Abstract
The facial nerve is one of the most commonly injured cranial nerves. Once injured, the effects on form, function, and psyche are profound. We review the anatomy of the facial nerve from the brain stem to its terminal branches. We also discuss the physical exam findings of facial nerve injury at various levels. Finally, we describe various reconstructive options for reanimating the face and restoring both form and function.
Keywords: Facial nerve, facial nerve palsy, facial paralysis, Bell's palsy, facial reanimation, smile surgery
The face is the most visible part of the body. Facial expressions allow us to interact with others and relay feeling and emotions.1 When facial movement is absent, so is the ability to convey these nonverbal cues and function normally in society. Unfortunately, the facial nerve, which controls the muscles of facial expression, is the most commonly paralyzed nerve in the human body.2 Although current state-of-the-art surgery for facial nerve palsy falls short of perfection, it does offer considerable improvement to these unfortunate patients.
ANATOMY AND FUNCTION OF THE FACIAL NERVE
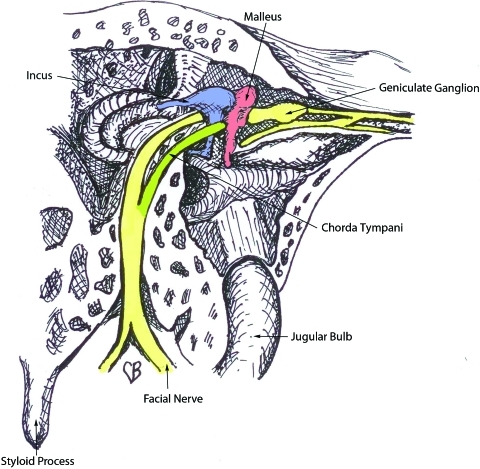
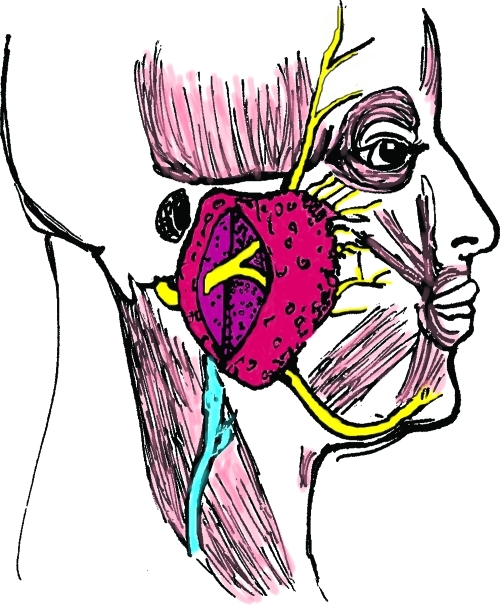
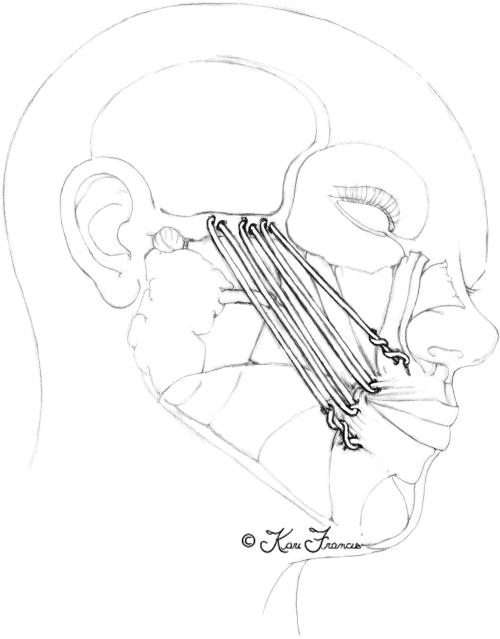
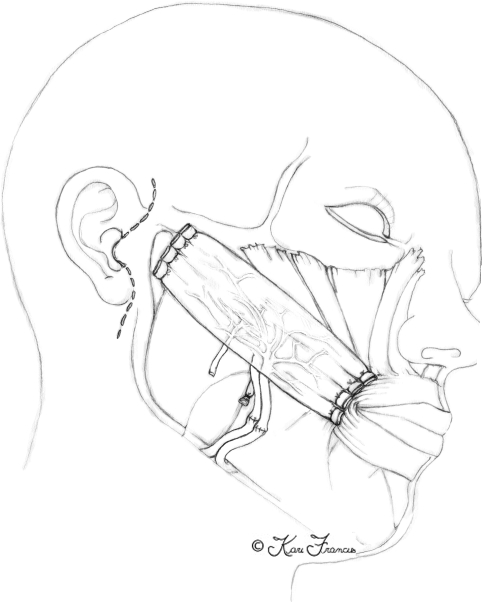
The facial nerve begins at the level of the pons, then exits the skull base at the petrous portion of the temporal bone. It then winds through the internal auditory meatus within the facial canal and exits the skull base at the stylomastoid foramen (Fig. 1). This foramen lies anterior and deep to the mastoid process.3 However, upon exiting, the nerve quickly becomes superficial and as it does, it becomes increasingly susceptible to injury. It then enters the parotid gland and divides into an upper and lower trunk (Fig. 2). The trunks divide further within the gland so that, by the time they exit the parotid, multiple branches make up the five divisions. Significantly for the surgeon, there is frequent duplication of branches providing the same function. This makes cross facial nerve grafting possible as a branch that innervates the same muscle or muscle group as another can safely be sacrificed and used for coaptation of the graft.4
Figure 1.
Anatomy of the facial nerve as it courses through the middle ear.
Figure 2.
Upper and lower trunk of the facial nerve within the parotid gland.
The temporal division runs across the undersurface of the temporal-parietal fascia after crossing over the zygomatic arch. It innervates the frontalis, corrugator, and upper orbicularis oculi muscles. As there is little fatty tissue between the nerves and skin at the lateral border of the frontalis, the nerves are very subject to injury from lacerations. The zygomatic and buccal divisions supply the lower eyelid, nasalis, zygomaticus major and minor, levator labii superioris, anguli oris, orbicularis oris, and buccinators with frequent overlap.
The marginal mandibular division, consisting of one to three branches, passes caudal to the angle of the mandible and then arches upward to cross the mandibular body approximately at the junction of body and parasymphyseal region. It innervates the depressor anguli oris, depressor labii inferioris, mentalis, and sometimes the upper platysma and lower orbicularis oris. The cervical division is the most caudal and innervates the platysma. Both marginal mandibular and cervical branches run on the deep surface of the platysma.3
There are 18 muscles of facial expression, each contributing in its unique way to create the distinctive appearance of an individual's facial animation. Seventeen of these are paired and the 18th, the orbicularis oris, is unpaired. The muscles are arranged in layers.5,6 The most superficial are the depressor anguli oris, zygomaticus minor, and orbicularis oculi. Nerves entering their deep surface innervate the superficial and middle layer muscles. The deepest layer is comprised of the buccinators, mentalis, and depressor anguli oris. These latter muscles receive their innervation by nerves that enter the superficial surface so that they are in the same plane as all the other nerve branches.
ETIOLOGY OF PARALYSIS
Given the complex anatomy of the facial nerve, it is no surprise that trauma is one of the main causes of injury along with Bell's palsy, iatrogenic injury, and idiopathic and congenital palsy.7 Traumatic injuries include lacerations, blunt crush injuries, temporal bone fractures, and birth trauma.8 The more lateral position of the stylomastoid foramen in newborns makes the nerve particularly susceptible to injury during delivery whether or not forceps are required. Although nearly 90% of neonatal palsies display a complete return of function,9 congenital disorders such as Möbius and craniofacial microsomia are unlikely to improve.
Bell's palsy is the most common cause of transient facial nerve paralysis. Though function often returns without intervention, permanent paresis, synkinesis, or paralysis can occur.10 The etiology in Bell's palsy is thought to be herpes simplex virus infection.11 Some improvement has been shown with acyclovir and steroid therapy12 as well as surgical decompression,13 but the ideal management remains elusive.
Intracranial injury due to trauma or stroke can bring about a sudden loss of facial nerve function. These etiologies should be managed with a neurologist and neurosurgeon. As with Bell's palsy, sufficient time must be given to allow spontaneous return of function before contemplating reanimation procedures.
A gradual onset of facial nerve weakness is an ominous sign and always merits further workup. Such symptoms can occur from tumor growth anywhere along the course of the nerve including the brain stem,14 the middle ear, and the parotid gland.15 Once the tumor is removed, any residual facial nerve deficiency can be assessed and treated.
PATIENT ASSESSMENT
Once onset of symptoms has been addressed and the paralysis is determined to be long-standing and irreversible, the patient's needs are assessed. These needs are grouped into functional, aesthetic, and emotional, all of which are equally important.
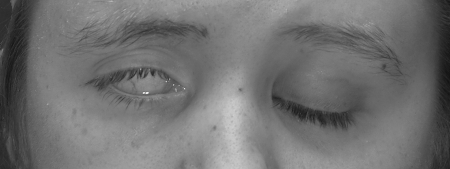
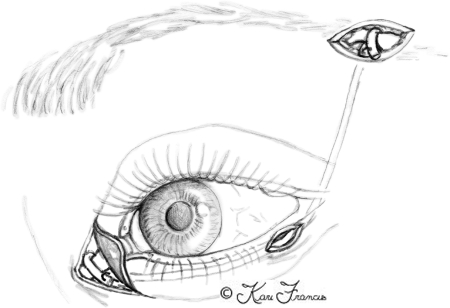
Functional deficiencies center upon the eye and the mouth. Eye symptoms are partially due to the denervation of the orbicularis oculi muscle and loss of the blink reflex. This puts the eye at risk for trauma and reduces the spread of lacrimal secretions across the eye, resulting in dry eyes. Corneal protection, therefore, relies on an intact Bell phenomenon16 (Fig. 3), which, if absent, can lead to corneal keratitis, ulcerations, and blindness. Lacrimal gland innervation can also be affected, resulting in decreased tear production.17,18 Alternatively, a hyperlacrimation can result in the crocodile tears phenomenon, which responds to botulinum toxin injection.19,20 Last, the loss of muscle tone to the orbicularis oculi muscle can allow the lower lid to pull away from the globe. This ectropion disables the lower cannalicular system thereby creating excess tears. The paradoxical situation of dry eyes and epiphora can affect visual acuity and make the globe prone to injury.
Figure 3.
An intact Bell's phenomenon on the patient's right protects the cornea despite inadequate lid closure.
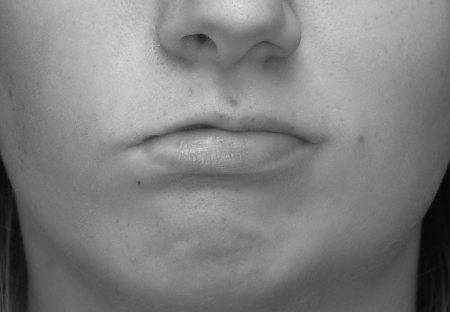
Functional deficits of the mouth are also due to loss of tone. Loss of tone to the muscles that control the oral sphincter leads to drooping of the commissure (Fig. 4). This can cause drooling and difficulty eating. Lack of tone in the cheek can also affect speech, contributing to problems of articulation. Patients will often physically support the cheek with their hand to make their speech more intelligible.
Figure 4.
Drooping of the right commissure coupled with a flaccid cheek can affect speech and cause loss of oral competence and drooling.
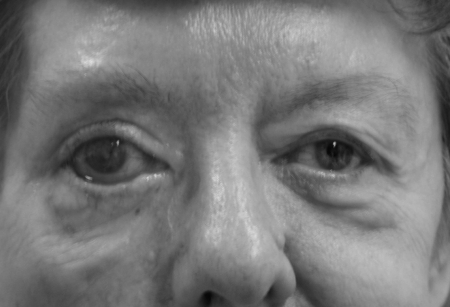
Aesthetic concerns also center upon the eyes, cheeks, and mouth. Loss of frontalis function can lead to brow ptosis. Initially, brow position is maintained in repose and it is the asymmetry of motion that is most noticeable. But, with time, gravity will draw the brow downward, making the eye look heavy and eventually obstructing visual fields with excess skin (Fig. 5). The palpebral fissure is generally rounder and more open on the affected side with excess scleral show and possibly ectropion. This coupled with the epiphora, scleral irritation, and conjunctival exposure can draw attention to the affected side (Fig. 6).
Figure 5.
Loss of frontalis function leads to brow ptosis, which can obstruct the visual field. This patient is marked for a direct brow lift on the right side. (From Neligan PC, Wei F-C. Microsurgical Reconstruction of the Head and Neck. St. Louis: Quality Medical Publishing, Inc.; 2009.)
Figure 6.
Loss of orbicularis oculi tone leads to ectropion, scleral irritation, and epiphora.
In the cheek and mouth, there is a loss of nasolabial fold and sagging of the commissure. The cheek is flaccid and often droops. The lips also sag and are less full on the affected side. This accentuates the asymmetry and deformity and can be quite distressing to the patient.
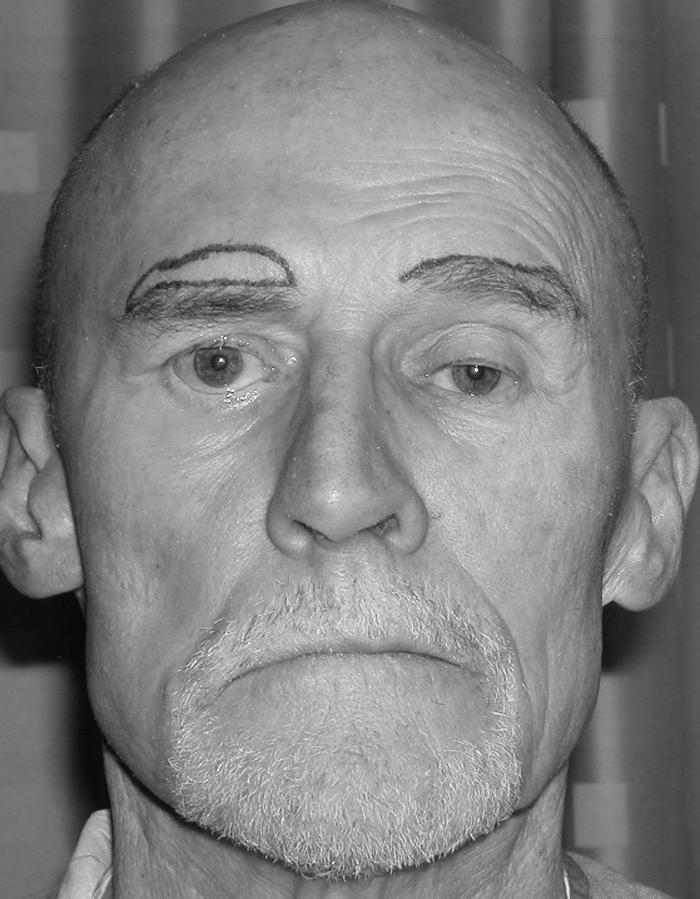
Emotional concerns are not to be overlooked and can be devastating to the patient's daily life. Successful professionals are suddenly treated as intellectual inferiors, despite normal intelligence, based solely on their facial appearance (Fig. 7). Many patients withdraw from social situations, change jobs to those with less interpersonal contact, and avoid intimate relationships.21,22 They report an awareness of being stared at, avoid eating in public so as not to drool, and abhor being photographed. They also learn not to smile and laugh to avoid accentuating their deformity. These psychological issues are easily overlooked but being aware of them is extremely important for the team of care providers looking after these patients.
Figure 7.
Loss of bilateral facial nerve function has negatively affected the facial appearance in this successful professional. (From Neligan PC, Wei F-C. Microsurgical Reconstruction of the Head and Neck. St. Louis: Quality Medical Publishing, Inc.; 2009.)
Examination of the Paralyzed Face
The face should be examined in a systematic, orderly fashion, starting from the top and working down. The examiner should sit at eye level and carefully measure differences between the facial sides. This is best done with two clear plastic rulers using defined facial features.23 Points to be measured include the midbrow, the palpebral opening, the amount of scleral show, and the position of the oral commissure relative to the other side. Examination of the eye should include an assessment of visual acuity as well as measurement of the amount of closure achieved. It is also important to measure facial movement in the lower face, and this can be done at the oral commissure, the mid upper lip, and the philtrum. The vector of lower-facial movement is also important to appreciate, particularly if one is considering a functioning muscle transfer to produce a smile. Some people have a very horizontal smile and others have a more vertical one, with greater tooth show. Measuring the degree and direction of movement can help the surgeon select the appropriate reanimation procedure.
NONSURGICAL THERAPY
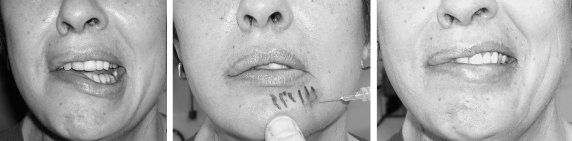
The first step in treating facial nerve paralysis is often nonsurgical. Neuromuscular retraining by an experienced therapist can have a very beneficial effect in treating patients with partial paralysis and especially in treating the patient with synkinesis. For patients with ocular findings, ophthalmic drops and ointment should be used to protect the cornea. Asymmetry of facial movement can be treated with botulinum toxin. For instance, the intact frontalis muscle can be chemically denervated with Botox® (Botox Allergan, Inc., Irvine, CA) to balance with the affected side. Additionally, Botox® can be used to denervate the intact lip depressors to balance the smile. This can first be simulated in the clinic with an injection of local anesthetic (Fig. 8). Botox® is also useful in treating synkinesis. However, the effects of botulinum toxin are transient and patients often seek a more permanent treatment.
Figure 8.
The strong pull of intact lip depressors on the left side enhance the lip asymmetry. A “test drive” with local anesthetic temporarily paralyzes the intact depressors and balances out the smile.
SURGICAL THERAPY
Brow
Eyebrow symmetry can be attained by permanently disabling the intact frontal branch with a neurectomy. This will improve kinetic balance, but will make both brows prone to sag in repose. A brow-lift can be used to improve brow symmetry at rest. Although an endoscopic brow-lift may be adequate for mild brow ptosis, a direct excision is often necessary to correct severe deformity. In this operation, an elliptical excision is designed just along the main line of hair follicles (Fig. 5). Skin and frontalis muscle are directly excised, and the defect should be closed in layers so that the skin edges evert. The resulting scar is quite visible, but can be acceptable in elderly patients and in men who have more prominent brows. Dynamic procedures to provide brow movement through transfer of the frontalis muscle24 and the platysma25 have been described, but their use has not been reproducible or widespread.
Upper Eyelid
Although the orbicularis oris muscle is paralyzed, the levator palpebrae superioris is still intact as it is innervated by cranial nerve III. Thus, the patient maintains the ability to raise the upper eyelid. The surgeon can capitalize on this by inserting a device into the eyelid to allow closure. The simplest device is a gold weight.26,27 By choosing the appropriate weight, the patient can close the upper eyelid by relaxing the levator muscle. An alternative approach is to insert a spring device in the upper eyelid.28,29 This functions like an open safety pin. The levator is strong enough to close the spring and when the levator is relaxed the spring opens, thus closing the lid.
Lower Eyelid
Lid laxity and ectropion are most frequently treated with medial and or lateral tarsorrhaphy30 or lid-tightening procedures.31 However, in our experience, use of a tendon sling for the lower eyelid32 in combination with a gold weight for the upper-lid produces the most consistent correction. A thin strip of palmaris longus tendon is passed on a Keith needle just beneath the lash line. It is then wrapped around the medial canthal tendon and secured to itself (Fig. 9), then elevated up around the lateral canthal tendon to the superior orbital rim where it is fixed to a drill hole. Dynamic procedures to create lid closure have been described,33 most using the temporalis muscle or its fascia to pull the upper and lower lids laterally. Although this procedure can provide eyelid movement, the closure is abnormal and may not correct the lid malposition and lagophthalmos, which cause the patients' epiphora and dry eye symptoms.
Figure 9.
A lower-eyelid tendon sling supports the lid and reestablishes contact of the punctum to the globe, thus enhancing tear drainage.
Lower Lip
Asymmetry of smile can be caused by lip depressor inactivity due to marginal mandibular paralysis. Dynamic procedures have been described to correct this34 but are technically difficult and have not become widespread. Typically, the intact lip depressors are deanimated to balance the smile. This can be done medically with Botox® or surgically with a myomectomy35 or marginal mandibular neurectomy36 to produce a permanent result.
Midface
Reconstruction of the flaccid cheek and sagging, adynamic commissure can either utilize static or dynamic procedures. Patient's needs, goals, and medical condition should be weighed when making the treatment plan. Static options involve placing a sling to restore symmetry at rest, and dynamic procedures require transfer of a functioning muscle.
Static
Fascia lata slings have been the traditional means of supporting the face.4,37 Slips of fascia are connected to the commissure and upper and lower lip, then suspended to the zygomatic arch to elevate the mouth and create a nasolabial fold. This technique produces a good immediate correction but is prone to attenuate with time. Alloplastic material such as Gore-tex® (W.L. Gore & Associates, Inc., Flagstaff, AZ) can be used to prevent this attenuation but runs the risk of inflammation, infection, scarring, and bowstringing. A preferred technique utilizes the plantaris tendon. The plantaris is present in ~85% of patients and can easily be obtained through a single short transverse incision just behind the medial malleolus. Once harvested, the tendon is placed through a face-lift incision and is woven through orbicularis oris and the zygomatic fascia and then woven through itself in a Pulvertaft weave38 (Fig. 10). This provides multiple vectors of pull to recreate the normal nasolabial fold on the unaffected side. Incisions at the nasolabial fold should be avoided as they are unnecessary and can detract from the goal of symmetry.
Figure 10.
A static sling tendon weave reestablishes lip and commissure position.
Dynamic
Dynamic reconstruction offers the advantage of providing movement in addition to static repositioning of the mouth. Dynamic procedures require placement of a muscle and a nerve to power that muscle. This can be done with local flaps, such as the temporalis or masseter, or with distant flaps using microsurgical techniques. The advantage of local flaps is the relative ease of surgery, and the muscles bring their own nerve with them. The disadvantages are the donor site deformity on the face and the constraints on the vector of pull. As mentioned earlier, not all smiles are alike. It is a distinct advantage to be able to adjust the vector of pull without being constrained by a muscle origin or a pedicle as can happen with local muscle flaps.
Numerous muscles have been used to reanimate the face. These include the gracilis,39,40 the pectoralis minor,41 rectus abdominis,42 latissimus dorsi,43,44 extensor carpal radialis brevis, serratus anterior,45,46,47,48 tensor fascia lata, and abductor hallucis.49 Choice of muscle should be made based on length (10 to 12 cm is needed), size, strength, pedicle length, predictability of motor nerve, donor site morbidity, and patient positioning. The gracilis is preferentially used at our center due to its predictable anatomy, adequate length and strength, minimal donor morbidity, unobtrusive scar, and ease of two-team approach.
The gracilis is exposed and an adequate length is marked out centered on the pedicle. The muscle is then split longitudinally, taking more muscle in heavier patients and less in pediatric patients. The muscle is then introduced into the face via a face-lift incision. It is secured to the commissure and the orbicularis oris of the upper and lower lip. Placement of these sutures is critical as they must re-create a normal nasolabial fold. Typically, one suture is placed at the commissure, one below, and two above into the upper lip using 0 Vicryl. The facial artery and vein are used for recipient vessels, and the buccal fat pad is removed to locate the facial vein and reduce bulk. The obturator nerve is then connected to either the cross-face nerve graft or the nerve to the masseter muscle, depending on the reconstructive approach. The muscle is then attached to the superficial muscular aponeurotic system and zygomatic periosteum along the vector of pull, applying resting tension onto the commissure and creating a normal-appearing nasolabial fold (Fig. 11). An implantable Doppler is placed to monitor patency of the microvascular anastamosis.
Figure 11.
Gracilis free flap for facial reanimation attached to the commissure and orbicularis distally and the zygoma proximally.
Selection of the motor nerve to power the gracilis muscle depends on the patient. If possible, we use either the nerve to the masseter or the contralateral facial nerve via cross-face nerve graft. Each has its advantages and disadvantages. The nerve-to-masseter muscle has the advantage of single-stage surgery, on the same side of the face, without risk to the contralateral, intact facial nerve. The donor site morbidity is minimal as patients can chew adequately with the other masseter and temporalis muscles. Additionally, our experience has been that this motor nerve provides a stronger contraction of the gracilis muscle, thereby creating greater excursion of the commissure and a bigger smile. The downside is that the smile created is not spontaneous and requires some learning for the patient to make it appear natural. Yet, even older patients are able, with practice, to make a smile without conscious thought that appears normal to the bystander. The cross-face nerve graft has the advantage of a totally spontaneous smile without the need for learning or practice. Its disadvantages are two stages separated by many months, donor site scars on the face and leg with numbness of the lateral foot (if the sural nerve is used), and risk to the intact facial nerve. Generally, a buccal branch of the intact facial nerve is identified with a nerve stimulator that has crossover innervation with another branch. This branch can then be cut and attached to the sural graft without deanimating the normal side. The graft is then tunneled to the upper lip at the frenum, where it is marked and banked until axonal ingrowth has occurred. This short graft has more predictable results than does a long graft to the contralateral face. Axonal growth is then followed by Tinel's sign, and once the end of the graft is reached, a biopsy is performed at the time of second-stage surgery to insure axonal ingrowth has occurred. In general, the two-stage cross-face nerve graft gives the best results, but the force of contraction tends to be less than with the nerve to the masseter muscle, especially in older adults. In our practice, we typically reserve the two-stage cross-face nerve graft to patients under the age of 20 and offer the single-stage procedure to patients over 20 using the nerve to the masseter muscle.
CONCLUSION
Patients with facial nerve paralysis suffer devastating functional, aesthetic, and emotional consequences due to their inability to move their face. With loss of movement comes loss of eye protection, loss of intelligible speech, loss of oral competence, and loss of normal interactions with others in daily life. For the surgeon, a chance to reanimate the face and correct the functional deficits can be extremely rewarding. A systematic analysis and a combination of static and dynamic procedures can improve these deficits and return the patient back into society. A patient's heartfelt smile is always rewarding for the surgeon, especially when it is the first smile they have ever made.
References
- Ambadar Z, Cohn J F, Reed L I. All smiles are not created equal: morphology and timing of smiles perceived as amused, polite, and embarrassed/nervous. J Nonverbal Behav. 2009;33:17–34. doi: 10.1007/s10919-008-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C G, von Doersten P G. The facial nerve. Current trends in diagnosis, treatment, and rehabilitation. Med Clin North Am. 1999;83:179–195, x. doi: 10.1016/s0025-7125(05)70096-1. [DOI] [PubMed] [Google Scholar]
- Rosson G D, Redett R J. Facial palsy: anatomy, etiology, grading, and surgical treatment. J Reconstr Microsurg. 2008;24:379–389. doi: 10.1055/s-0028-1082897. [DOI] [PubMed] [Google Scholar]
- Lee K K, Terzis J K. Management of acute extratemporal facial nerve palsy. Clin Plast Surg. 1984;11:203–210. [PubMed] [Google Scholar]
- Gassner H G, Rafii A, Young A, Murakami C, Moe K S, Larrabee W F., Jr Surgical anatomy of the face: implications for modern face-lift techniques. Arch Facial Plast Surg. 2008;10:9–19. doi: 10.1001/archfacial.2007.16. [DOI] [PubMed] [Google Scholar]
- Freilinger G, Gruber H, Happak W, Pechmann U. Surgical anatomy of the mimic muscle system and the facial nerve: importance for reconstructive and aesthetic surgery. Plast Reconstr Surg. 1987;80:686–690. doi: 10.1097/00006534-198711000-00005. [DOI] [PubMed] [Google Scholar]
- May M, Klein S R. Differential diagnosis of facial nerve palsy. Otolaryngol Clin North Am. 1991;24:613–645. [PubMed] [Google Scholar]
- Chang C Y, Cass S P. Management of facial nerve injury due to temporal bone trauma. Am J Otol. 1999;20:96–114. [PubMed] [Google Scholar]
- Smith J D, Crumley R L, Harker L A. Facial paralysis in the newborn. Otolaryngol Head Neck Surg. 1981;89:1021–1024. doi: 10.1177/019459988108900628. [DOI] [PubMed] [Google Scholar]
- Peitersen E. The natural history of Bell's palsy. Am J Otol. 1982;4:107–111. [PubMed] [Google Scholar]
- Murakami S, Mizobuchi M, Nakashiro Y, Doi T, Hato N, Yanagihara N. Bell palsy and herpes simplex virus: identification of viral DNA in endoneurial fluid and muscle. Ann Intern Med. 1996;124(1 Pt 1):27–30. doi: 10.7326/0003-4819-124-1_part_1-199601010-00005. [DOI] [PubMed] [Google Scholar]
- Adour K K, Ruboyianes J M, Von Doersten P G, et al. Bell's palsy treatment with acyclovir and prednisone compared with prednisone alone: a double-blind, randomized, controlled trial. Ann Otol Rhinol Laryngol. 1996;105:371–378. doi: 10.1177/000348949610500508. [DOI] [PubMed] [Google Scholar]
- Gantz B J, Rubinstein J T, Gidley P, Woodworth G G. Surgical management of Bell's palsy. Laryngoscope. 1999;109:1177–1188. doi: 10.1097/00005537-199908000-00001. [DOI] [PubMed] [Google Scholar]
- Jullian H. [Tumors of the Cerebellopontine Angle] Fr Medicine. 1963;26:401–403. [PubMed] [Google Scholar]
- Conley J, Selfe R W. Occult neoplasms in facial paralysis. Laryngoscope. 1981;91:205–210. doi: 10.1288/00005537-198102000-00004. [DOI] [PubMed] [Google Scholar]
- Yoon J S, Lew H, Lee S Y. Bell's phenomenon protects the tear film and ocular surface after frontalis suspension surgery for congenital ptosis. J Pediatr Ophthalmol Strabismus. 2008;45:350–355. doi: 10.3928/01913913-20081101-17. [DOI] [PubMed] [Google Scholar]
- Silverstein H. Surgery of the facial nerve. J Otolaryngol. 1981;10:449–458. [PubMed] [Google Scholar]
- May M, Hardin W B. Facial palsy: interpretation of neurologic findings. Laryngoscope. 1978;88(8 Pt 1):1352–1362. doi: 10.1288/00005537-197808000-00019. [DOI] [PubMed] [Google Scholar]
- Zaidi F H, Gregory-Evans K, Acheson J F, Ferguson V. Familial Bell's palsy in females: a phenotype with a predilection for eyelids and lacrimal gland. Orbit. 2005;24:121–124. doi: 10.1080/01676830490916082. [DOI] [PubMed] [Google Scholar]
- Kizkin S, Doganay S, Ozisik H I, Ozcan C. Crocodile tears syndrome: botulinum toxin treatment under EMG guidance. Funct Neurol. 2005;20:35–37. [PubMed] [Google Scholar]
- Jørgensen B G, Pedersen C B. Acoustic neuroma. Follow-up of 78 patients. Clin Otolaryngol Allied Sci. 1994;19:478–484. doi: 10.1111/j.1365-2273.1994.tb01273.x. [DOI] [PubMed] [Google Scholar]
- Kane N M, Kazanas S, Maw A R, et al. Functional outcome in patients after excision of extracanalicular acoustic neuromas using the suboccipital approach. Ann R Coll Surg Engl. 1995;77:210–216. [PMC free article] [PubMed] [Google Scholar]
- Manktelow R T, Zuker R M, Tomat L R. Facial paralysis measurement with a handheld ruler. Plast Reconstr Surg. 2008;121:435–442. doi: 10.1097/01.prs.0000297648.90717.bd. [DOI] [PubMed] [Google Scholar]
- Burgess L, Goode R L. Reanimation of the Paralyzed Face. New York: Thieme Medical Publishers; 1994. [Google Scholar]
- Terzis J K, Bruno W. Outcomes with eye reanimation microsurgery. Facial Plast Surg. 2002;18:101–112. doi: 10.1055/s-2002-32200. [DOI] [PubMed] [Google Scholar]
- Sobol S M, Alward P D. Early gold weight lid implant for rehabilitation of faulty eyelid closure with facial paralysis: an alternative to tarsorrhaphy. Head Neck. 1990;12:149–153. doi: 10.1002/hed.2880120210. [DOI] [PubMed] [Google Scholar]
- Seiff S R, Sullivan J H, Freeman L N, Ahn J. Pretarsal fixation of gold weights in facial nerve palsy. Ophthal Plast Reconstr Surg. 1989;5:104–109. doi: 10.1097/00002341-198906000-00005. [DOI] [PubMed] [Google Scholar]
- May M. Gold weight and wire spring implants as alternatives to tarsorrhaphy. Arch Otolaryngol Head Neck Surg. 1987;113:656–660. doi: 10.1001/archotol.1987.01860060082020. [DOI] [PubMed] [Google Scholar]
- Terzis J K, Kyere S A. Experience with the gold weight and palpebral spring in the management of paralytic lagophthalmos. Plast Reconstr Surg. 2008;121:806–815. doi: 10.1097/01.prs.0000299919.18076.b4. [DOI] [PubMed] [Google Scholar]
- Maas C S, Benecke J E, Holds J B, Schoenrock L D, Simo F. Primary surgical management for rehabilitation of the paralyzed eye. Otolaryngol Head Neck Surg. 1994;110:288–295. doi: 10.1177/019459989411000305. [DOI] [PubMed] [Google Scholar]
- Chang L, Olver J. A useful augmented lateral tarsal strip tarsorrhaphy for paralytic ectropion. Ophthalmology. 2006;113:84–91. doi: 10.1016/j.ophtha.2005.06.038. [DOI] [PubMed] [Google Scholar]
- Terzis J K, Kyere S A. Minitendon graft transfer for suspension of the paralyzed lower eyelid: our experience. Plast Reconstr Surg. 2008;121:1206–1216. doi: 10.1097/01.prs.0000305520.07311.fb. [DOI] [PubMed] [Google Scholar]
- Frey M, Giovanoli P, Tzou C H, Kropf N, Friedl S. Dynamic reconstruction of eye closure by muscle transposition or functional muscle transplantation in facial palsy. Plast Reconstr Surg. 2004;114:865–875. doi: 10.1097/01.prs.0000133028.02303.16. [DOI] [PubMed] [Google Scholar]
- Terzis J K, Kalantarian B. Microsurgical strategies in 74 patients for restoration of dynamic depressor muscle mechanism: a neglected target in facial reanimation. Plast Reconstr Surg. 2000;105:1917–1931. discussion 1932–1934. doi: 10.1097/00006534-200005000-00001. [DOI] [PubMed] [Google Scholar]
- Chen C K, Tang Y B. Myectomy and botulinum toxin for paralysis of the marginal mandibular branch of the facial nerve: a series of 76 cases. Plast Reconstr Surg. 2007;120:1859–1864. doi: 10.1097/01.prs.0000287136.22709.77. [DOI] [PubMed] [Google Scholar]
- Breslow G D, Cabiling D, Kanchwala S, Bartlett S P. Selective marginal mandibular neurectomy for treatment of the marginal mandibular lip deformity in patients with chronic unilateral facial palsies. Plast Reconstr Surg. 2005;116:1223–1232. doi: 10.1097/01.prs.0000182219.40800.56. [DOI] [PubMed] [Google Scholar]
- Rose E H. Autogenous fascia lata grafts: clinical applications in reanimation of the totally or partially paralyzed face. Plast Reconstr Surg. 2005;116:20–32. discussion 33–35. doi: 10.1097/01.prs.0000169685.54862.18. [DOI] [PubMed] [Google Scholar]
- Pulvertaft G. Repair of tendon injuries in the hand. Rev Bras Cir. 1953;26:295–303. [PubMed] [Google Scholar]
- O'Brien B M, Pederson W C, Khazanchi R K, Morrison W A, MacLeod A M, Kumar V. Results of management of facial palsy with microvascular free-muscle transfer. Plast Reconstr Surg. 1990;86:12–22. discussion 23–24. [PubMed] [Google Scholar]
- Sassoon E M, Poole M D, Rushworth G. Reanimation for facial palsy using gracilis muscle grafts. Br J Plast Surg. 1991;44:195–200. doi: 10.1016/0007-1226(91)90126-5. [DOI] [PubMed] [Google Scholar]
- Terzis J K. Pectoralis minor: a unique muscle for correction of facial palsy. Plast Reconstr Surg. 1989;83:767–776. [PubMed] [Google Scholar]
- Sajjadian A, Song A Y, Khorsandi C A, et al. One-stage reanimation of the paralyzed face using the rectus abdominis neurovascular free flap. Plast Reconstr Surg. 2006;117:1553–1559. doi: 10.1097/01.prs.0000206378.90174.6b. [DOI] [PubMed] [Google Scholar]
- Dellon A L, Mackinnon S E. Segmentally innervated latissimus dorsi muscle. Microsurgical transfer for facial reanimation. J Reconstr Microsurg. 1985;2:7–12. doi: 10.1055/s-2007-1007040. [DOI] [PubMed] [Google Scholar]
- Harii K, Asato H, Yoshimura K, Sugawara Y, Nakatsuka T, Ueda K. One-stage transfer of the latissimus dorsi muscle for reanimation of a paralyzed face: a new alternative. Plast Reconstr Surg. 1998;102:941–951. doi: 10.1097/00006534-199809040-00001. [DOI] [PubMed] [Google Scholar]
- Godat D M, Sanger J R, Lifchez S D, et al. Detailed neurovascular anatomy of the serratus anterior muscle: implications for a functional muscle flap with multiple independent force vectors. Plast Reconstr Surg. 2004;114:21–29. discussion 30–31. doi: 10.1097/01.prs.0000129072.11466.c3. [DOI] [PubMed] [Google Scholar]
- Lifchez S D, Sanger J R, Godat D M, Recinos R F, LoGiudice J A, Yan J G. The serratus anterior subslip: anatomy and implications for facial and hand reanimation. Plast Reconstr Surg. 2004;114:1068–1076. doi: 10.1097/01.prs.0000135327.92121.c5. [DOI] [PubMed] [Google Scholar]
- Derby L D, Bartlett S P, Low D W. Serratus anterior free-tissue transfer: harvest-related morbidity in 34 consecutive cases and a review of the literature. J Reconstr Microsurg. 1997;13:397–403. doi: 10.1055/s-2007-1006419. [DOI] [PubMed] [Google Scholar]
- Whitney T M, Buncke H J, Alpert B S, Buncke G M, Lineaweaver W C. The serratus anterior free-muscle flap: experience with 100 consecutive cases. Plast Reconstr Surg. 1990;86:481–490. discussion 491. [PubMed] [Google Scholar]
- Jiang H, Guo E T, Ji Z L, Zhang M L, Lu V. One-stage microneurovascular free abductor hallucis muscle transplantation for reanimation of facial paralysis. Plast Reconstr Surg. 1995;96:78–85. [PubMed] [Google Scholar]