Abstract
Cerebrospinal fluid (CSF) leak closure remains one of the most difficult surgeries for skull base surgeons, particularly with frontal sinus involvement. Technological advances in endoscopic surgery increasingly allow for less morbid approaches to the frontal sinus. We describe a series of patients who underwent endoscopic frontal sinus CSF leak repair utilizing a unilateral approach, to evaluate the utility and outcomes of this method. We performed a retrospective review of four cases in tertiary care centers. Participants included patients with CSF leak involving the frontal sinus. Main outcome measures included cessation of CSF leak and frontal sinus patency. Three patients were closed on the first surgical attempt; one with a communicating hydrocephalus required a revision procedure. Leak etiologies included prior craniotomy for frontal sinus mucopyocele, spontaneous meningoencephalocele, erosion due to mucormycosis, and prior endoscopic sinus surgery. The frontal sinus remained patent in three of four patients. No patients have evidence of a leak at a minimum of 1 year after surgery. The repair of frontal sinus CSF leaks is possible in specific cases with an endoscopic unilateral approach in leaks with multiple etiologies. Surgeons should consider this approach when selecting the appropriate procedure for repair of frontal sinus CSF leaks.
Keywords: Cerebrospinal fluid leak, frontal sinus, frontal recess, endoscopic repair, unilateral
Cerebrospinal fluid (CSF) leaks of the frontal sinus present one of the more difficult challenges in endoscopic sinonasal surgery, involving an area that is anatomically complicated and technically demanding to access. Surgical approaches that have been utilized for dural repair include intracranial and extracranial approaches, endonasal approaches, and most recently in the historical progression, endoscopic approaches to the skull base. The first successful repair of a dural defect was reported in 1926 by Dandy,1 with a bifrontal craniotomy and intracranial approach to a frontal sinus posterior table leak for repair of the dura with fascia lata. Extracranial repair approaches were described through the mid-twentieth century2 with progression to endonasal surgery3,4 for access to the paranasal sinuses and skull base. Endoscopic techniques were initially used in repairs of small localized leaks5 and have been utilized more frequently over the past three decades with relatively high success rates as the reach of endoscopic instrumentation steadily increases.6 Evaluation of the endoscopic repair of sinonasal CSF leaks has shown high success rates of 90% for first attempts at repair and up to 97% following a second endoscopic repair.7
Despite this progress, the unique anatomy of the frontal sinus has made endoscopic approach to this area particularly challenging with acute angles of access from the anterior nasal cavity and complex and variable frontal sinus and recess landmarks. Frontal sinus giraffe instruments and 70-degree endoscopes improve visualization, but endoscopic access to more superior and lateral reaches of the frontal sinus posterior table remains technically complicated and often beyond the ability of available instrumentation. Frontal sinus repairs are especially challenging due to the risk of frontal recess stenosis and sinus outflow obstruction. Maintaining a functioning, draining sinus is preferable to sinus obliteration as it allows the surgeon to endoscopically evaluate the area for mucocele formation, but in more advanced frontal sinus defects this can be difficult to achieve. This study describes an endoscopic approach to the frontal sinus and frontal recess for repair of dural defects using only a unilateral approach, aiming to evaluate the viability of this technique for surgical utility and outcomes.
METHODS
We retrospectively analyzed 2 years of our institution's experience from 2007 to 2009 with frontal sinus CSF leaks repaired with unilateral endoscopic approaches, following approval from the institutional review board. Inclusion criteria included CSF leaks involving the frontal sinus or frontal recess, with repair through a unilateral endoscopic approach. Cases with varying etiologies of CSF leaks qualified for inclusion: meningoencephalocele, postoperative leak after removal of a large intracranial frontoethmoidal mucopyocele, postoperative leak following functional endoscopic sinus surgery, and sinonasal mucormycosis following surgical debridement. These cases were evaluated for etiology, location, patient age, gender, surgical approach, method of dural repair, and outcomes including resolution of CSF leak and patency of the frontal sinus (Table 1). Average age at presentation was 64 years old, ranging from 53 to 74 years old. Defect locations included the posterior table of the frontal sinus as well as the posterior portion of the frontal recess.
Table 1.
Patient Data Including Patient Age, Gender, Etiology, Location, Surgical Approach, Method of Dural Repair, Outcome, and Follow-Up Length
| Patient | Age/Gender | Etiology | Location | Repair | Result | Months of Follow-Up |
|---|---|---|---|---|---|---|
| 1 | 74/male | Meningoencephalocele | Right frontal sinus, anterior ethmoid | Duragen overlay graft, Gelfoam countersink, overlying septal free graft, DuraSeal closure | No recurring leak, patent frontal sinus | 20 |
| 2 | 69/male | Mucormycosis erosion, surgical debridement | Left frontal sinus and frontal recess | Alloderm overlay, Gelfilm, Tisseal, and Gelfoam closure | Graft reinforced 6 days postoperatively, no recurring leak, patent frontal sinus | 22 |
| 3 | 59/male | FESS ethmoidectomy | Frontal recess, anterior ethmoid | Overlay free septal graft, DuraSeal and Gelfoam closure | No recurring leak, patent frontal sinus | 24 |
| 4 | 53/male | Frontoethmoid mucopyocele | Right frontal sinus posterior table | Fat graft and Duragen overlay, Tisseal, Gelfoam, and FloSeal closure | Recurring leak 2 wk postoperatively with meningitis | 17 |
FESS, functional endoscopic sinus surgery.
Preoperatively patients underwent various evaluations to confirm CSF leak and location, including thorough history and physical with nasal endoscopy and testing including magnetic resonance imaging (MRI), computed tomography (CT), intrathecal fluorescein dye injection, and β-2 transferrin testing for confirmation of CSF rhinorrhea. In all cases, image guidance systems were used for intraoperative navigation.
RESULTS
Case 1
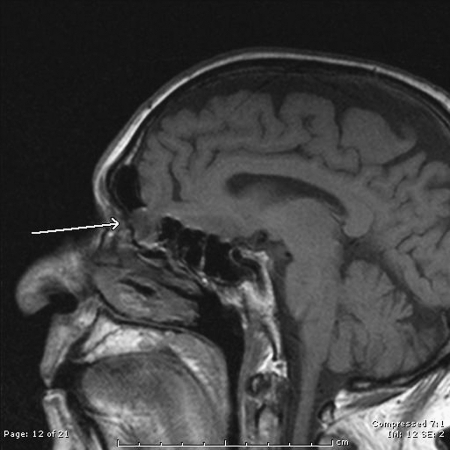
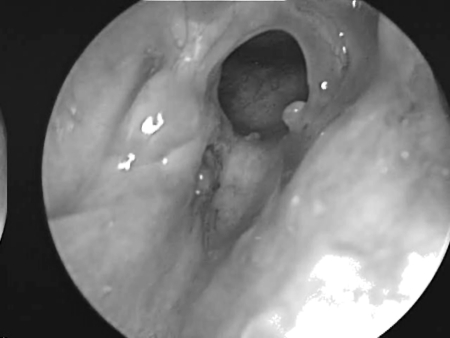
A 74-year-old man with no history of trauma presented with increasing right-sided rhinorrhea over several months. Nasal endoscopy revealed a small, shiny, white lesion in the right anterior ethmoid region, and an MRI confirmed a meningoencephalocele with a defect through the skull base at the junction of the right frontal and anterior ethmoid sinuses (Fig. 1). Intraoperatively, the meningoencephalocele was easily visualized and resected using bipolar cautery. The frontal sinus was then further opened using a 70-degree scope and curved instrumentation. This was accomplished by performing a complete ethmoidectomy and identifying the skull base posteriorly. The skull base was then tracked anteriorly, and the anterior ethmoid artery was identified, marking the posterior extent of the frontal recess. The agger nasi cell was completely opened, using a Kerrison rongeur to remove the anterior wall of the agger nasi cell in the axilla (anterior-superior insertion of the middle turbinate at the lateral nasal wall) of the middle turbinate. This portion is essential to provide adequate visualization of the defect and enable postoperative patency. The defect site was prepared with removal of a rim of surrounding mucosa and freeing underlying dura from the bony defect, followed by a DuraGen (Integra Life Sciences, Plainsboro, NJ) overlay graft and Gelfoam (Pfizer, New York, NY) countersink to reconstruct the ethmoid and frontal defects. An overlying septal mucosal free graft was placed carefully to leave the frontal sinus os patent, and the repair was sealed with DuraSeal (Confluent Surgical, Waltham, MA). A complete resection and reconstruction was completed with no recurring cerebrospinal leak, and postoperative evaluations showed a fully patent right frontal sinus with a well-healed graft site (Fig. 2).
Figure 1.
Sagittal magnetic resonance image showing meningoencephalocele protruding through anterior skull base defect into frontal and anterior ethmoid sinuses (arrow).
Figure 2.
Three-month postoperative result with patent right frontal sinus and healed graft reconstruction site.
Case 2
A 69-year-old diabetic man presented for outpatient evaluation with a 2-week history of left facial pain and persistent sinusitis resistant to multiple outpatient courses of antibiotics. The patient underwent a renal transplant 1 year prior to presentation with posttransplant immunosuppression and had a history of right-sided visual loss, leaving only left-sided vision for daily functioning. Office nasal endoscopy by another surgeon showed areas of necrosis over the left middle turbinate, ethmoid cells, and lateral nasal wall, along with necrotic involvement of the posterior nasal septal mucosa. Biopsies were taken during endoscopy for histological confirmation of fungal elements and mucormycosis, prompting admission for aggressive antifungal treatment and debridement and reversal of the immunosuppression. Initial surgical resection resulted in removal of the left lamina papyracea, ethmoid cells, left middle turbinate, along with a partial posterior septectomy and resection of tissue in the frontal recess.
During a second operation 3 days later, a left frontal sinus CSF leak was noted after debridement of mucosa of the frontal sinus. Dehiscent areas were seen radiologically including the posterior table of the frontal sinus and the frontal recess and extending to the cribriform area. The total bony dehiscence extended ~1 × 3 cm along the anterior skull base. Intraoperatively, dural pulsations were visible in the frontal sinus, along with movement of the orbital contents with pressure delineating a large orbital dehiscence from erosion and excision of lamina papyracea. Further debridement of necrotic tissue revealed leakage of CSF from the frontal sinus. Subsequently, after debridement of all necrotic tissue, the leak was repaired by completely removing the diseased mucosa of the frontal sinus and recess and repairing the defect with an Alloderm (Life Cell, Branchburg, NJ) overlay, using Tisseel (Biosurgery, Deerfield, IL) and Gelfoam to secure the graft in position.
Six days postrepair, a third-look intraoperative endoscopy noted further spread of necrotic involvement with the graft in appropriate position and no evidence of spinal fluid leaking. Necrotic tissue was further debrided from the left ethmoid cells, maxillary sinus, and sphenoid prior to reinforcing the graft with additional Gelfoam.
Following the third total procedure, the patient required no further surgical debridements postoperatively, with complete resolution of mucormycosis sinusitis and preserved visual function of his left eye. Close outpatient follow-up with office endoscopic evaluations showed no further necrosis, no recurrence of CSF leak, and appropriate healing of the graft site at 2-year follow-up.
Case 3
A 59-year-old man presented to his initial surgeon with postoperative rhinorrhea following extensive endoscopic sinus surgery for chronic sinusitis. Intraoperative findings consisted of extensive purulence. He was referred to our institution and was found on endoscopy to have extensive inflammation over the anterior skull base and orbit with a large area of orbital dehiscence. Beta-2 transferrin was tested and positive. CT evaluation showed thinned bone along the anterior ethmoid roof and floor of anterior cranial fossa to the medial frontal sinus and frontal recess; however, no single site could be identified on imaging.
Image guidance was used intraoperatively for a unilateral endoscopic repair of the CSF leak through an assumed anterior skull base defect. Intrathecal fluorescein was used intraoperatively; however, this was unable to identify the location of the leak. On intraoperative endoscopic exam, an obvious bony dehiscence of the skull base was identified in the frontal recess against the posterior wall of the frontal sinus, and mucosa was removed from this area. A free septal graft was placed over the defect extending from the inferior portion of the frontal sinus through the frontal recess and onto the skull base at the anterior ethmoid sinus. This was sealed with DuraSeal and Gelfoam. Postoperatively, the patient had complete resolution of the CSF leak, and a fully healed graft and patent frontal sinus were visible on postoperative endoscopy with the donor septal graft site well healed more than 2 years after surgery.
Case 4
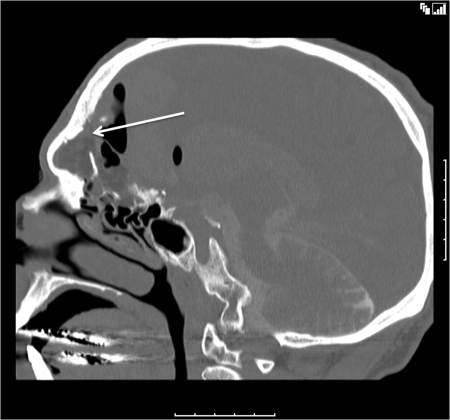
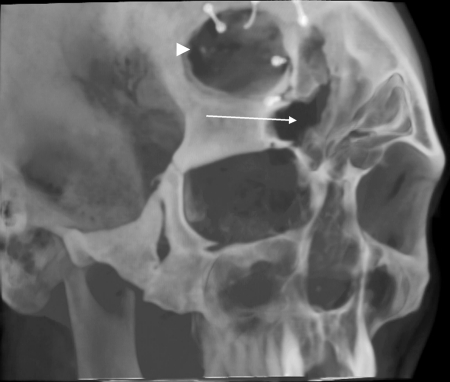
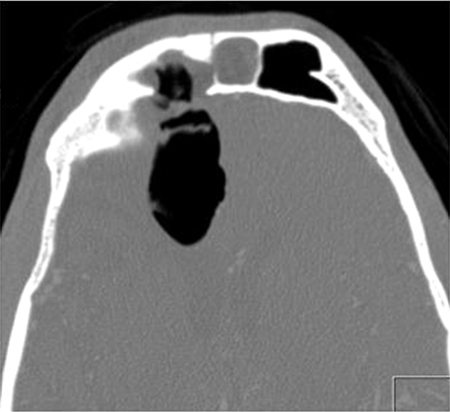
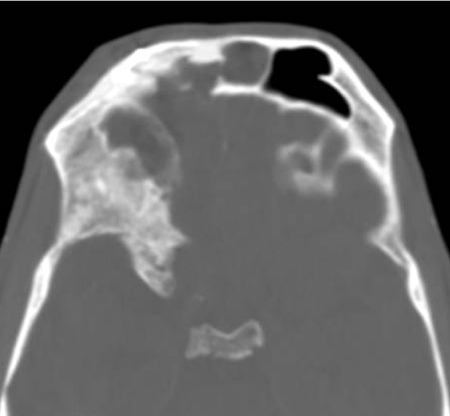
A 53-year-old man presented with CSF rhinorrhea and persisting mild headache 3 weeks after neurosurgical repair of a large frontoethmoidal mucopyocele with orbital and intracranial extension secondary to orbital trauma as a child. This was completed with a bicoronal approach and frontal craniotomy for drainage of the mucopyocele and was closed with a pericranial flap after removing the mucosa from the frontal sinus, which was entered to drain and remove the mucopyocele. Initial treatment of the postoperative rhinorrhea consisted of a lumbar drain without resolution of the leak. A CT scan showed enlarging pneumocephalus with a bony defect in the posterior table of right frontal sinus (arrow, Fig. 3) and exposed orbital contents with a dehiscent lamina papyracea. Figure 4 shows CT image data compiled into a 3-D reconstruction of the defect (arrow) adjacent to the frontal craniotomy site (arrowhead) utilizing InVivoDental5.0® software (Anatomage®, San Jose, CA). A decision was made to approach this defect endoscopically using image guidance. Intraoperatively, an uncinectomy, anterior ethmoidectomy, and frontal recess dissection were performed to gain access to the frontal sinus. This was accomplished by performing a complete ethmoidectomy, identifying the skull base and anterior ethmoid artery, and opening the axilla of the middle turbinate to expose the frontal recess completely. A frontal sinusotomy was completed, which required removal of muscle that had been used to plug the frontal duct previously by the neurosurgeon. Upon removal of the muscle plug, a large release of CSF stained with fluorescein was encountered. A large defect was then identified of the posterior wall of the frontal sinus using a 70-degree scope. Plugging of the posterior table defect was completed with abdominal fat graft packed into the defect. Due to the distance required to reach the defect, the fat was placed carefully into the frontal sinus using a frontal sinus seeker probe and a quarter-inch cottonoid against the fat packing to position it into the defect appropriately. Once the fat was in place, it was covered with a piece of DuraGen laid against the posterior portion of the sinus and recess, to keep the sinus opening patent. This was then sealed with Tisseal, Gelfoam, and FloSeal (Baxter, Deerfield, IL) for closure. A preoperative CT with significant pneumocephalus (Fig. 5) contrasts with a postoperative CT showing resolution of the pneumocephalus within days (Fig. 6).
Figure 3.
Computed tomographic sagittal view showing right frontal sinus posterior table defect (arrow) with anterior fossa pneumocephalus.
Figure 4.
Three-dimensional reconstruction of maxillofacial computed tomography showing posterior table defect of right frontal sinus (arrow) adjacent to the frontal craniotomy site (arrowhead).
Figure 5.
Axial computed tomography showing right frontal sinus posterior table defect with pneumocephalus.
Figure 6.
Axial computed tomography demonstrating obliterated right frontal sinus with resolution of pneumocephalus following repair.
Two weeks postoperatively, the patient presented with fever and headaches and was diagnosed with meningitis and a recurring CSF leak. He was taken for a second repair following treatment and resolution of the meningitis with intravenous antibiotics. A leak was again visualized with fluorescein coming from the frontal sinus, just posterior to the original DuraGen graft site, which no longer appeared to be flush to the posterior wall of the frontal sinus. The sinus was then obliterated, leaving the fat plug in place and adding a piece of rolled DuraGen into the frontal sinus to buttress the fat. An additional piece of DuraGen was then placed over the frontal sinus extending to the skull base in the anterior ethmoid sinus and covered with DuraSeal. Following the second procedure, the patient had no further CSF leak. Postoperative outpatient nasal endoscopy showed healed graft site over the obliterated right frontal sinus. An MRI scan 17 months postoperatively showed no recurrence of the mucocele, with further surveillance follow-up planned yearly.
DISCUSSION
Frontal sinus CSF leaks can have many etiologies including spontaneous, tumor-related, traumatic (accidental or iatrogenic), or congenital leaks. Etiology affects the risk of recurrence and thus the method of repair by having an impact on the defect size, location, degree of dural involvement, the likelihood of elevated intracranial pressure (ICP), and the possibility of meningoencephalocele protrusion.8 Spontaneous frontal sinus CSF leaks tend to occur in anatomic sites of weakness that will release under increased hydrostatic pressure from elevated ICP, including the ethmoid roof or anterior cribriform plate adjacent to frontal recess. By contrast, surgical trauma defects tend to occur in sites of bony disruption during endoscopic sinus surgery or neurosurgery such as the lateral lamella of the cribriform plate or the anterior ethmoid sinus.
Anterior skull base defects in the area of the frontal sinuses are often delineated by anatomic site into areas abutting the frontal recess such as the anterior ethmoid roof, the frontal recess, and the body of the frontal sinus,8 although defects may incorporate several areas. These anatomic categories correlate to accessibility and potential surgical approaches, with increasing difficulty moving from the frontal recess into the frontal sinus more laterally and posteriorly as endoscopic instrumentation reaches its limitations. In these cases, or in situations with larger or more complex defects, a frontal sinus drill-out, a combined endoscopic and intracranial approach, or an external approach may allow for better access to the site requiring repair. The size of the defect also has an impact on surgical planning for the type of grafting required, as smaller defects are more conducive to pliable overlay grafts, and larger (> 4 mm) sites can accommodate an underlay graft or multilayer closure with both underlay and overlay grafts. Other options for situations requiring a stronger reconstruction—very large defects or elevated ICP that could dislodge a soft graft—include bony underlay grafts and soft overlay grafts with bony countersinking techniques.9,10
Defect site preparation involves removal of a rim of mucosa around the defect edge to prevent mucus production underneath the graft from detaching it and removal of mucosa from sinus portions that will be obliterated or could be eventually affected by outflow obstruction. Once this is complete, the graft can be positioned. A variety of graft materials exist, including local mucosal flaps (turbinate rotational flap, septal mucoperichondrial flap) and free mucosal flaps, which benefit from a vascular supply in pedicled local flaps and/or natural tissue scaffolding that speed graft healing. In fact, the use of pedicled mucosal flaps has been shown to dramatically reduce the rate of graft failure and CSF leak recurrence to 5% in some studies.11 Mucosal grafting must be oriented carefully to ensure placement of the mucosal surface toward the nasal cavity or sinus to prevent mucocele formation or other intracranial complications. Nonmucosal soft graft materials consist of fascial grafts, fat, skin grafts, bone pate,12 Duragen, and Alloderm, and firm grafts include cartilage, bone, Medpor (Stryker, Newnan, GA), and hydroxyapatite cement grafts. Once the primary graft monolayer or multiple layers are positioned, a variety of sealants and packing can be utilized to stabilize the graft during its initial healing and adherence to the defect site, including DuraSeal, GelFilm (Pfizer, New York, NY), Tisseel, Gelfoam, and Surgicel (Ethicon, Cornelia, GA).
Four risk factors for graft failure have been identified previously by Lindstrom et al13: high body mass index, large defect size, spontaneous CSF leak etiology (implying potentially elevated ICP), and lateral sphenoid defect location. Using appropriate graft types with multilayer closures and firm graft underlays or countersinking, along with parallel measures to reduce elevated ICP (lumbar drains, acetazolamide) can reduce the risk of CSF leak recurrence or other complications.9 Also ensuring that grafts lie smoothly against the defect and cover a sufficient margin beyond the defect edge by at least 5 mm will reduce failure rates.14 Other technologies and techniques are under investigation that could expand the surgeon's endoscopic reach and improve the ease and durability of defect closure, such as improved frontal sinus instrumentation, chip tip endoscopes, high-definition digital optics,15 and laser tissue welding with biologic solders incorporating wavelength-specific chromophores for rapid, watertight defect closure.16
Accomplishing these established grafting and reconstruction techniques through a unilateral endoscopic method allows for a minimally invasive approach with several advantages. Unilateral transnasal approaches utilize the deep access of the endonasal corridor without additional resection of the nasal septum often involved in a bilateral surgical plan. This reduces soft tissue morbidity, limiting tissue removal to the area of pathology and immediate surrounding access, allowing for a faster postoperative recovery and less disruption of pediatric growth centers in the craniofacial skeleton of younger patients. The unilateral endoscopic approach can be used to achieve similar access as the bilateral approach to the full extent of the skull base with appropriate endoscopes and equipment. However, the surgeon's technical expertise is critical to success, as is appropriate patient selection for tumor or defect size and location.
Our study highlights the potential for a durable dural repair and complete cessation of CSF leaks in complex cases and the potential for maintaining frontal sinus patency in more clear-cut clinical situations. Outcomes of these cases over an average of more than 12 months of postsurgical follow-up show resolution of the CSF leaks and frontal sinus patency after the initial repair in three of four patients, and resolution of CSF leak after secondary repair in the fourth patient. These results demonstrate that fully endoscopic repair of frontal sinus, frontal recess, and posterior table CSF leaks is possible in select cases using a unilateral approach and utilizing graft overlay and countersinking techniques for a reliable, secure repair of bony and dural defects.
NOTE
Presented by poster at the 2009 North American Skull Base Society meeting, New Orleans, LA, October 15 to 17, 2009.
References
- Dandy W D. Pneumocephalus (intracranial pneumocele or aerocele) Arch Surg. 1926;12:949–982. [Google Scholar]
- Dohlman G. Spontaneous cerebrospinal rhinorrhea: case operated by rhinologic methods. Acta Otolaryngol Suppl. 1948;67:20–23. doi: 10.3109/00016484809129635. [DOI] [PubMed] [Google Scholar]
- Hirsch O. Successful closure of cerebrospinal fluid leak by endonasal surgery. Arch Otolaryngol. 1952;56:1–13. doi: 10.1001/archotol.1952.00710020018001. [DOI] [PubMed] [Google Scholar]
- Schick B, Ibing R, Brors D, Draf W. Long-term study of endonasal duraplasty and review of the literature. Ann Otol Rhinol Laryngol. 2001;110:142–147. doi: 10.1177/000348940111000209. [DOI] [PubMed] [Google Scholar]
- Wigand M E. Transnasal ethmoidectomy under endoscopical control. Rhinology. 1981;19:7–15. [PubMed] [Google Scholar]
- Mattox D E, Kennedy D W. Endoscopic management of cerebrospinal fluid leaks and cephaloceles. Laryngoscope. 1990;100:857–862. doi: 10.1288/00005537-199008000-00012. [DOI] [PubMed] [Google Scholar]
- Hegazy H M, Carrau R L, Snyderman C H, Kassam A, Zweig J. Transnasal endoscopic repair of cerebrospinal fluid rhinorrhea: a meta-analysis. Laryngoscope. 2000;110:1166–1172. doi: 10.1097/00005537-200007000-00019. [DOI] [PubMed] [Google Scholar]
- Schlosser R J, Bolger W E. Endoscopic management of cerebrospinal fluid rhinorrhea. Otolaryngol Clin North Am. 2006;39:523–538, ix. doi: 10.1016/j.otc.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Woodworth B A, Schlosser R J, Palmer J N. Endoscopic repair of frontal sinus cerebrospinal fluid leaks. J Laryngol Otol. 2005;119:709–713. doi: 10.1258/0022215054797961. [DOI] [PubMed] [Google Scholar]
- Leng L Z, Brown S M, Anand V K, et al. “Gasket-seal” watertight closure in minimal-access endoscopic cranial base surgery. Neurosurgery. 2008;62(5 Suppl):ONSE342–343. discussion ONSE343. doi: 10.1227/01.neu.0000326017.84315.1f. [DOI] [PubMed] [Google Scholar]
- Snyderman C H, Pant H, Carrau R L, Prevedello D, Gardner P, Kassam A B. What are the limits of endoscopic sinus surgery? The expanded endonasal approach to the skull base. Keio J Med. 2009;58:152–160. doi: 10.2302/kjm.58.152. [DOI] [PubMed] [Google Scholar]
- Chatrath P, Saleh H A. Endoscopic repair of cerebrospinal fluid rhinorrhea using bone pate. Laryngoscope. 2006;116:1050–1053. doi: 10.1097/01.MLG.0000217644.74806.8F. [DOI] [PubMed] [Google Scholar]
- Lindstrom D R, Toohill R J, Loehrl T A, Smith T L. Management of cerebrospinal fluid rhinorrhea: the Medical College of Wisconsin experience. Laryngoscope. 2004;114:969–974. doi: 10.1097/00005537-200406000-00003. [DOI] [PubMed] [Google Scholar]
- Mirza S, Thaper A, McClelland L, Jones N S. Sinonasal cerebrospinal fluid leaks: management of 97 patients over 10 years. Laryngoscope. 2005;115:1774–1777. doi: 10.1097/01.mlg.0000175679.68452.75. [DOI] [PubMed] [Google Scholar]
- Chandra R K, Conley D B, Kern R C. Evolution of the endoscope and endoscopic sinus surgery. Otolaryngol Clin North Am. 2009;42:747–752, vii. doi: 10.1016/j.otc.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Bleier B S, Palmer J N. Cranial-base repair using endoscopic laser welding. Otolaryngol Clin North Am. 2009;42:901–906, xi. doi: 10.1016/j.otc.2009.07.002. [DOI] [PubMed] [Google Scholar]