Abstract
We assessed the feasibility and safety of free flap reconstruction in children undergoing extensive surgical excision of malignant head and neck tumors. We performed a retrospective review in a tertiary referral center of all patients aged 18 years or younger who underwent free flap reconstruction following resection of malignant head and neck tumors at our institution. Main outcome measures included complications at the primary and donor sites, functional and esthetic outcome, and tumor control. Eight of the 237 (3.4%) free flap reconstructions were performed on children. All tumors were malignant sarcomas. Ablative surgery was via a transfacial (n = 4) or a combined approach (n = 4). Transferred free flaps were the rectus abdominis (n = 3), gracilis (n = 3), fibula (n = 1), and anterolateral thigh (n = 1). The mean follow-up was 25.5 months. The overall early and late complication rates were 50% and 25%, respectively. There were no flap losses and no donor site complications. Functional outcome, including mastication, deglutition, and speech, was satisfactory. Local tumor control rate at last follow-up was 87.5%. Free flap reconstruction is an efficient and relatively safe technique for reconstructing surgical defects of the head and neck in children undergoing extensive surgery for malignant disease.
Keywords: Head and neck surgery, skull base surgery, free flap reconstruction, pediatric oncology, sarcoma
Free tissue transfer has been widely reported as being an effective method for reconstructing surgical defects following resection of head and neck tumors in adults,1,2,3 but data on its application in the pediatric population are sparse. Children undergoing free flap reconstruction following resection of malignant head and neck tumors comprise a unique population from several aspects. Although most surgical resections for malignancies in adults are for the removal of squamous cell carcinoma, most of the malignant tumors requiring surgical excision in children are sarcomas. A large proportion of these children undergo preoperative chemotherapy or chemoradiation, both of which adversely affect wound healing and growth. Resection and reconstruction of the head and neck must take into account the anatomy and growth pattern of the craniofacial complex. Donor site morbidity might be substantial following the harvesting of tissue in the growing limb. Other concerns are related to the challenges of performing microsurgery in children whose blood vessel diameters are small, anatomic landmarks are inconsistent, and growth of anastomosed vessels is anticipated. Several retrospective studies4,5,6,7 have been conducted to understand the special considerations related to this age group, but the sample sizes were small, and data on the therapeutic management of children with malignant tumors are limited.
The aims of this study were to review our experience with free flap reconstruction after resection of head and neck cancers in children and to assess the feasibility and safety of this technique. Only cases of children treated for malignant tumors were reviewed so that we could focus on the unique challenges of ablative and reconstructive surgery in these children.
PATIENTS AND METHODS
This retrospective study included all patients who underwent free flap reconstruction following resection of head and neck tumors at the Tel-Aviv Sourasky Medical Center between 2000 and 2009. The study was approved by the institutional review board of the Tel-Aviv Sourasky Medical Center. The cases of free flap reconstruction for rehabilitation of facial paralysis were excluded from this series. The suitability of children aged 18 years or younger to be included in the analysis was identified by a search of a computerized database. Their medical records were retrieved to extract information on demographic characteristics, previous treatment, surgical records, and pathology reports. All of the selected patients had undergone ablative surgery and primary free flap reconstruction at our institution. Data on their postoperative course and early complications were collected from the medical charts. Follow-up notes from surgical and medical outpatient clinics were reviewed for late complications, evaluation of growth and function at the reconstructed and donor sites, and outcome. The mean follow-up was 25.5 months (range 6 to 77).
RESULTS
A total of 237 free flap transfers for reconstruction of surgical defects following surgery for malignant tumors of the head and neck were performed at our institution between 2000 and 2009. Eight of them (3.4%) involved children under the age of 18 years who included four boys and four girls whose mean age at the time of surgery was 12 years (range 2 to 18). All the tumors were malignant sarcomas. All the children had undergone preoperative chemotherapy or chemotherapy and radiation. Three children had prior surgery. Patient characteristics and tumor histological type are presented in Table 1.
Table 1.
Patient Characteristics, Tumor Histological Types, and Preoperative Treatments
| Patient | Gender | Age at Time of Surgery (y) | Pathology | Preoperative Treatment |
|---|---|---|---|---|
| 1 | F | 13 | RMS | Chemotherapy, radiotherapy |
| 2 | M | 14 | Ewing's sarcoma | Chemotherapy |
| 3 | F | 17 | Osteosarcoma | Surgery, chemotherapy, radiotherapy |
| 4 | M | 2 | RMS | Chemotherapy |
| 5 | M | 4 | RMS | Surgery, chemotherapy, radiotherapy |
| 6 | M | 14 | RMS | Chemotherapy |
| 7 | F | 16 | RMS | Chemotherapy, radiotherapy |
| 8 | F | 18 | Osteosarcoma | Surgery, chemotherapy |
RMS, rhabdomyosarcoma.
Surgical approach and extent of resection are detailed in Table 2. Ablative surgery was performed via transfacial or combined approach, which included a transfacial and orbitozygomatic approach, a transcervical and orbitozygomatic approach, or a transfacial and subcranial approach. Resections included maxillectomy, mandibulectomy, orbital exenteration, and resection of the involved anterior skull base and infratemporal fossa. The transferred free flaps (Table 2) were rectus abdominis muscle or myocutaneous flap, gracilis myocutaneous flap, fibula osteocutaneous flap and anterolateral thigh. Two cases in which maxillectomy included resection of hard palate were reconstructed with an obturator. Six children received postoperative adjuvant treatment that consisted of radiation alone or chemotherapy and radiation (Table 2).
Table 2.
Surgical Approach, Extent of Resection, Reconstruction, and Follow-Up
| Patient | Surgical Approach | Extent of Resection | Reconstruction | Postoperative Treatment | Follow-Up (mo) | Status at Last Follow-Up |
|---|---|---|---|---|---|---|
| 1 | Transfacial | Orbital exenteration | Anterolateral thigh | None | 8 | FOD |
| 2 | Combined: transfacial and subcranial | ASB, total maxillectomy, partial orbitectomy | Rectus abdominis muscle, obturator | Chemotherapy, radiotherapy | 77 | FOD |
| 3 | Transfacial | Total maxillectomy, orbital exenteration, nasal bone | Rectus abdominis myocutaneous flap, iliac bone graft, obturator | Chemotherapy, radiotherapy | 37 | AWD |
| 4 | Transfacial | Orbital exenteration | Gracilis myocutaneous flap | None | 17 | DOD |
| 5 | Combined: transfacial and orbitozygomatic | Orbital exenteration, partial maxillectomy, ITF | Rectus abdominis, PTSG, iliac bone graft | Radiotherapy | 6 | FOD |
| 6 | Combined: transcervical and orbitozygomatic | Segmental mandibulectomy, partial maxillectomy, ITF | Fibula Osteocutaneous flap | Chemotherapy, radiotherapy | 29 | AWD |
| 7 | Transfacial | Orbital exenteration | Gracilis myocutaneous flap | Chemotherapy, radiotherapy | 15 | FOD |
| 8 | Combined: transfacial and subcranial | ASB, orbital exenteration | Gracilis myocutaneous flap | Radiation | 15 | FOD |
ASB, anterior skull base; AWD, alive with disease; DOD, dead of disease; FOD, free of disease; ITF, infratemporal fossa; PTSG, partial thickness skin graft.
The overall early complication rate was 50% (4/8), and the complications included venous thrombosis requiring revision of the anastomosis (n = 1), transient flap congestion requiring decompression (n = 1), minor wound breakdown (n = 1), and wound infection at the site of resection (n = 1). There were no flap losses and no donor site complications. The overall late complication rate was 25% (2/8): one case of osteoradionecrosis of the frontonasal bone segment following subcranial surgery and adjuvant chemoradiation was successfully reconstructed with a lateral thigh free flap, and one case of antrocutaneous fistula after maxillectomy was closed with conservative treatment. Functional outcome, including mastication, deglutition, and speech, was satisfactory. Following the early postoperative period, all children resumed a full oral diet and were able to masticate and swallow solid food. There was no need for a feeding gastrostomy in any of the cases. All children had intelligible speech. Cosmetic results varied and were related to the extent of resection. There was no impairment of donor site growth or function. All children could resume normal physical activities, and there were no reports of limb length discrepancies. At last follow-up, five children were free of disease, one child was alive with metastatic disease, one child was alive with recurrent local disease, and one child was dead of metastatic disease, for a local control rate of 87.5% (7/8).
DISCUSSION
Considerable progress has been made in the field of reconstructive surgery during the last three decades. Compared with local and regional flaps, free flaps are more effective in achieving the goals of reconstructive surgery (i.e., promoting wound healing, restoring function, and maintaining esthetics). Free flap reconstruction is now the gold standard for reconstructing major surgical defects following resection of malignant head and neck tumors.
Although data on free flap reconstruction in adults are abundant,1,2,3 few studies have assessed the feasibility of free tissue transfer in children.4,5,6,7 All of the available studies are retrospective reviews, and the cohorts are relatively small, probably due to the rarity of the disease, which is usually treated nonsurgically. The aim of the current study was to investigate the unique surgical considerations and postoperative clinical course in a group of children who were all treated surgically for malignant tumors. This is in contrast to most previous studies in which some, or most, of the children had benign disease. Cases of free flap reconstruction for rehabilitation of facial paralysis that had been included in some of the previous studies were excluded from ours since treatment for this group does not pose similar challenges. Unlike the other cohorts that had been described in the literature, all of our study children received preoperative chemotherapy or chemotherapy and radiation, factors recognized as adversely affecting wound healing.8 Although the gracilis free flap is a versatile option for reconstructing soft tissue defects, data on its use in children are very limited.
There are several concerns unique to pediatric patients. Pediatric microsurgery is challenging because vascular structures are small. Most flap losses reportedly occurred in smaller children.7 In the current series, the only case requiring revision of a venous anastomosis was a 17-year-old. Microsurgery in the smaller children (i.e., aged 2 and 4 years) was more difficult but nevertheless successful. There is a question as to whether vessel growth could cause flow issues at the anastomotic site, but we did not encounter any subsequent events to support these concerns. In our experience, there were no late revisions and no signs of differential growth at the site of reconstruction that could be attributed to impaired blood flow. It is our impression that it is possible to achieve a reasonable microsurgical complication rate in children. Other concerns are related to the donor site. Although donor site morbidity is generally minor in adults, the risk of long-term functional deficits must be considered when the patients are children. In addition to the inevitable compromise at the site of resection, esthetic defects at the donor site can have a major psychological impact on a child. Commonly used donor sites, such as the fibula, iliac, and scapula, possess epiphyseal growth centers that are central to skeletal growth and development, and soft tissue architecture must be considered as well.
We encountered no short- or long-term donor site complications. The children who underwent free fibula transfer reported no difficulties during daily or recreational activities or any evidence of leg length discrepancy. Gracilis muscle harvest caused no functional impairment and a minimal esthetical flaw. Rectus abdominis harvest required a longer period of rehabilitation, but there were no reports of pain or restriction when normal activities were resumed.
Although there were no flap losses, there was a relatively high rate (50%) of early complications among our patients, and they all were related to the reconstructed site (infection, dehiscence, and microsurgical complications). In our experience with 229 free flap reconstructions in adults, the rate of early complications was 20%, and only 14% of them were related to the surgical site. This difference could be attributed to some of the factors described earlier, such as impaired wound healing after chemotherapy and radiation, difficulties in handling delicate tissues of a child, and problems of wound care and immobilization in a small child who might be less cooperative than an adult.
Craniofacial development is a complex process involving several mechanisms of growth.9 Our experience in treating children with skull base tumors10,11,12 has helped us understand the considerable anatomic differences between children and adults, including the small size and fragility of neurovascular elements, the inconsistency of anatomic landmarks, and the need to avoid disruption of permanent dentition. Wide resection is crucial when treating sarcomas,13 and reconstruction is the key to maintaining anatomic and physiological relationships of the craniofacial complex after such resection. In the children who were included in the current study, resection of large malignant tumors inevitably resulted in major craniofacial defects, necessitating complex reconstruction (Figs. 1 and 2). Reconstruction of these defects with free tissue transfer enabled the achievement of satisfactory symmetry and function. The children did not suffer from functional problems in terms of deglutition, speech, and mastication. All children resumed a full oral diet and were able to masticate and swallow solid food. There was no need for a feeding gastrostomy in any of the cases. All children had intelligible speech. Importantly, there was no evidence of differential growth at the reconstructed site. Finally, most malignant tumors in the pediatric population are sarcomas that are treated by chemotherapy and radiation, both before and after surgery. Adequate reconstruction with free tissue transfer can minimize late sequelae of medical treatment, such as facial hypoplasia and asymmetry.14
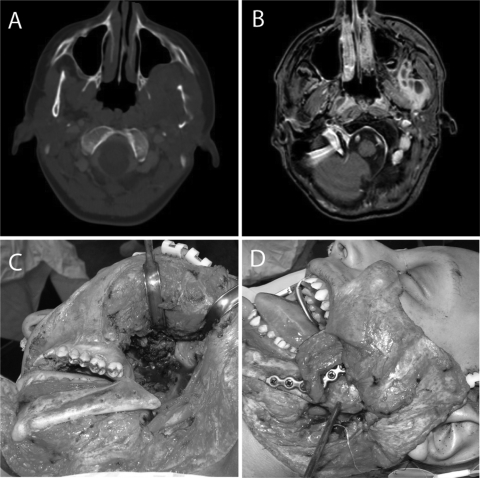
Figure 1.
A 14-year-old boy with Ewing's sarcoma. Axial computed tomography (A) and magnetic resonance (B) images show the tumor involving the left mandible and infratemporal fossa. Tumor resection included a segmental mandibulectomy (C), and reconstruction was preformed with a fibula free flap and titanium plate (D).
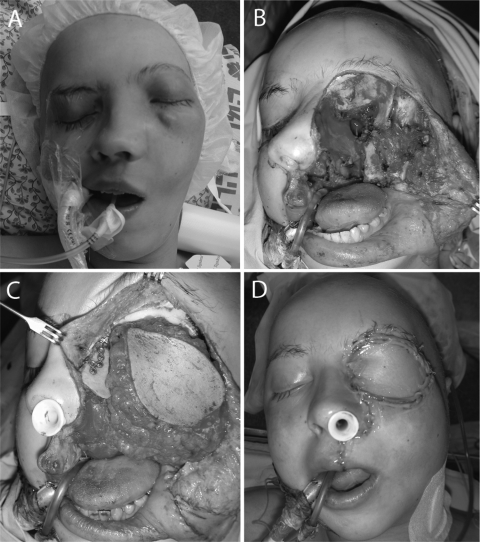
Figure 2.
A 17-year-old girl with osteosarcoma of the left maxillary sinus and orbit (A). The resection included maxillectomy with orbital exenteration (B). The large defect was reconstructed with a rectus abdominis free flap, iliac bone graft, and titanium mini-plates (C, D).
Our study has several limitations. The cohort is small and it bears the usual shortcomings of a retrospective analysis. Evaluation of growth and function of the reconstructed and donor sites was based on physical examination and subjective patient/family reports as well as on indirect measures, such as the lack of late revision surgery, need for a feeding gastrostomy, or prolonged treatment by a speech pathologist. Serial radiological evaluations, cephalometrics, and donor limb measurements as well as quality-of-life questionnaires would have been of interest and value.
CONCLUSION
Published data on reconstruction surgery in children undergoing surgical excision of malignant head and neck tumors are sparse. The results of this study demonstrate that free flap reconstruction is an efficient and relatively safe technique for reconstructing surgical defects of the head and neck in children undergoing extensive surgery and adjuvant therapy for malignant disease. Despite the difficulties in performing the surgery and in the postoperative care of these children, free flap reconstruction provides satisfactory functional and esthetic results.
ACKNOWLEDGMENT
We thank Esther Eshkol, M.A., the institutional medical copy editor, for editorial assistance.
References
- Eckardt A, Fokas K. Microsurgical reconstruction in the head and neck region: an 18-year experience with 500 consecutive cases. J Craniomaxillofac Surg. 2003;31:197–201. doi: 10.1016/s1010-5182(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Amarante J, Reis J, Costa-Ferreira A, et al. Head and neck reconstruction: a review of 117 cases. Eur J Plast Surg. 2000;23:404–412. [Google Scholar]
- Cordeiro P G, Disa J J, Hidalgo D A, Hu Q Y. Reconstruction of the mandible with osseous free flaps: a 10-year experience with 150 consecutive patients. Plast Reconstr Surg. 1999;104:1314–1320. doi: 10.1097/00006534-199910000-00011. [DOI] [PubMed] [Google Scholar]
- Boyd J B. Mandibular reconstruction in the young adult using free vascularized iliac crest. Microsurgery. 1988;9:141–149. doi: 10.1002/micr.1920090216. [DOI] [PubMed] [Google Scholar]
- Nakatsuka T, Harii K, Yamada A, et al. Immediate free flap reconstruction for head and neck pediatric malignancies. Ann Plast Surg. 1998;40:594–599. doi: 10.1097/00000637-199806000-00004. [DOI] [PubMed] [Google Scholar]
- Genden E M, Buchbinder D, Chaplin J M, Lueg E, Funk G F, Urken M L. Reconstruction of the pediatric maxilla and mandible. Arch Otolaryngol Head Neck Surg. 2000;126:293–300. doi: 10.1001/archotol.126.3.293. [DOI] [PubMed] [Google Scholar]
- Arnold D J, Wax M K, Microvascular Committee of the American Academy of Otolaryngology–Head and Neck Surgery Pediatric microvascular reconstruction: a report from the Microvascular Committee. Otolaryngol Head Neck Surg. 2007;136:848–851. doi: 10.1016/j.otohns.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Sassler A M, Esclamado R M, Wolf G T. Surgery after organ preservation therapy. Analysis of wound complications. Arch Otolaryngol Head Neck Surg. 1995;121:162–165. doi: 10.1001/archotol.1995.01890020024006. [DOI] [PubMed] [Google Scholar]
- Thilander B. Basic mechanisms in craniofacial growth. Acta Odontol Scand. 1995;53:144–151. doi: 10.3109/00016359509005964. [DOI] [PubMed] [Google Scholar]
- Gil Z, Constantini S, Spektor S, et al. Skull base approaches in the pediatric population. Head Neck. 2005;27:682–689. doi: 10.1002/hed.20226. [DOI] [PubMed] [Google Scholar]
- Gil Z, Patel S G, Cantu G, et al. International Collaborative Study Group Outcome of craniofacial surgery in children and adolescents with malignant tumors involving the skull base: an international collaborative study. Head Neck. 2009;31:308–317. doi: 10.1002/hed.20958. [DOI] [PubMed] [Google Scholar]
- Shlomi B, Chaushu S, Gil Z, Chaushu G, Fliss D M. Effects of the subcranial approach on facial growth and development. Otolaryngol Head Neck Surg. 2007;136:27–32. doi: 10.1016/j.otohns.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Gil Z, Patel S G, Singh B, et al. International Collaborative Study Group Analysis of prognostic factors in 146 patients with anterior skull base sarcoma: an international collaborative study. Cancer. 2007;110:1033–1041. doi: 10.1002/cncr.22882. [DOI] [PubMed] [Google Scholar]
- Raney R B, Asmar L, Vassilopoulou-Sellin R, et al. IRS Group of the Children's Cancer Group and the Pediatric Oncology Group Late complications of therapy in 213 children with localized, nonorbital soft-tissue sarcoma of the head and neck: A descriptive report from the Intergroup Rhabdomyosarcoma Studies (IRS)-II and - III. Med Pediatr Oncol. 1999;33:362–371. doi: 10.1002/(sici)1096-911x(199910)33:4<362::aid-mpo4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]