Summary
Cerebral venous thrombosis (CVT) is a potentially serious disease, with nonspecific clinical symptoms and an unpredictable outcome. Despite adequate anticoagulation, a patient’s clinical condition can rapidly deteriorate. The aim of this study was to evaluate the efficacy of local thrombolysis in these patients.
Consecutive patients with progressive cerebral venous thrombosis between October 2008 and January 2011 were enrolled prospectively. Progressive CVT was defined as the persistence of neurologic findings (headache, blurred vision, and visual field defects) despite at least four days (or 48 hours in patients with involvement of more than one sinus) on full anticoagulation therapy with heparin and development of focal neurologic deficits or cortical hemorrhage. We excluded patients with large hematomas and predisposing malignancies like leukemia. All patients underwent local thrombolysis with 30 mg recombinant tissue plasminogen activator (rtPA).
Overall, 26 patients were enrolled with a mean age of 35.5 years (range 18 to 56 years). Six patients (23%) were male and twenty patients (77%) were female. The most common presenting feature was headache and the most common neurologic finding was papilledema, which was present in all patients. Eighty-five percent of women had a history of oral contraceptive pill consumption. Successful recanalization was achieved in all patients except one (96.2%). Neurological examinations and follow-up assessments were based on a modified Rankin scale (mRS). Favorable outcome and recovery was defined as a mRS score of 0–1. Follow-up assessments at the third week showed that 25 out of 26 recovered, with 18 having a mRS score of 0 and 7 with a mRS score of 1. There were no procedure-related neurological complications.
Our results show that local thrombolysis is a safe and effective treatment modality for patients suffering from progressive CVT.
Key words: cerebral venous thrombosis, recombinant tissue plasminogen activator, local thrombolysis
Introduction
Cerebral venous thrombosis (CVT) is an uncommon and potentially serious disease, with variable and nonspecific clinical symptoms at presentation and an unpredictable outcome. Although there is no definite evidence for the best choice of treatment, intravenous heparin is used as the first line treatment modality 1-4 in order to prevent thrombus extension and promote spontaneous thrombus dissolution. However, heparin does not always treat acute thrombosis 5,6 and despite adequate anticoagulation the patient's clinical condition can rapidly deteriorate. Patients in this subgroup need rapid recanalization and have a high rate of mortality 4, suggesting the need for a more effective treatment modality.
Thrombolysis is an option for providing rapid recanalization. Local thrombolysis involves removal of the thrombus and can restore the patency of the involved sinuses. Local intra sinus thrombolysis can be an effective and relatively safe treatment for acutely deteriorating patients who have not responded to conventional anticoagulant therapy 7-9.
The purpose of this study was to evaluate the efficacy and safety of catheter-directed thrombolysis in the management of carefully selected patients suffering from progressive CVT.
Materials and Methods
We prospectively enrolled consecutive patients with progressive cerebral venous thrombosis between October 2008 and January 2011. All patients gave written informed consent and the study was approved by our local Ethics Committee. Diagnosis of CVT was based on relevant clinical and neurologic findings and confirmed by imaging studies, including brain CT and MRI scans and magnetic resonance venography (MRV).
The criteria for inclusion were: 1) confirmed acute thrombosis in at least one of the cerebral venous sinuses; 2) clinical and neurologic findings of increased intracranial pressure; 3) progression of neurologic findings (headache, blurred vision, and visual field defects) despite at least four days (or 48 hours in the patients with evidence of involvement of more than one sinus) of full anticoagulation therapy with heparin; and 4) development of focal neurologic deficits, cortical hemorrhage, or any other complications secondary to sinus thrombosis like focal seizure due to cortical ischemia during heparin therapy. Exclusion criteria included any evidence of an accompanying space occupying lesion other than hemorrhage, good clinical response to heparin despite persistence of thrombosis in the imaging studies, patients with a large hematoma that caused transtentorial herniation or profound loss of consciousness, CVT secondary to malignancy such as leukemia, and inability to obtain informed consent.
Patients had detailed laboratory and hematologic work ups, including liver, thyroid, and kidney function tests, and full coagulation tests, including antithrombin III, antiphospholipid antibody, anticardiolipin antibody, and proteins C and S. Clinical, neurological, laboratory, and imaging findings were examined independently by two experienced neurologists. Progressive CVT was defined as the presence of deteriorating neurological symptoms that were unresponsive to at least 4 days of heparin therapy or at least 48 hours when more than one sinus was involved.
These patients underwent digital subtraction angiography (DSA) for further confirmation of involved sinuses and planning of local thrombolysis. The target sinus was catheterized using a right transfemoral vein approach. A 6 F guiding catheter (JR 3.5) was placed in the jugular bulb and 30 mg T-PA was injected through the microcatheter during the time period of 30 minutes. In the case of transverse and sigmoid sinus thrombosis, the guiding catheter was placed in the ipsilateral jugular bulb.
Blood pressure, oxygen saturation, and clinical condition were monitored continuously throughout the procedure. All procedures were done under light anesthesia. The angiographic endpoint was ante grade flow within the dural sinus and not the total absence of thrombus. After the procedure, heparin was continued for at least 24 hours. Patients without predisposing factors were maintained on warfarin daily for the following three months. Those with known risk factors, such as antiphospholipid antibody (APA) syndrome and antithrombin III or protein C deficiency, were continued on heparin and then switched to warfarin for at least six months or indefinitely.
Control MRVs were performed for all cases three days after the procedure and patients were followed by a neurologist on a monthly basis for at least three months. In the patients with visual field defects, control and follow-up perimetry was requested. Functional outcome was assessed using the modified Rankin Scale (mRS) 10. A favorable outcome and recovery was defined as a mRS score of 0-1.
Results
Overall, we enrolled 26 patients. Detailed characteristics of these patients are shown in Table 1. The age of the patients ranged from 18 to 56 years, with a mean of 35.5 ± 10.8. Six patients (6/26, 23%) were male, with a mean age of 37.0 ± 12.5, and twenty patients (20/26, 77%) were female, with a mean age of 35.1 ± 10.5 years (Table 1).
Table 1.
Baseline patient characteristics.
| No. | Age/ sex |
Clinical presentation |
Risk factor |
Location of thrombosis |
Duration of heparin therapy |
Predisposing factor |
Neurologic findings |
Follow-up/ months |
|---|---|---|---|---|---|---|---|---|
| 1 | 35 /F | Headache, blurred vision |
– | Left transverse | 3 days | Antithrombin III deficiency |
Papilledema | 11 |
| 2 | 44/F | Headache, right side weakness |
OCP | Left transverse and SSS |
7 days | – | Papilledema, right hemiparesis |
23 |
| 3 | 21/M | Headache, seizure | Head trauma |
SSS | 4 days | – | Papilledema | 22 |
| 4 | 41/F | Headache | OCP | Left transverse | 5 days | – | Papilledema | 21 |
| 5 | 47/F | Headache | OCP | Left transverse | 6 days | – | Papilledema | 18 |
| 6 | 44/F | Headache | OCP | Right transverse and SSS |
6 days | – | Papilledema,visual field defects |
16 |
| 7 | 23/F | Headache | OCP | Right transverse | 10 days | – | Papilledema | 13 |
| 8 | 45/M | Headache, left side weakness |
Opium | Right transverse | 2 weeks | – | Papilledema and left hemiparesis |
11 |
| 9 | 51/F | Headache,blurred vision |
– | Left transverse | 1 week | Antiphospholipid ab syndrome |
Papilledema | 12 |
| 10 | 48/F | Headache, seizure, drowsiness |
OCP | Left transverse | 7 days | – | Papilledemal | 12 |
| 11 | 36/M | Headache, seizure | – | SSS | 8 days | Antiphospholipid ab syndrome |
Papilledema | 9 |
| 12 | 18/F | Headache, blurred vision |
OCP | Left transverse | 14 days | – | Papilledema | 7 |
| 13 | 24/F | Headache, seizure | Post partum |
Left transverse SSS |
4 days | Protein c deficiency |
Papilledema, visual field defect |
7 |
| 14 | 45/F | Headache, right side weakness |
OCP | Left transverse | 6 days | – | Papilledema,right side paresis |
6 |
| 15 | 26/F | Headache, blurred vision |
OCP | Left and right transverse, posterior part of SSS |
2 days | – | Papilledema, six nerve palsy bilateral and l.o.c |
10 |
| 16 | 20/F | Headache, tinnitus, vertigo |
OCP | Right transverse | 14 days | – | Papilledema | 9 |
| 17 | 37 /M | Headache, focal seizure |
– | SSS | 10 days | APA syndrome | Papilledema | 11 |
| 18 | 33/F | Headache, tinnitus, diplopia |
OCP | SSS | 14 days | – | Papilledema, right six nerve palsy |
4 |
| 19 | 29/F | Headache, blurred vision |
OCP | Left transverse | 8 days | – | Papilledema | 3 |
| 20 | 40/F | Headache, blurred vision |
OCP | Right transverse and sigmoid |
7 days | – | Papilledema | 5 |
| 21 | 44/F | Headache, blurred vision |
OCP | Left transverse sinus | 21 days | – | Papilledema | 4 |
| 22 | 22/F | Headache, tinnitus | OCP | Left transverse sinus | 14 days | – | Papilledema | 3 |
| 23 | 27/M | Headache, dizziness | – | Right transverse and SSS |
16 days | – | Papilledema | 7 |
| 24 | 39/F | Headache, | OCP | Left transverse | 7 days | APA | Papilledema | 5 |
| 25 | 29 /F | Headache, seizure, SAH |
OCP | Left transverse | 8 days | – | Papilledema | 7 |
| 26 | 56/M | Headache, right side paresis |
– | Right transverse | 10 days | APA | Papilledema | 4 |
| OCP: oral contraceptive pill; SSS: superior sagittal sinus; APA syndrome: antiphospholipid antibody syndrome; SAH: subarachnoid hemorrhage; LOC loss of consiousness. | ||||||||
The most common presenting feature was headache followed by blurred vision, focal neurologic signs, seizure, drowsiness, tinnitus, and diplopia. One patient presented with subarachnoid hemorrhage (SAH). In this particular case imaging studies ruled out other possible causes of hemorrhage, including aneurysm or vascular malformation. The most common neurologic finding was papilledema, which was present in all patients. Other findings included hemiparesis, visual field defects, and sixth nerve palsy.
Eighty-five percent (17/20) of women had a history of oral contraceptive pill (OCP) consumption. All patients had a history of heparin therapy, ranging from two to 21 days with a mean duration of 8.9 ± 4.8 days. In 15 patients the left side transverse sinus was involved followed by the right transverse and superior sagittal sinus (SSS). In five patients there was a combination of transverse sinus and SSS involvement.
Successful recanalization was achieved in all of the cases except for one (25/26, 96.2%). These patients showed marked and progressive clinical improvement after thrombolysis. Recanalization failed in one 44-year-old patient with severe headache and visual field defects secondary to thrombosis in the right transverse and superior sagittal sinuses due to OCP consumption. She was maintained on anticoagulation therapy without any further clinical or imaging improvement. There was one case with postpartum CVT. The 24-year-old had a history of protein C deficiency and developed a headache and blurred vision one week after normal vaginal delivery. Imaging studies revealed left transverse and superior sagittal sinus thrombosis.
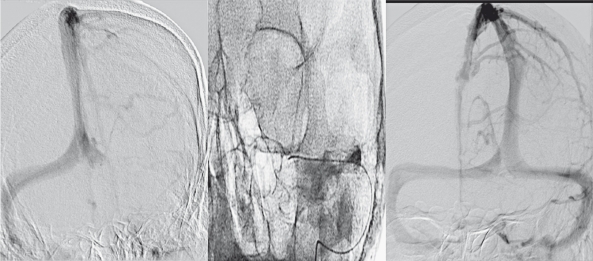
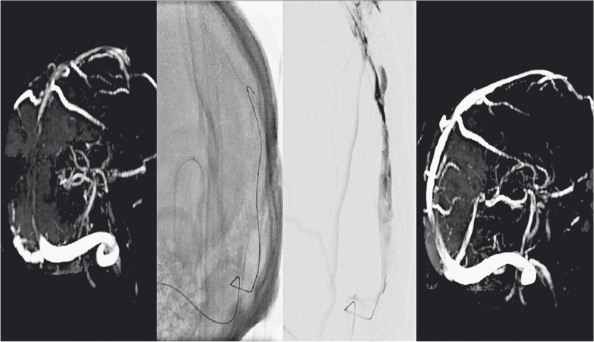
Five cases (5/26, 19.2%) had a history of antiphospholipid antibody syndrome (APA). Cerebral venous thrombosis developed while they were on antiplatelet therapy. One case had severe headache and papilledema due to left transverse sinus thrombosis and further work-up revealed antithrombin III deficiency (Figure 1). Two male patients had a history of head trauma and opium addiction as an etiologic factor. A 21-year-old patient developed acute thrombosis in the SSS five days after a closed head trauma. He presented with severe headache and blurred vision. He showed evidence of cortical venous infarction in the right frontoparietal region followed by focal seizure while under treatment with full dose heparin. Antiepileptic medication was started and local thrombolysis was performed immediately (Figure 2).
Figure 1.
A 35-year-old woman presented with headache and blurred vision secondary to acute thrombosis in the left lateral sinus (left). The microcatheter was advanced up to the distal segment of sinus (middle). Control image 20 min after thrombolysis showed excellent recanalization (right). Further work-up revealed Antitrombin III deficiency.
Figure 2.
MRV showed SSS thrombosis secondary to closed head trauma (left) in a 21-year-old male patient. The microcatheter was advanced into the site of thrombosis and 30 mg rtPA was injected (middle). Control MRV showed patent sinus (right).
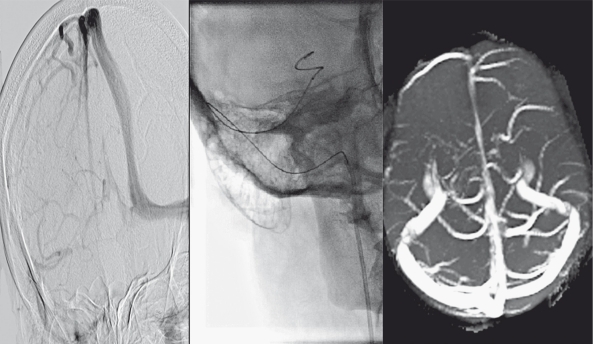
Another patient was 45 years old with a history of chronic opium addiction and major depression. He had stopped taking his antidepressant medication without doctor approval and recently increased the amount of opium he was taking. He was diagnosed with right transverse sinus thrombosis and treated with full anticoagulation therapy. He developed left-sided hemiparesis due to a small hemorrhage in the right temporal lobe two weeks later and was referred to our institute for further management. He underwent local thrombolysis with 30 mg recombinant tissue plasminogen activator (rtPA). He responded well to treatment and control brain MRV showed complete patency of involved the sinus (Figure 3).
Figure 3.
Right transverse sinus thrombosis in a 45-year-old opium addicted man (left). While the guiding catheter is in the jugular bulb, the microcatheter was advanced into the transverse sinus (middle). The patient showed significant improvement and control MRV showed good recanalization (right).
The mean follow-up time period was 10.0 ± 5.9 months (range 3-23 months). Follow-up assessments at the third week showed that 25 out of 26 recovered, with 18 having a mRS score of 0 and 7 having a mRS score of 1. In patients with successful recanalization, the control MRV was scheduled for 72 hours later. MRV findings in these patients revealed continued sinus patency and normal flow in the dural sinuses. Control perimetry was performed in only 12 patients who showed significant improvement in comparison with baseline. The patient with failed recanalization developed severe visual loss and visual field defects. She remained handicapped, with a mRS score of 4. There were no procedure-related neurological complications. Two patients developed small puncture site hematomas that resolved spontaneously.
Discussion
We found a dramatic response to local thrombolysis and significant clinical improvement in the patients with progressive CVT, which otherwise have high rates of mortality and morbidity. Imaging revealed complete recanalization in 25 patients (96.2%). All patients were under treatment with heparin, which was continued for a long period in some patients without results, and they responded positively to local thrombolysis, emphasizing the benefits of a more aggressive treatment for this type of CVT.
We did not achieve recanalization in one patient with a poor prognosis and she developed severe visual loss, highlighting the importance of rapid and successful recanalization in patients with progressive CVT. According to our results, CVT is more common in women and in the left transverse sinus. OCP consumption was the most common etiologic factor in our cases. We found opium addiction and head trauma as a suspected etiologic factors in two male patients.
In addition to a high success rate for recanalization and good clinical recovery, we did not observe any major complications related to the interventional procedure. This could be due to early intervention in the more severe cases, exclusion of patients with large hematomas and resulting mass effects or malignant predisposing factors like leukemia, correction of the predisposing factor whenever possible, and appropriate post-procedural management by our team.
One possible limitation of our study is that baseline and control perimetry was performed in less than of half of the cases. In addition, there is the possibility of selection bias in our patient group since most of our cases had an isolated transverse sinus thrombosis. These patients may have a better clinical outcome regardless of the treatment applied than patients with superior sagittal sinus thrombosis or bilateral transverse sinus thrombosis. However, the clinical condition of all the patients was deteriorating despite the administration of optimal medical treatment.
The widespread use of magnetic resonance imaging (MRI) and rising clinical awareness has increased the recognition of cerebral sinovenous thrombosis in recent years. Although anticoagulation is the first choice for treatment and improved outcome in patients, it is only able to prevent thrombus propagation and does not lyse the thrombus that occludes the sinus 9,11. In contrast, local thrombolysis can reestablish venous drainage actively and prevent grave consequences such as cerebral edema and hemorrhage.
Several reports have shown that endovascular management of progressive CVT, including direct thrombolysis with or without mechanical thrombus extraction, is a safe and effective treatment 12-19. These reports were based on nonrandomized uncontrolled case series or case reports and the risk to benefit ratio of this approach remains unknown. There is also a report on the safety and efficacy of local intrasinus heparin infusion with or without adjunctive balloon thrombectomy in the patients for which systemic anticoagulation treatment has failed or is contraindicated 20.
In a systematic review of the literature up to June 2010, which included 15 studies and 156 patients, Dentali et al. 21 found that thrombolysis is associated with a non-negligible incidence of major bleeding complications, including intracranial bleeding, potentially affecting patients' outcome. However, a careful analysis of all reported cases suggests that the high-risk group of patients selected as candidates for thrombolysis may ultimately develop such complications even without any intervention.
In another study, Canhao et al. 22 systematically reviewed 72 studies on the effect of urokinase as a fibrinolytic therapy for treatment of cerebral venous and sinus thrombosis. Intracerebral hemorrhage (ICH) was reported in 17% of the patients and in 5% it caused clinical deterioration. They concluded that local thrombolysis appeared to be safe but its efficacy cannot be adequately assessed from the available data.
Stam et al. 23 reviewed endovascular treatment in CVT patients prospectively. They enrolled 20 patients, 12 of whom were comatose, and 14 had hemorrhagic infarcts. They selected patients with altered mental status, coma, straight sinus thrombosis, or large space-occupying lesions and used urokinase infusion as a thrombolytic agent or the combination of thrombectomy with urokinase infusion. Twelve patients recovered and six patients died, five of whom had large infarcts and impending herniation prior to thrombolysis. Another factor that was associated with a fatal outcome was patients with leukemia. They concluded that local thrombolysis is an effective treatment modality for patients with severe sinus thrombosis, but their condition may worsen because of increased cerebral hemorrhage, and there was no clear benefit for cases presenting with large infarcts and impending herniation. Based on this study's findings, we excluded patients with large infarcts, hematoma, or leukemia.
In another nonrandomized study, Wasay et al. 24 reviewed 40 consecutive patients with SSS thrombosis that were treated with local urokinase (thrombolysis group) or systemic heparin anticoagulation (heparin group). They found that hemorrhagic complications were higher in the thrombolysis group (10% vs. 0%), but local thrombolysis with urokinase was fairly well-tolerated and may be more effective than systemic heparin anticoagulation.
In our study we used rtPA as a thrombolytic agent. rtPA has many considerable advantages over urokinase, including a shorter half-life, lower rate of antigenecity, and more selectivity for fibrin clot dissolution 25. Lee et al. from Toronto University used a rapid-pulsed direct-infusion technique of 30 to 50 mg of rtPA through a microcatheter over 15 to 20 minutes. They found that endovascular treatment in patients that show clinical deterioration despite 24 hours of heparin therapy and reestablishment of ante grade flow with continued anticoagulation is sufficient to facilitate clinical improvement 26,27. Our method of injecting 30 mg rtPA through a microcatheter over 30 minutes was largely based on their findings.
In the guidelines proposed by the European Federation of Neurological Societies (EFNS 28), the recommended duration of oral anticoagulation therapy varies depending on the underlying etiology. In this study, the patients' treatments were based on their predisposing factors. For transient risk factors like OCP consumption, head trauma, and opium addiction the treatment was continued for three months. For idiopathic variety and mild thrombophilia, like patients suffering from protein C and antithrombin III deficiencies, warfarin was continued for six to 12 months. For those with severe thrombophilia, such as APA patients that developed CVT while on antiplatelet therapy, the therapy should be continued indefinitely.
In conclusion, our results show that local thrombolysis is a safe and effective treatment modality for progressive CVT. These promising results may be explained in part by careful case selection and exclusion of patients with a high probability of failed recanalization and complications after thrombolysis based on previously published reports. Although this type of treatment provided rapid recanalization of occluded sinuses, further comparative and randomized studies are needed to clarify its efficacy versus other therapeutic modalities for the treatment of severe CVT.
References
- 1.Lewis MB, Bousser MG. Cerebral venous thrombosis: Nothing, heparin, or local thrombolysis? Stroke. 1999;30:1729. [PubMed] [Google Scholar]
- 2.DeBruijn SFT, deHaan RJ, Stam J, >for the Cerebral Venous Sinus Thrombosis Study Group. Clinical features and prognostic factors of cerebral venous sinus thrombosis in a prospective series of 59 patients. J Neurol Neurosurg Psychiatry. 2001;70:105–108. doi: 10.1136/jnnp.70.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brucker AB, Vollert-Rogenhofer H, Wagner M, et al. Heparin treatment in acute cerebral sinus venous thrombosis: A retrospective clinical and MR analysis of 42 cases. Cerebrovasc Dis. 1998;8:331–337. doi: 10.1159/000015876. [DOI] [PubMed] [Google Scholar]
- 4.Einhaupl KM, Villringer A, Meister W, et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991;338:597–600. doi: 10.1016/0140-6736(91)90607-q. [DOI] [PubMed] [Google Scholar]
- 5.Ferro JM, Lopes MG, Rosas MJ, Cerebral Venous Thrombosis Portuguese Collaborative Study Group (VENOPORT). et al. Long-term prognosis of cerebral vein and dural sinus thrombosis: results of the VENOPORT study. Cerebrovasc Dis. 2002;13:272–278. doi: 10.1159/000057855. [DOI] [PubMed] [Google Scholar]
- 6.Ferro JM, Canhao P, Stam J, et al. Prognosis of cerebral vein and dural sinus thrombosis: Result of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT) Stroke. 2004;35:664–677. doi: 10.1161/01.STR.0000117571.76197.26. [DOI] [PubMed] [Google Scholar]
- 7.Canhao P, Falcao F, Ferro JM. Thrombolytics for cerebral sinus thrombosis: A systematic review. Cerebrovasc Dis. 2003;15:159–166. doi: 10.1159/000068833. [DOI] [PubMed] [Google Scholar]
- 8.Tsai FY, Higashida RT, Matovich V, et al. Acute thrombosis of the intracranial dural sinus: Direct thrombolytic treatment. Am J Neuroradiol. 1992;13:1137–1141. [PMC free article] [PubMed] [Google Scholar]
- 9.Bousser MG, Chiras J, Bories J, et al. Cerebral venous thrombosis: A review of 38 cases. Stroke. 1985;16:199–213. doi: 10.1161/01.str.16.2.199. [DOI] [PubMed] [Google Scholar]
- 10.Bonita R, Beaglehole R. Modification of Rankin Scale: recovery of motor function after stroke. Stroke. 1988;19:1497–1500. doi: 10.1161/01.str.19.12.1497. [DOI] [PubMed] [Google Scholar]
- 11.Chow K, Gobin YP, Saver J, et al. Endovascular treatment of dural sinus thrombosis with rheolytic thrombectomy and intra-arterial thrombolysis. Stroke. 2000;31:1420–1425. doi: 10.1161/01.str.31.6.1420. [DOI] [PubMed] [Google Scholar]
- 12.Smith TP, Higashida RT, Barnwell SL, et al. Treatment of dural sinus thrombosis by urokinase infusion. Am J Neuroradiol. 1994;15:801–807. [PMC free article] [PubMed] [Google Scholar]
- 13.Scott JA, Pascuzzi RM, Hall PV, et al. Treatment of dural sinus thrombosis with local urokinase infusion. Case report. J Neurosurg. 1988;68:284–287. doi: 10.3171/jns.1988.68.2.0284. [DOI] [PubMed] [Google Scholar]
- 14.Barnwell SL, Higashida RT, Halbach VV, et al. Direct endovascular thrombolytic therapy for dural sinus thrombosis. Neurosurgery. 1991;28:135–142. doi: 10.1097/00006123-199101000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Horowitz M, Purdy P, Unwin H, et al. Treatment of dural sinus thrombosis using selective catheterization and urokinase. Ann Neurol. 1995;38:58–67. doi: 10.1002/ana.410380112. [DOI] [PubMed] [Google Scholar]
- 16.Wasay M, Bakshi R, Kojan S, et al. Nonrandomized comparison of local urokinase thrombolysis versus systemic heparin anticoagulation for superior sagittal sinus thrombosis. Stroke. 2001;32:2310–2316. doi: 10.1161/hs1001.096192. [DOI] [PubMed] [Google Scholar]
- 17.Dowd CF, Malek AM, Phatouros CC, et al. Application of a rheolytic thrombectomy device in the treatment of dural sinus thrombosis: a new technique. Am J Neuroradiol. 1999;20:568–570. [PMC free article] [PubMed] [Google Scholar]
- 18.Kirsch J, Rasmussen PA, Masaryk TJ, et al. Adjunctive rheolytic thrombectomy for central venous sinus thrombosis: technical case report. Neurosurgery. 2007;60:E577–E578. doi: 10.1227/01.NEU.0000255339.26027.68. [DOI] [PubMed] [Google Scholar]
- 19.Mohammadian R, Najaran A, Mansourizadeh R, et al. Progressive cerebral venous thrombosis: Report of three cases treated with local thrombolysis. Med J Tabriz Univ Med Sci. 2011;33:72–76. (in Persian) [Google Scholar]
- 20.La Barge DV, 3rd, Bishop FS, Stevens EA, et al. Intrasinus catheter-directed heparin infusion in the treatment of dural venous sinus thrombosis. Am J Neuroradiol. 2009;30:1672–1678. doi: 10.3174/ajnr.A1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dentali F, Squizzato A, Gianni M, et al. Safety of thrombolysis in cerebral venous thrombosis. A systematic review of the literature. Thromb Haemost. 2010;104:1055–1062. doi: 10.1160/TH10-05-0311. [DOI] [PubMed] [Google Scholar]
- 22.Canhao P, Falcão F, Ferro JM. Thrombolytics for cerebral sinus thrombosis: A systematic review. Cerebrovasc Dis. 2003;15:159–166. doi: 10.1159/000068833. [DOI] [PubMed] [Google Scholar]
- 23.Stam J, Majoie CBLM, Van Delden OM, et al. Endovascular thrombectomy and thrombolysis for severe cerebral sinus thrombosis: A prospective study. Stroke. 2008;39:1487–1490. doi: 10.1161/STROKEAHA.107.502658. [DOI] [PubMed] [Google Scholar]
- 24.Wasay M, Bakshi R, Kojan S, et al. Nonrandomized comparison of local urokinase thrombolysis versus systemic heparin anticoagulation for superior sagittal sinus thrombosis. Stroke. 2001;32:2310–2317. doi: 10.1161/hs1001.096192. [DOI] [PubMed] [Google Scholar]
- 25.Eisenberg PR, Sherman LA, Tiefenbrunn AJ, et al. Sustained fibrinolysis after administration of t-PA despite its short half-life in the circulation. Thromb Haemost. 1987;57:35–40. [PubMed] [Google Scholar]
- 26.Lee SK, Terbrugge KG. Cerebral venous thrombosis in adults: The role of imaging evaluation and management. Neuroimag Clin N Am. 2003;13:139–152. doi: 10.1016/s1052-5149(02)00095-3. [DOI] [PubMed] [Google Scholar]
- 27.Lee SK, Kim BS, Terbrugge KG. Clinical presentation, imaging and treatment of cerebral venous thrombosis (CVT) Intervent Neuroradiol. 2002;8:5–14. doi: 10.1177/159101990200800102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Einhaupl K, Stam J, Bousser MG, et al. EFNS guideline on the treatment of cerebral venous and sinus thrombosis in adult patients. Eur J Neurol. 2010;17:1229–1235. doi: 10.1111/j.1468-1331.2010.03011.x. [DOI] [PubMed] [Google Scholar]