOVA was used to assess differences between baseline and all subsequent VAS measurements for each cement-type cohort. (Repeated measures ANOVA could not be used due to several data points lost to follow up). Comparisons between the bio-ceramic and PMMA groups at each corresponding time point were by Student's t-test. Data analysis software (Microsoft Excel 2007) was used for all analyses. Results were considered statistically significant at the p <0.05 level.
Results
Human Clinical Series
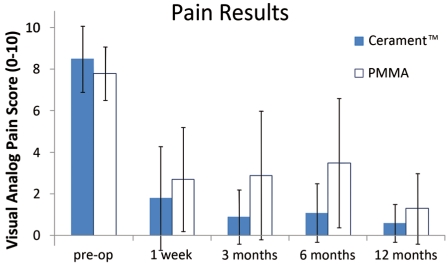
A total of 20 patients, evenly balanced male to female, were treated between May 2008 and July 2009. Eighteen patients were treated for single level fractures and two patients were treated at two adjacent levels. The average volume of bio-ceramic material injected per level was 2.5 ± 1.6 ml. and the average patient age was 74.7 years (range: 54-90). The pre-operative VAS pain score was 8.5 ± 1.6 and one-week VAS was 1.8 ± 2.5. Six month VAS for 14 of the 20 patients (two deceased and four seasonally re-located) was 1.1 ± 1.4. Twelve month VAS for 17 of the 20 patients (two deceased and one LTF) was 0.6 ± 0.9.
For comparison, a similar cohort of patients previously treated by the same physician using PMMA cement for percutaneous vertebroplasty had the following statistics: age: 78.5 (range: 57-92); pre-procedure VAS: 7.8 ± 1.3; one-week VAS: 2.7 ± 2.5; six months VAS: 2.7 ± 2.8; and final 12 month follow-up VAS: 1.3 ± 1.7 (Figure 1). None of VAS scores were significantly different when compared between Cerament™ and PMMA.
Figure 1.
Average VAS scores over time from pre-op to 12 months post-op showing improvement in pain relief with time.
There was no evidence in the follow-up period of a re-fracture of any of the levels treated with the bio-ceramic material. There were remote fractures in two patients and adjacent level fractures in the same two patients (Table 2). In one of the patients the adjacent level fracture (T8) was also located adjacent to a vertebra (T7) treated with PMMA six months prior to this fracture. In the other patient, the adjacent level fracture occurred 11 months after the Cerament™ treatment. The adjacent and remote fractures in these patients were treated to protocol with vertebroplasy using PMMA.
Table 2.
Two patients experienced both adjacent and remote fractures in the 12 month follow-up period.
| Fracture type | Patient 1 | Time after VP (Months) |
Patient 2 | Time after VP (Months) |
|---|---|---|---|---|
| Primary fracture | T9 | 0 | L3 | 0 |
| Adjacent fractures | T8 | 3 | L2 | 11 |
| Remote fractures | T5 | 5.5 | T10, L1, L5 | 7, 10, 10 |
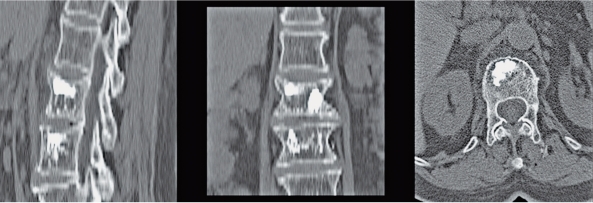
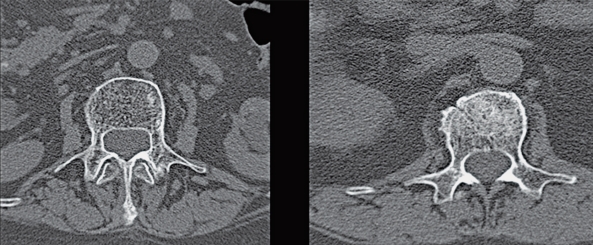
The transverse images and the sagittal reconstructions of the post procedure CT scans show local distribution of the bio-ceramic in each case (Figure 2). The radiocontrast was not visible on six and twelve month follow-up CT scans, with signs of fracture healing as indicated by disappearance of the fracture surfaces with time as well as incorporation of the bio-ceramic (Figure 3).
Figure 2.
Sagittal and transverse CT scans showing fill of bi-phasic ceramic to seal fractured elements.
Figure 3.
Transverse CT scans of 2 patients at 6 and 12 months post-op show healing with no evidence of radiocontrast.
Animal Study
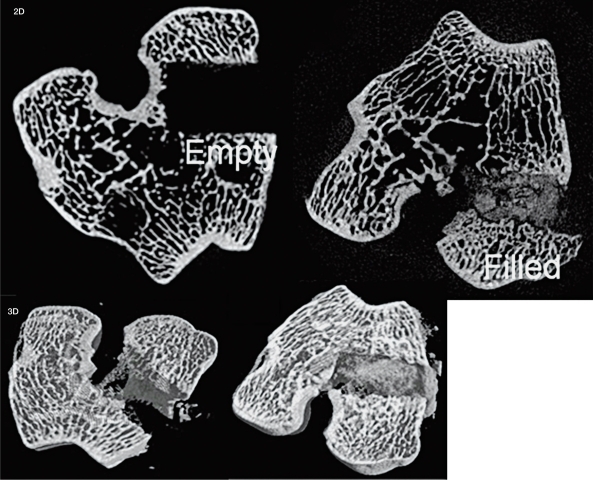
The rabbit μCT analysis showed significantly more mineralized material in the filled defects (FILLED) compared to the empty defects (EMPTY) at three weeks: 56 ± 7% and 12 ± 5%; seven weeks: 58 ± 10% and 14 ± 5%; and 12 weeks: 38 ± 18% and 13 ± 6%, p<0.05 (Figure 4). Decalcified histology healing and the amount of new bone formation were significantly greater after 12 weeks in the FILLED (22.5 ± 12.6%) compared to EMPTY (5.8 ± 2.6%), p<0.05 (Figure 5). Undecalcified sections observed under fluorescence microscopy demonstrate new bone formation (Figure 6).
Figure 4.
Images (both 2-D and 3-D) of rabbit μCT scans of EMPTY (left) and FILLED (Right) defects after 12 weeks demonstrate the difference in appearance of the two different conditions.
Figure 5.
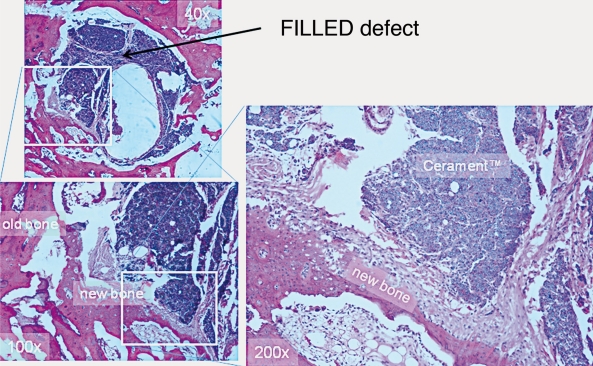
Decalcified histological section through a bi-phasic ceramic filled rabbit defect at 12 weeks shows the extent of new bone formation and residual material.
Figure 6.
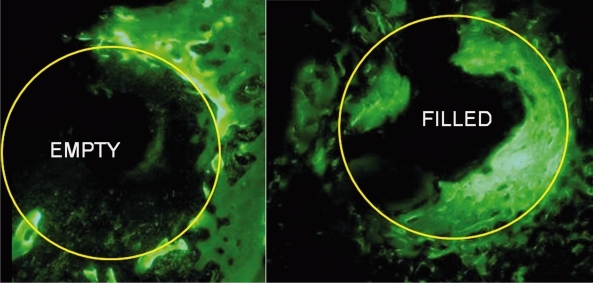
Undecalcified sections observed under fluorescence microscopy through defects at 12 weeks shows the recently formed new bone labeled with calcein.
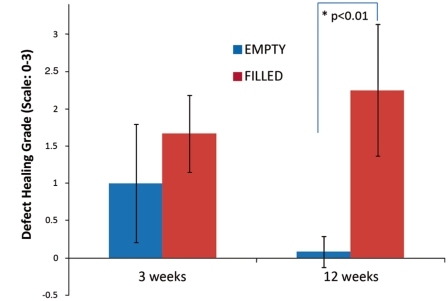
Qualitative grading of defect healing was performed at three and 12 weeks. A grade from 0 to 3 [0=no cellular activity, 1=minimal cellular activity only at the defect boundary, 2=cellular activity with new bone formation at the defect boundary, 3=extensive cellular activity with new bone extending to the defect center] was assigned to each histological section. In the EMPTY defects, an early bone healing response subsided between three and 12 weeks, while in the bio-ceramic FILLED defects the healing response was greater and continued to 12 weeks with a statistically significant difference at 12 weeks (Figure 7).
Figure 7.
Analogue scale grading of the extent of defect healing was significantly different at twelve weeks between EMPTY and FILLED defects.
Although no PMMA control group was studied directly in this rabbit study, a previous study using the same model and similar time points (four and 12 weeks) showed no new bone formation within the PMMA cement-filled defects 13.
Discussion
The primary goal of vertebroplasty and vertebral augmentation procedures is pain relief. In this pilot study of 20 patients with the bio-ceramic bone substitute, the pain scores improved statistically significant and clinically relevant, within one week, with an average VAS score change of 6.7, which is on the same level as VAS score changes found in the retrospective comparison with the same neuroradiologist (HPH) and in the same setting with PMMA vertebroplasty. The pain relief is consistent with improvement reported generally for PMMA vertebroplasty 14 and was maintained at all time points within the 12 month follow-up period, with a tendency towards further improvement during the time period.
In addition to pain relief, the general purpose of fracture treatment is to stabilize the fracture to allow bone healing in a correct position 15. The fast and sustained pain relief demonstrated in this study indicates that this novel injectable bi-phasic ceramic bone substitute has sufficient mechanical strength to stabilize the compression fractures investigated and suggests healing of the vertebral fracture.
PMMA polymerizes through an exothermic process, resulting in a fibrous tissue layer surrounding the PMMA 8, and this fibrous layer may compromise the bony fusion of the fractured bone. The bio-ceramic bone substitute hardens by a process with a moderate temperature increase of 4°C, allowing fracture healing. This was demonstrated in this study in both CT scans of human vertebroplasty and in the rabbit bone model.
The gradual absorption of the bio-ceramic material allows new bone in-growth, which further strengthens the fractured bone. The bone healing demonstrated in both this clinical series and in rabbits provides for significantly improved architecture of the VCF after augmentation with the bio-ceramic bone substitute. Radiocontrast analysis of Cerament™-treated animals in the rabbit model has previously demonstrated that there was minimal iodine detectable in the blood after one week in vivo 16. This indicates that the material observed with μCT was in fact a combination of newly mineralized tissue and residual HA. This further supports the observations from the human six and twelve month CT scans that enhanced density of the vertebral bodies is due to residual HA and new bone and not due to the radiocontrast agent.
The primary measure of success for a new cement used for vertebroplasty should be pain relief. The historical success of PMMA has largely been attributed to mechanical stability 7. On the other hand, PMMA is permanent and changes little if any over time in the vertebra. The use of a remodeling bio-ceramic material for vertebroplasty differs fundamentally from PMMA. The bio-ceramic bone substitute has been shown with CT in humans and in histology in rabbits to produce new bone growth and a more normal bone architectural appearance. It has been shown clearly in rabbits to have the capacity to be resorbed and replaced by living healthy bone.
A major problem in treating VCF with vertebral augmentation has been the incidence of new fractures 17-19. The odds of experiencing new VCF after PMMA vertebroplasty is approximately 20% in the first year 9,20, and the odds of experiencing adjacent level fractures with vertebral body augmentation using PMMA is significantly increased compared to conservative treatment, particularly within the first 90 days 18. Adjacent fractures are more likely to occur sooner than remote fractures following PMMA vertebroplasty 19,21,22.
The mechanism for adjacent level fractures is still unclear, but Baroud et al. 20 hypothesize, based on experimental and computational studies, that vertebral cancellous bone augmented with PMMA is approximately 12 times stiffer and 35 times stronger than untreated osteoporotic cancellous bone. Their work concludes that a change in mechanical loading of the adjacent vertebrae occurs following augmentation with PMMA of the treated compressed fracture. The “pillar” effect of the injected cement is hypothesized to decrease the end-plate bulge in the augmented vertebra, causing an increase in adjacent disc pressure that is communicated to the adjacent vertebra.
The newly developed bio-ceramic bone substitute has an immediate mechanical strength similar to that of healthy cancellous bone 11, which potentially minimizes the risk of implant-induced adjacent fractures. In the current study no adjacent level fractures occurred within 30 days. One occurred at three months in a patient recently also treated with PMMA at an adjacent level and the other occurred at 11 months. Although no statistical significance can be drawn from these results, fracture healing and gradual absorption of the bio-ceramic bone substitute allowing for new bone growth in a VCF should provide a more biomechanically appropriate environment over time, and thereby potentially reduce the number of adjacent fractures that has been experienced with the use of PMMA. However, this has to be further investigated in a larger clinical trial.
One of the questions regarding a less mechanically substantial cement is whether it can meet the main objective of stability in order to provide pain relief. This investigation has shown that the bio-ceramic provides excellent pain relief comparable to PMMA 11. This builds on the first reported clinical study with this material in Europe where 80% of 15 patients maintained pain relief over 18 months, and with no adjacent level fractures observed 15. A secondary benefit of the material may be its ability to resorb and be replaced by living bone, compared to PMMA that may entail a risk of future device-related complications. A previous rabbit study, using a model very similar to the model presented in the present study, showed that PMMA cement remains intact with no resorption or remodeling. There is only biologic activity at the cement bone interface, and in most cases toxicity from the monomer hinders new bone formation 13. This investigation has shown that the bio-ceramic material not only provides sufficient stability to meet the primary objective of pain relief, but that it also remodels within six months such that there is normal appearing bone in the healed vertebra. However, it might be over time in an osteoporotic patient, that the vertebrae could again become osteopenic and re-fracture, which would be unlikely when PMMA is used. Our rabbit data show that the material incorporates rapidly with new bone formation infiltrating the space previously occupied by the ceramic. Larger and longer term clinical studies are recommended to show the time course benefit to bone quality in the osteoporotic patient.
Conclusion
A prospective clinical series of a novel bi-phasic ceramic bone substitute for vertebroplasty has been evaluated with 20 patients. Significant and clinically relevant pain relief was achieved in patients at one week and this was sustained through 12 months. Histologic and μCT evidence of bone growth has been demonstrated in the rabbit model. CT scans of patients have indicated healing of the osteoporotic fractures and new bone in-growth. Reinforcement of vertebral bodies with new bone growth and healing provides for architecture similar to the remaining vertebral bodies, potentially reducing the risk of new VCF and adjacent fractures. Longer term clinical studies are recommended to show the time course benefit to bone quality in the osteoporotic patient.
Acknowledgments
We are grateful for the support of this project by BONESUPPORT A.B., Lund, Sweden who provided research grants. Within the terms of these grants the sponsoring company has the opportunity to review the manuscript before submission.
References
- 1.Watts NB, Harris ST, Genant HK. Treatment of painful osteoporotic vertebral fractures with percutaneous vertebroplasty or kyphoplasty. Osteoporosis Int. 2001;12:429–437. doi: 10.1007/s001980170086. [DOI] [PubMed] [Google Scholar]
- 2.Obuchowski AM, Curcin A, Kostuik JP. Osteoporosis: medical and surgical options. In: Mathis JM, Deramond H, Belkoff SM, editors. Percutaneous vertebroplasty. Heidelberg: Springer-Verlag; 2002. pp. 25–40. [Google Scholar]
- 3.Diamond TH, Bryant C, Browne L, et al. Clinical outcomes after acute osteoporotic vertebral fractures: A 2-year non-randomised trial comparing percutaneous vertebroplasty with conservative therapy. Med J Aust. 2006;184:113–117. doi: 10.5694/j.1326-5377.2006.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 4.Bostrom MPG, Lane JM. Future directions: Augmentation of osteoporotic vertebral bodies. Spine. 1997;22:38–42. doi: 10.1097/00007632-199712151-00007. [DOI] [PubMed] [Google Scholar]
- 5.Galibert P, Deramond H, Rosar P, et al. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. 1987;33:166–168. [PubMed] [Google Scholar]
- 6.Belkoff SM, Mathis JM, Jasper LE, et al. The biomechanics of vertebroplasty. The effect of cement volume on mechanical behavior. Spine. 2001;26:1537–1541. doi: 10.1097/00007632-200107150-00007. [DOI] [PubMed] [Google Scholar]
- 7.Rousing R, Anderson MO, Jespersen SM, et al. Percutaneous vertebroplasty compared to conservative treatment in patients with painful acute or subacute osteoporotic vertebral fractures. Spine. 2009;34:1349–1354. doi: 10.1097/BRS.0b013e3181a4e628. [DOI] [PubMed] [Google Scholar]
- 8.Lewis G. Properties of acrylic bone cement: State of art review. J Biomed Mater Res Appl Biomat. 1997;38:152–182. doi: 10.1002/(sici)1097-4636(199722)38:2<155::aid-jbm10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 9.Syed MI, Patel NA, Jan S, et al. Intradiscal extravasation with low volume cement filling in percutaneous vertebroplasty. Am J Neuroradiol. 2005;26:2397–2401. [PMC free article] [PubMed] [Google Scholar]
- 10.Furtado N, Oakland RJ, Wilcox RK, et al. A biomechanical investigation of vertebroplasty in osteoporotic compression fractures and in prophylactic vertebral reinforcement. Spine. 2007;32:E480–E487. doi: 10.1097/BRS.0b013e31811ea2ee. [DOI] [PubMed] [Google Scholar]
- 11.Nilsson M, Wang J-S, Wielanek L, et al. Biogradation and biocompatibility of a calcium sulphate-hydroxyapatite bone substitute. J Bone Joint Surg. 2004;86-B:120–125. [PubMed] [Google Scholar]
- 12.Wang JS, Zhang M, McCarthy I, et al. Biomechanics and bone integration on injectable calcium sulphate and hydroxyapatite in large bone defect in rat. Abstract; American Orthopedic Research Society; 18-22 March, 2006; Chicago. 2006. [Google Scholar]
- 13.de la Torre B, Salvado M, Corchón MA, et al. Biological response of new activated acrylic bone cements with antiseptic properties. Histomorphometric analysis. J Mater Sci Mater Med. 2007;18:933–941. doi: 10.1007/s10856-006-0066-1. [DOI] [PubMed] [Google Scholar]
- 14.Frankel BM, Monroe T, Wang C. Percutaneous vertebral augmentation: An elevation in adjacent level fracture risk in kyphoplasty as compared with vertebroplasty. Spine J. 2007;7:575–582. doi: 10.1016/j.spinee.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Rauschmann M, Vogl T, Verheyden A, et al. Bioceramic vertebral augmentation with a calcium sulphate/hydroxyapatite composite (Cerament™ SpineSupport) in vertebral compression fractures due to osteoporosis. Eur Spine J. 2010;19:887–892. doi: 10.1007/s00586-010-1279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voor MJ, Borden J, Burden RL, et al. Cancellous bone defect healing with a novel calcium sulfate - hydroxyapatite composite bone substitute cement. Proceedings of the Orthopaedic Research Society 56th Annual Meeting; 2010; New Orleans, LA. 2010. [Google Scholar]
- 17.Kim SH, Kang HS, Choi J-A, et al. Risk factors of new compression fractures in adjacent vertebrae after percutaneous vertebroplasty. Acta Radiologica. 2004;45:440–445. doi: 10.1080/02841850410005615. [DOI] [PubMed] [Google Scholar]
- 18.Mudano AS, Bian J, Cope JU, et al. Vertebroplasty and kyphoplasty are associated with an increased risk of secondary vertebral compression fractures: A population-based cohort study. Osteoporosis Int. 2009;20:819–826. doi: 10.1007/s00198-008-0745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uppin AA, Hirsch JA, Cemenera LV, et al. Occurrence of a new vertebral body fracture after percutaneous vertebroplasty in patients with osteoporosis. Radiology. 2003;226:119–124. doi: 10.1148/radiol.2261011911. [DOI] [PubMed] [Google Scholar]
- 20.Baroud G, Vant C, Wilcox R. Long-term effects of vertebroplasty: Adjacent vertebral fractures. J Long Term Eff Med Implants. 2006;16:265–280. doi: 10.1615/jlongtermeffmedimplants.v16.i4.10. [DOI] [PubMed] [Google Scholar]
- 21.Trout AT, Kallmes DF, Kaufmann TJ. New fractures after vertebroplasty: adjacent fractures occur significantly sooner. Am J Neuroradiol. 2006;27:217–223. [PMC free article] [PubMed] [Google Scholar]
- 22.Tseng Y-Y, Yang T-C, Tu P-S, et al. Repeated and multiple new vertebral compression fractures after percutaneous transpedicular vetrebroplasty. Spine. 2009;34:1917–1922. doi: 10.1097/BRS.0b013e3181ac8f07. [DOI] [PubMed] [Google Scholar]