Summary
Endovascular treatment has assumed a role of first choice in the management of ruptured intracranial aneurysms. We describe the clinical and morphological data after the treatment of 258 ruptured intracranial aneurysms in 241 patients, in order to evaluate the safety and the efficacy of the endovascular treatment.
Two hundred and forty-one patients with saccular ruptured aneurysms were treated at our institution between 2000 and 2005. After the endovascular treatment a clinical and angiographic follow-up was conducted. The clinical follow-up was carried out with a medical examination and telephonic interviews and mRS was used for evaluation.
Two hundred and forty-nine acutely ruptured aneurysms were successfully treated and immediately after the endovascular procedure 81.9% of the aneurysms resulted completely occluded, 12.1% had a residual neck and 6% revealed a residual sac. The evolution of each grade was evaluated at six months and two years. During the follow-up we observed five early and one late re-bleedings. Twenty-four patients underwent a second procedure. After the discharge and up to ten years 73.1% of patients had a good clinical outcome (mRS0-1), 8.9% died and the remainder showed moderate-severe disability (mRS2-3).
The long-term stability of the anatomical result is a critical issue of this approach because eventual re-bleedings may occur even after several months or years. A careful clinical and radiological follow-up for up to two years after the embolization may prevent recurrences but may not be sufficient.
Key words: subarachnoid haemorrhage, endovascular treatment, ruptured aneurysms
Introduction
Endovascular treatment has progressively assumed a role of first choice in the management of ruptured intracranial aneurysms 1. The rationale of this approach is to exclude aneurysms from the physiological circulation and to reduce the invasiveness of the treatment compared with the surgical approach. However, the choice of the most adequate therapeutic option, endovascular or surgical, depends on certain features of aneurysms, such as size, localisation, morphological characteristics, clinical conditions and extension of bleeding. Nevertheless, some studies, such as the ISAT study compared these two different approaches and concluded that the outcome is significantly better with endovascular coiling, concerning patients with a ruptured intracranial aneurysm that were eligible for both approaches 2. The only concern about endovascular treatment is the long-term stability and exclusion of the aneurysm (anatomical result) and the consequent capability to avoid a recurrence of rupture. Aneurysmal re-bleeding may occur in an early time, due to the incomplete coiling or, less frequently, to other factors such as fibrinolytic or anti-platelet therapy or the dissecting nature of the aneurysm. Late re-bleeding, even after several years, is frequently secondary to recanalization. In the literature, recanalization and late re-bleeding rate are not yet well-established especially if we consider the very long-term follow-up (over five years). For this reason we reviewed our population with a clinical (at least five years) and a radiological follow-up (digital subtraction angiography, DSA, or magnetic resonance angiography, MRA) of a minimum of two years. Our aim was to describe the data we obtained by treating 258 ruptured intracranial aneurysms in 241 patients, focusing on complications and long-term clinical (5-10 years) and radiological follow-up results in order to evaluate the stability of the initial grade of occlusion.
Materials and Methods
Subjects
A total of 241 patients with saccular ruptured aneurysms were treated at our Interventional Neuroradiology Unit from January 2000 to December 2005. We chose an endovascular procedure whenever possible. More complex aneurysms and patients with life-threatening haematoma underwent surgery. Patients with fusiform, dissecting aneurysms or post-surgical remnants were excluded from this population. Mean age was 55.7 years (age range: 13-86 years), 34% were males (82/241) and 66% were females (159/241), 5.8% (14/241) underwent coiling of more than one aneurysm during the same procedure when the offending lesion could not be identified. One patient (0.4%) had a concomitant arteriovenous malformation (AVM). All patients included had a subarachnoid haemorrhage (SAH) on admission, documented by CT scan or lumbar puncture. SAH was evaluated with Fischer Scale (Grade 1 in 8.7%, 2 in 30.7%, 3 in 29.9% and 4 in 30.7%). Hunt and Hess (HH) Grade at admission was I in 11.6% (28/241), II in 37.3% (90/241), III in 22.4% (54/241), IV in 14.9% (36/241) and V in 13.7% (33/241) (Table 1). In 7.9% (19/241) vasospasm was documented during the pre-treatment angiography.
Table 1.
Study Population
| No. of patients |
No. of aneurysms |
||||
|---|---|---|---|---|---|
| HH grade at admission |
Localization of aneurysms |
||||
| HH1 | 28 (11.6%) | Anterior circulation |
ACoA | 94 (36.4%) | |
| HH2 | 90 (37.3%) | ICA | 88 (34.1%) | ||
| HH3 | 54 (22.4%) | MCA | 21 (8.1%) | ||
| HH4 | 36 (14.9%) | ACA | 16 (6.2%) | ||
| HH5 | 33 (13.7%) | PCoA | 9 (3.5%) | ||
| Total | 241 | Posterior circulation |
BA. VA | 14 (5.4%) | |
| PICA | 11 (4.3%) | ||||
|
Fischer Scale evaluation of SAH at admission |
PCA | 4 (1.5%) | |||
| Grade 1 | 21 (8.7%) | SCA | 1 (0.4%) | ||
| Grade 2 | 74 (30.7%) | Total | 258 | ||
| Grade 3 | 72 (29.9%) | ||||
| Grade 4 | 74 (30.7%) |
Size of aneurysms |
|||
| Total | 241 | Small | 106 (41.4%) | ||
| Medium | 121 (46.9%) | ||||
| Large | 27 (10.5%) | ||||
| Giant | 4 (1.5%) | ||||
| Total | 258 | ||||
|
Notes: HH (Hunt & Hess), SAH (Subarachnoid Haemorrhage), ACoA (Anterior Communicating Artery), ICA (Internal Carotid Artery), MCA (Middle Cerebral Artery), ACA (Anterior Cerebral Artery), PCoA (Posterior Communicating Artery), BA (Basilar Artery), VA (Vertebral Artery), PICA (Postero-inferior Cerebellar Artery), PCA (Posterior Cerebral Artery), SCA (Superior Cerebellar Artery), | |||||
Protocol
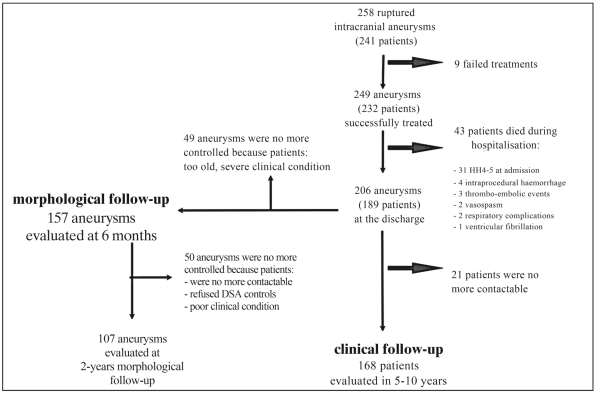
In all 241 patients a digital complete angiographic examination was performed. Informed consent was obtained. Timing of the treatment was between six hours and six days. All patients underwent endovascular treatment on admission and 73.4% (177/241) within 12-24 hours from initial bleeding. Each case was evaluated by both the neurosurgeon and the interventional neuroradiologist to choose the most appropriate therapeutic approach. Selection criteria for surgical treatment were the complexity of the aneurysmal architecture, the basal involvement of arterial bifurcations, a critical vascular access, a haematoma associated to aneurysm that required an immediate evacuation (Figure 1).
Figure 1.
Flow chart of the study.
Interventional Technique
Endovascular procedures were performed under general anaesthesia and with systemic heparinization (Activated Clotting Time, ACT: 200-250 seconds). After femoral arterial access (in one case, carotid access was executed), aneurysms were embolized with platinum coils. When an artery originated from the ground of the sac, a residual part of the neck was left to maintain the patency of the vessel; 45% (116/258) of aneurysms (104/241 patients) were treated with balloon-assisted coiling (Remodeling Technique, RT) and in one case was stent-assisted. All patients had intravenous administration of Nimodipine as preventive protocol for vasospasm. In case of perforation of the sac during embolization the systemic inflow of heparin was reverted with protamine sulphate to promote the thrombosis of the remnant part and to reduce haemorrhagic complications. After the angiographic procedure, all patients underwent a CT scan and afterwards they were hospitalized in the Department of Neurosurgery or Neurosurgical Intensive Care Unit.
Description of Aneurysms
Two hundred and fifty-eight aneurysms were identified by angiographic examination: 88.4% (228/258) were harboured in the anterior circulation and 11.6% (30/258) had a posterior localisation. Further sub-classifications are summarised in Table 1. As far as size is concerned, 41.4% (106/258) of aneurysms were small (<5 mm), 46.9% (121/258) were medium-sized (5-15 mm), 10.5% (27/258) were large (15-25 mm) and 1.5% (4/258) were giant (>25 mm).
Grade of Occlusion at the End of Procedure
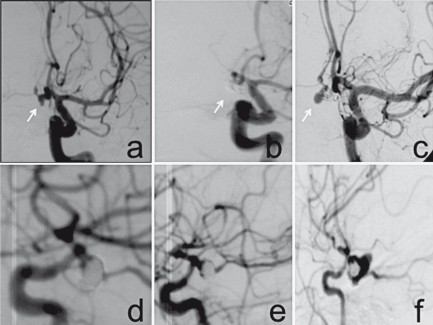
The angiographic result of endovascular treatment was classified in grades of occlusion with Raymond's classification, considering grade I as a total filling of the aneurysm, grade II as incomplete occlusion of the aneurysm with a residual neck (<2 mm) and grade III as an incomplete occlusion with a residual part of aneurismal sac (Figure 2).
Figure 2.
Late rebleeding. A) Pre-embolization angiography, small in size aneurysm of ACoA. B) Complete exclusion after treatment (G0). C) De novo aneurysm after DSA control. D-F) Early rebleeding, D) DSA evaluation after treatment of haemorrhagic aneurysm with residual neck . E) Control after stability of result. F) Angiography after rebleeding due to the dissecting evolution of the coiled aneurysm, successively clipped.
Follow-up
After the endovascular treatment a clinical and radiological follow-up was conducted. One hundred and one aneurysms were excluded because the patients were too old, in a poor clinical condition, deceased and did not undergo DSA examination (Figure 1). All the other patients underwent a two-year clinical and radiological follow-up. An MRA was performed at three, 12 and 18 months and DSA at six months and two years after the procedure. Only the DSA findings are reported. The stability and modifications of the grade of occlusion were evaluated comparing the angiographic findings at the end of the procedure, at six months and two years. To follow these variations, aneurysms with stability of complete occlusion were considered grade I→I, aneurysms with neck recanalization I→II and aneurysms with sac rehabitation I→III. The same rationale was used to evaluate aneurysms with different initial grades of occlusion (II, III). Concerning the evaluation of the stability of the initial treatment, patients who underwent a second treatment due to recanalization of the aneurysm, were not subsequently considered. The clinical follow-up was carried out with a medical examination and telephonic interviews and modified Rankin Scale (mRS) was used for evaluation. The total period of the follow-up was 60-120 months.
Results
Anatomic Results and Grades of Occlusion
The aim of the endovascular treatment was to obtain complete exclusion of the aneurysms from the physiological circulation. Treatment failed in 3.5% (9/258 aneurysms, 9/241 patients): in these patients catheterization was performed but coils were not released because of their instability due to a wide aneurysmal neck (>4 mm or dome-to-neck ratio≤2). Out of the nine failed cases eight have been treated with surgical clipping, one was not treated because of a critical condition on admission. Two hundred and forty-nine aneurysms were successfully treated and immediately after the endovascular procedure 81.9% (204/249) resulted as grade I, 12.1% (30/249) as II and 6% (15/249) as III (Table 2).
Table 2.
Results and complications.
| No. of aneurysms |
No. of aneurysms |
No. of aneurysms |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
Anatomical results |
at the end of the procedure |
at 6 months | at 2 years | ||||||
| G0 | 204 (81.9%) | 114 (72.6%) | 76 (71%) | ||||||
| G1 | 30 (12.1%) | 27 (17.2%) | 22 (20.6%) | ||||||
| G2 | 15 (6%) | 16 (10.2%) | 9 (8.4%) | ||||||
| Total (excluding 9 failed treatments) |
249 | 157 | 107 | ||||||
|
Evolution of aneurysms after treatment |
|||||||||
| Occlusion grade at the end of the procedure |
No. of aneurysms |
Occlusion grade at 6 months |
No. of aneurysms |
Occlusion grade at 2 years |
No. of aneurysms |
||||
| G0 | 64 | ||||||||
| G0 | 106 | G1 | 10 | ||||||
| G2 | - | ||||||||
| G0 | 3 | ||||||||
| G0 | 204 | G1 | 22 | G1 | 11 | ||||
| G2 | 1 | ||||||||
| G2 | 8 | retreated | |||||||
| G0 | 7 | ||||||||
| G0 | 7 | G1 | - | ||||||
| G2 | - | ||||||||
| G0 | 1 | ||||||||
| G1 | 30 | G1 | 4 | G1 | 3 | ||||
| G2 | - | ||||||||
| G2 | 4 | retreated | |||||||
| Rebleeding | 1 | ||||||||
| G0 | 1 | ||||||||
| G0 | 1 | G1 | - | ||||||
| G2 | - | ||||||||
| G0 | - | ||||||||
| G2 | 15 | G1 | 1 | G1 | 1 | ||||
| G2 | - | ||||||||
| G2 | 1 | ||||||||
| G2 | 4 | retreated | 1 | ||||||
| Complications | |||||||||
| No. of patients |
Morbility | Mortality | |||||||
| Thrombo- embolic |
11/241 (4.6%) |
3/241 (1.2%) |
6/241 (2.5%) |
||||||
| Haemorrhagic | 33/241 (12.8%) |
15/241 (6.2%) |
7/241 (2,9%) |
||||||
Peri-Procedural Complications
Thrombo-embolism occurred in 11 out of 241 patients (4.6%). Six of the 11 patients subsequently died: three of them were classified with HH grade 4-5 and three with HH1-3 on admission; only the three patients with HH1-3 could be considered deceased secondary to thrombo-embolism, while in the other three patients (HH4-5), the critical clinical condition prevented us establishing if embolism was the direct cause of death. Three out of 11 had no or minimal sequelae (2 mRS0 and 1 mRS1 at discharge) and two had a severe disability (one was HH5 at admission). Independently from the clinical condition at admission, mortality related to ischemic events was 2.5% (6/241 patients) and morbidity was 1.2% (3/241 mRS1-5). In 33 out of 241 patients (12.8%) the aneurysmal sac was perforated, with or without bleeding. Seven out of the 33 patients died (four were HH4-5), 12 had moderate-severe permanent disabilities (mRS2-5) and 14 had no clinically relevant consequences (three were mRS1 11 mRS0). Mortality related to haemorrhagic complications was 2.9% (7/241) and morbidity was 6.2% (15/241 mRS 1-5). Coil fracture occurred in three out of 241 patients (1,2%): in one case the aneurysm was not treated (failure), in the other two the aneurysm was excluded with clinical consequences secondary to ischemic events (one patient was mRS1 and one was mRS2). Hypertensive crisis (without clinical consequences, mRS 0) occurred in one case (0.42%) as well as retroperitoneal haematoma secondary to the femoral access. In this case, the patient subsequently underwent vascular surgical intervention. Procedure-related mortality and morbidity, excluding deceased patients or those with severe disabilities because of their severe clinical conditions at admission, were 2.4% (6/241) and 4.1% (10/241) respectively. Total procedure-related mortality and morbidity, including also patients with a severe acute clinical condition were 5.4% (13/241) and 7.4% (18/241 mRS1-5).
Clinical Evaluation at Discharge
The outcome was evaluated with mRS at discharge; failed treatments (9/241 patients) were excluded. Out of the 232 successfully treated patients, 55.7% (129/232) were discharged with mRS0; 4.7% (11/232) with mRS1; 4.3% (10/232) with mRS2; 3% (7/232) with mRS3; 10.8% (25/232) with mRS4 and 3% (7/232) with mRS 5, 18.5% (43/232) died (Table 3). The causes of death were: the severity of the clinical condition at the admission (HH4-5) in 72.1% (31/43), intra- or peri-procedural haemorrhage in 9.2% (4/43; three died because of sac perforation and one died two days after the treatment with uneventful stent-assisted coiling); thrombo-embolic events 7% (3/43), vasospasm in 4.7% (2/43) as well as respiratory complications (2/43), cardiologic alterations (ventricular fibrillation) in 2.3% (one case).
Table 3.
Clinical evolution.
| mRS at discharge | No. of patients | mRS at 5-10 years | No. of patients |
|---|---|---|---|
| 0 | 129 (55.7%) | 0 | 112 (66.6%) |
| 1 | 11 (4.7%) | 1 | 11 (6.5%) |
| 2 | 10 (4.3%) | 2 | 9 (5.4%) |
| 3 | 7 (3%) | 3 | 8 (4.7%) |
| 4 | 25 (10.8%) | 4 | 7 (4.1%) |
| 5 | 7 (3%) | 5 | 6 (3.5%) |
| 6 | 43 (18.5%) | 6 | 15 (8.9%) |
| Total | 232* | 168** | |
| * excluding failed treatments (9/241 patients) ** excluding patients previously deceased (43) and patients no longer contacted (21) | |||
Radiological Follow-up
At the end of the endovascular treatment 249/258 aneurysms were evaluated and failed treatments (9/258) were excluded. Finally, 92/249 aneurysms did not undergo radiological follow-up so that only 157 treated aneurysms were included in the evaluation. Aneurysms controlled with DSA were 157 at six months and 107 at two years (Figure 1). At six months, aneurysms classified as grade I were 72.6% (114/157), grade II 17.2% (27/157) and grade III 10.2% (16/157). After the first angiographic control nine out of 157 patients were retreated (5.7%). At two years, excluding re-treatments, aneurysms that resulting still totally excluded (grade I) were 71% (76/107), 20.6% (22/107) resulted as grade II and 8.4% (9/107) as III. Furthermore, we considered the evolution of the aneurysms related to the grade of occlusion obtained at the end of the procedure (Table 2).
Retreatments
All the retreatments were performed after the first DSA control at six months (15.2%, 24/157) because of early or late recanalization (“elective” re-treatments, 21/24, or re-bleeding, 3/24). A second embolization regarded I→II aneurysms in 38% (8/21), I→III in 33% (7/21), II→III in 19% (4/21) and in 9.5% (2/21) aneurysms that were not completely occluded at the end of the procedure. Considering elective re-treatments, 47.6% (10/21) regarded aneurysms harboured in the anterior communicating artery (ACoA), 25% (5/21) in the carotid siphon, 10% (2/21) in the middle cerebral artery (MCA) and the other four cases respectively in the postero-inferior cerebellar artery (PICA), posterior communicating artery (PCoA), basilar artery (BA) and anterior cerebral artery (ACA). As far as size is concerned, retreated aneurysms were small in 40% (9/21), medium in 30% (6/21), large in 23.9% (5/21) and giant in 4.7% (1/21). After the second treatment, at the angiographic control 90.5% (18/21) resulted as I and in two cases as II (Table 2) In the latter two cases one patient died because of a neoplasm and the other did not undergo further examinations because of a critical general condition. Re-treatments were performed without complications.
Rebleedings
We experienced six rebleedings after the initial treatment (Table 2). After relapsing bleeding three patients were coiled and two were clipped, one patient was not treated because of a severe clinical condition. In three out of six cases haemorrhage occurred during hospitalisation (2.1%, 3/189, excluding deceased patients and failed treatments) and in two of them the rebleeding was certainly secondary to the rupture of the treated aneurysm: one aneurysm of the vertebrobasilar junction apparently completely treated with stent and coil rebled probably because of the double anti-platelet therapy, one medium-sized aneurysm located in the carotid siphon that was sub-totally occluded showed an evolution to a blister dissecting aneurysm. In the other case the relation between the re-bleeding and the rupture was doubtful: in one patient with two aneurysms the rebleeding occurred in the aneurysm that was not treated. Three out of six rebleedings were detected after discharge (1.6%, 3/189 patients) and in all of them the haemorrhage occurred in the same localisation as the treated aneurysm after one month (medium-sized, ACoA, grade II at the end of procedure during which a coil was stretched, outcome mRS1), two months (medium-sized, ICA, grade III at discharge not controlled or retreated because of renal failure, outcome mRS0) and four years, respectively. The rebleeding after four years occurred in a de novo aneurysm grown next to the ACoA small-sized aneurysm (grade I of occlusion at the end of the procedure resulted completely occluded after DSA and MRA at two years) and a good outcome has been obtained (mRS0). Even if three rebleeds occurred after discharge, two of them may be considered early (one and two months) and one of them was suboptimally treated (class III). Thus the number of rebleeds due to recurrence is only one.
Clinical Follow-up
Clinical evaluation up to five to ten years from the endovascular treatment was assessed (Table 3): 168 patients were included in the long-term follow-up, since 43 died had within the time of discharge and 21 were no longer contacted. During the clinical follow-up 8.9% (15/168) of the patients died (mRS6) but only 5.4% (9/168) could be related to a SAH (8/9 secondary to worsening of initial general condition and one out of 11 had respiratory complications). At five to ten years, in 66.6% (112/168) the outcome was classified as mRS0, in 6.5% (11/168) as mRS1, in 5.3% (9/168) as mRS2, in 4.7% (8/168) as mRS3, in 4.1% (7/168) as mRS4 and in 3.5% (6/168) as mRS5.
Discussion
An endovascular approach in case of ruptured aneurysms, as an alternative to surgical intervention, was technically possible in 96.5% of the intention-to-treat cases without relevant differences from the data reported in the literature 3,4. Concerning the exclusion of the aneurysmal sac in emergency, a total (I) or sub-total (II) filling was obtained in 90.7% of cases and these data confirm those of other studies in which the rate of an adequate treatment is 79-97% 5-8. However, considering the efficiency of the endovascular treatment in relation to clinical outcome at discharge, we observed a good outcome (mRS0-1) in 85% of the patients admitted with HH1-2 grades, in 70% (mRS0-2) of the patients admitted with HH3 grade and 25% (mRS0-3) of the most critical patients (grades HH4-HH5). After five to ten years we observed that 90% of the patients with a good outcome at the discharge (mRS0-1) were stable and the patients with a severe disability (mRS4-5) showed a clinical worsening. In literature, good clinical outcomes are reported in 90% of HH1-HH2, in 49% of HH3, and 52% of HH4-HH5 5. According to previous literature studies, such as the ISAT study 2,9, our study reveals lower rates of morbidity (21% vs 44% ISAT) but higher mortality rates (18% vs 7.5% ISAT). These data could be explained by the tendency of our Unit to treat patients also classified as HH4-HH5 on admission (28.6%, 69/241 patients, 71/258 aneurysms) and by the different types of treated aneurysms. In patients admitted with HH4-5 grades the total mortality was 22%. The study by Bracard et al. 10, that included patients admitted with grade HH5, noted that 62% of them died secondarily to the severity of their clinical condition. These data confirm that the severity of the haemorrhage and the consequences related to it (vasospasm, hydrocephalus, brain damage) are factors determining the final outcome in patients with ruptured intracranial aneurysms.
Safety and Efficacy of the Procedure
If we do not consider the group of critical patients and consider only patients whose decease or clinical deficits are certainly related to procedural complications, the mortality is 2.5% and morbidity is 4.1%. The total rate of intraprocedural complications found in the literature was between 9% and 14% with a mortality range of 1.4%-2.9% and morbidity of 2.5%-8.6% 3-5,11-13.
The stability of the occlusion grade of the aneurysm remains a critical issue. A stable complete occlusion resulted in only 77.9% (106/136) at the six months angiographic follow-up and in 86.4% (64/74) after two years. Therefore, in our experience, about one out of five of the grade I aneurysms at the end of the procedure revealed signs of recanalization after six months and after two years 1/3 of these controlled aneurysms showed a modification of the occlusion. In literature, the percentage of adequate occlusion at the end of the procedure is 45-85% 5,6,14. If the aneurysms are not completely excluded at the end of the procedure, the progression of recanalization is more frequent. Indeed, in our study, in about one out of three (5/16) of the aneurysms classified as grade II after the treatment, at six months an increased rehabitation of the sac was observed. However, other events may also occur, such as the spontaneous thrombosis of the aneurysmal sac (in our experience in 41.2% of II→I at six months and in 72.7% at two years). Only 10.3% (14/136) of the grade I aneurysms needed to be retreated. These data are similar to those reported in the literature 4,13,15. Our criteria for retreatment were the progression of rehabitation of the sac and rebleedings. Although the endovascular procedure is considered an effective approach to reduce the risk of recurrence of rupture, we experienced five early and one late rebleedings. Relapsing bleedings that occur before discharge after the procedure may be secondary to incomplete filling of the sac or to the drugs that interfere with the thrombosis of the aneurysms or to the dissecting nature of the aneurysm itself. If we consider the timing of the events of rebleeding, we observed that five out of six cases occurred before the first angiographic control and in the other case after the two year DSA evaluation. This experience suggests that the first morphological control (three months) may be too late and the last one (two years) may be too early. In the literature this percentage ranges from 0.5 to 3.6% 5,7,12,16-20. In some other studies, since the number of patients was small, no re-bleedings were reported 21. Despite the limitation of the recanalization and the rebleedings, endovascular treatment resulted an effective approach ion terms of the long-term clinical outcome. A good clinical result (mRS0-1) was assessed in 77.3% of the interviewed patients (130/168), a disabling neurological deficit (mRS2-3) was observed in 11.3% (19/168) and a prolonged hospitalisation (mRS4-5) in 7.7% (13/168). These data are in agreement with those in literature: mRS0-2 is reported in 75-88% (79% in our experience) and mRS3-6 in 16-25% (21% in our experience) 16,21-24. Despite the retrospective nature of our study, long-term clinical follow-up was possible in 88,8% of discharged patients (168/189). However, a six-month angiographic evaluation was possible only in 157 of the successful treatments and a two year one in 107: this should be considered a limitation of our study.
Conclusions
After the publication of the ISAT study 2 the endovascular treatment of ruptured intracranial aneurysms is to be considered the first therapeutic approach. Long-term stability of the anatomical result is the limit of this therapeutic approach because rehabitation of the sac may lead to rebleeding even after several months or years 25. A careful clinical and radiological follow-up (MRA and DSA) for at least two years after the embolization may prevent recurrences but may not be sufficient. Therefore, we recommend a long-term (three to four years) MRA follow-up and an early radiological investigation (before three months) in incompletely occluded aneurysms, especially in patients in which antiplatelet therapy is administered.
References
- 1.Hoya K, Hyodo A. Endovascular treatment for cerebral aneurysms. Brain Nerve. 2009;61:1029–1041. [PubMed] [Google Scholar]
- 2.Molyneux A, Kerr R, ISAT Collaborative Group International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. J Stroke Cerebrovasc Dis. 2002;11:304–314. doi: 10.1053/jscd.2002.130390. [DOI] [PubMed] [Google Scholar]
- 3.Fang C, Li MH, Zu YQ, et al. The effectiveness and feasibility of endovascular coil embolization for very small cerebral aneurysms; mid- and long-term follow-up. Ann Vasc Surg. 2010;24:400–407. doi: 10.1016/j.avsg.2009.10.005. Epub 2009 Dec 29. [DOI] [PubMed] [Google Scholar]
- 4.Renowden SA, Benes V, Bradley M, et al. Detachable coil embolisation of ruptured intracranial aneurysms: a single center study, a decade experience. Clin Neurol Neurosurg. 2009;111:179–188. doi: 10.1016/j.clineuro.2008.09.026. Epub 2008 Nov 13. [DOI] [PubMed] [Google Scholar]
- 5.Willinsky RA, Peltz J, da Costa L, et al. Clinical and angiographic follow-up of ruptured intracranial aneurysms treated with endovascular embolization. Am J Neuroradiol. 2008;30:1035–1040. doi: 10.3174/ajnr.A1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grunwald IQ, Papanagiotou P, Struffert T, et al. Recanalization after endovascular treatment of intracerebral aneurysms. Neuroradiology. 2007;49:41–47. doi: 10.1007/s00234-006-0153-5. [DOI] [PubMed] [Google Scholar]
- 7.Holmin S, Krings T, Ozanne A, et al. Intradural saccular aneurysms treated by Guglielmi detachable bare coils at single institution between 1993 and 2005 Clinical long term follow-up for a total of 1810 patients-years in relation to morphological treatment results. Stroke. 2008;39:2288–2297. doi: 10.1161/STROKEAHA.107.508234. [DOI] [PubMed] [Google Scholar]
- 8.Ferns SP, Sprengers ME, van Rooij WJ, et al. Coiling of intracranial aneurysms: a systematic review on initial occlusion and reopening and retreatment rates. Stroke. 2009;40:e523–529. doi: 10.1161/STROKEAHA.109.553099. Epub 2009 Jun 11. [DOI] [PubMed] [Google Scholar]
- 9.Molyneux AJ, Kerr RS, Birks J, et al. ISAT Collaborators. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol. 2009;8:427–433. doi: 10.1016/S1474-4422(09)70080-8. Epub 2009 Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bracard S, Lebedinsky A, Anxionnat R, et al. Endovascular treatment of Hunt and Hess grade IV and V aneurysms. Am J Neuroradiol. 2002;23:953–957. [PMC free article] [PubMed] [Google Scholar]
- 11.Melake MS, Yamamoto M, Yoshida K, et al. A retrospective clinical and angiographic study of the coiling outcome of ruptured intracranial aneurysms. J Clin Neurosci. 2010;17:328–333. doi: 10.1016/j.jocn.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 12.Gallas S, Pasco A, Cottier JP, et al. A multicenter study of 705 ruptured intracranial aneurysms treated with Guglielmi detachable coils. Am J Neuroradiol. 2005;26:1723–1731. [PMC free article] [PubMed] [Google Scholar]
- 13.Henkes H, Fischer S, Weber W, et al. Endovascular coil occlusion of 1811 intracranial aneurysms: early angiographic and clinical results. Neurosurgery. 2004;54:268–280. doi: 10.1227/01.neu.0000103221.16671.f0. discussion 280-285. [DOI] [PubMed] [Google Scholar]
- 14.Tailor J, Goetz P, Chandrashekar H, et al. Stability of ruptured intracranial aneurysms treated with detachable coils: is delayed follow-up angiography warranted? Br J Neurosurg. 2010;24:405–409. doi: 10.3109/02688697.2010.487130. [DOI] [PubMed] [Google Scholar]
- 15.Ries T, Siemonsen S, Thomalla G, et al. Long-term follow-up of cerebral aneurysms after endovascular therapy-prediction and outcome of retreatment. Am J Neuroradiol. 2005;26:1723–1731. doi: 10.3174/ajnr.A0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molyneaux AJ, Kerr RS, Yu LM, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups and aneurysm occlusion. Lancet. 2005;366:809–817. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 17.Jartti P, Isokangas JM, Karttunen A, et al. Early rebleeding after coiling of ruptured intracranial aneurysms. Acta Radiol. 2010;51:1043–1049. doi: 10.3109/02841851.2010.508172. [DOI] [PubMed] [Google Scholar]
- 18.Schaafsma JD, et al. Long-term recurrent subarachnoid hemorrhage after adequate coiling versus clipping of ruptured intracranial aneurysms. Stroke. 2009;40(5):1758–63. doi: 10.1161/STROKEAHA.108.524751. Epub 2009 Mar 12. [DOI] [PubMed] [Google Scholar]
- 19.Sluzewski M, van Rooij WJ, Beute GN, et al. Late rebleeding of ruptured intracranial aneurysms treated with detachable coils. Am J Neuroradiol. 2005;26:2542–2549. [PMC free article] [PubMed] [Google Scholar]
- 20.Gallas S, Januel AC, Pascoe A, et al. Long-term follow-up of 1036 cerebral aneurysms treated by bare coils: a multicentric cohort treated between 1998 and 2003. Am J Neuroradiol. 2009;30:1986–1992. doi: 10.3174/ajnr.A1744. Epub 2009 Aug 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedmann JA, Douglas AN, Meyer FB. Guglielmi detachable coil treatment of ruptured saccular cerebral aneurysms: retrospective review of a 10 year single center experience. Am J Neuroradiol. 2003;24:526–533. [PMC free article] [PubMed] [Google Scholar]
- 22.Sluzewski M, van Rooij WJ, Rinkel GJ, et al. Endovascular treatment of ruptured intracranial aneurysms with detachable coils: long term clinical and serial angiographic results. Radiology. 2003;227:720–724. doi: 10.1148/radiol.2273020656. [DOI] [PubMed] [Google Scholar]
- 23.Kremer C, Groden C, Lammers G, et al. Outcome after endovascular therapy of ruptured intracranial aneurysms: morbidity and impact of rebleeding. Neuroradiology. 2002;44:942–945. doi: 10.1007/s00234-002-0849-0. [DOI] [PubMed] [Google Scholar]
- 24.Medjoubi M, Gigaud M, Trémoulet M, et al. Initial primary endovascular treatment in the management of ruptured intracranial aneurysms: a prospective consecutive series. Neuroradiology. 2006;48:899–905. doi: 10.1007/s00234-006-0144-6. [DOI] [PubMed] [Google Scholar]
- 25.Ferns SP, Majoie CB, Sluzewski M, et al. Late adverse events in coiled ruptured aneurysms with incomplete occlusion at 6-month angiographic follow-up. Am J Neuroradiol. 2010;31:464–469. doi: 10.3174/ajnr.A1841. Epub 2009 Oct 15. [DOI] [PMC free article] [PubMed] [Google Scholar]