Abstract
There has been increasing experience in the utilization of intraoperative magnetic resonance imaging (iMRI) for intracranial surgery. Despite this trend, only a few U.S centers have examined the use of this technology for transsphenoidal resection of tumors of the sella. We present the largest series in North America examining the role of iMRI for pituitary adenoma resection. We retrospectively reviewed our institutional experience of 59-patients who underwent transsphenoidal procedures for sellar and suprasellar tumors with iMRI guidance. Of these, 52 patients had a histological diagnosis of pituitary adenoma. The technical results of this subgroup were examined. A 1.5-T iMRI was integrated with the BrainLAB (Feldkirchen, Germany) neuronavigation system. The majority (94%) of tumors in our series were macroadenomas. Seventeen percent of tumors were confined to the sella, 49% had suprasellar extensions without involvement of the cavernous sinus, 34% had frank cavernous sinus invasion. All patients underwent at least one iMRI, and 19% required one or more additional sets of intraoperative imaging. In 58% of patients, iMRI led to the surgeon attempting more resection. A gross total resection was obtained in 67% of the patients with planned total resections. There was one case of permanent postoperative diabetes insipidus and no other instances of new hormone replacement. In summary, iMRI was most useful for tumors of the sella with and without suprasellar extension where the information from the iMRI extended the complete resection rate from 40 to 72% and 55 to 88%, respectively. As one would expect, it did not substantially increase the rate of resection of tumors with cavernous sinus invasion. Overall, iMRI was particularly useful in guiding resection safely, aiding in clinical decision making, and allowing identification and preservation of the pituitary stalk and normal pituitary gland. Limitations of the iMRI include a need for additional personnel and training as well as additional operative time, which diminishes over time as personnel learn to optimize workflow efficiency. Additional costs are mitigated in part by using the iMRI as an immediate postoperative scan. Other data emerging from our experience suggest that preservation of normal gland and thus avoidance of hypopituitarism may be improved by iMRI use, but longer follow-up periods are required to test this conclusion. iMRI can detect unsuspected complications sooner than routine postoperative imaging, potentially leading to improved outcomes. However, larger studies are needed.
Keywords: Intraoperative MRI, pituitary, transsphenoidal
Tumors arising from the sellar area represent ∼10 to 15% of all intracranial neoplasms.1 Such lesions present with a unique symptomatology related to the anatomy of the sella and the surrounding structures as well as to the functions of the pituitary gland. Endocrine abnormalities secondary to aberrant secretion or dysregulation of pituitary hormones and visual deficits related to compression of the optic apparatus represent common findings in patients with such lesions.2 Surgical resection is often the first line of treatment in most cases with the exception of prolactinomas. Additional therapeutic modalities include medical therapy and radiation.3,4
The transsphenoidal approach is the most appropriate surgical route for resection of the majority of pituitary adenomas. The basic principles of this approach were first established by Schlofer in 1907 and further popularized by Cushing in 1909.5,6 The procedure has evolved in parallel with technological advances in surgery, adopting the operating microscope and then the neuroendoscope for better illumination and visualization.7,8 Ultrasound and vascular Doppler probes are also included in the repertoire of operative adjuncts for tissue visualization.9 Intraoperative localization has long been limited to the use of fluoroscopy but has encompassed computed tomography or magnetic resonance imaging (MRI) image guidance in the past decade, first with low-field magnets and more recently advances in operating room setups have allowed for high-field magnets to be used.10,11
This surgical approach would seem most conducive to technologies like the intraoperative MRI (iMRI) or endoscope, which improves visualization of the operative field and surrounding anatomic structures. Here we present our experience in utilizing high-field iMRI for a series of 59 consecutive transsphenoidal procedures for sellar and parasellar tumors. We examine the advantages of iMRI as they manifested in specific examples, and we point out some of the limitations of overreliance on this technology. It would be premature to draw conclusions as to the cost-benefit analysis of iMRI or the contribution to long-term patient outcomes in view of the short duration of follow-up and the limited number of patients. Our preliminary data suggest an important role for imaging during the procedure in improving visualization of residual tumors and diagnosing potential complications, as well as sparing normal gland tissue.
PATIENTS AND METHODS
This is a retrospective analysis of a prospectively constructed database consisting of 59 patients who underwent transsphenoidal procedures at Memorial Sloan-Kettering Cancer Center by a single neurosurgeon (V.T.) in a 24-month period from February 2008 to February 2010. A complete endocrine profile was documented both pre- and postoperatively on all patients. An endocrinologist was involved in all cases where an endocrinopathy was identified. Preoperative visual field (VF) testing was performed on all patients who presented with visual complaints. Postoperative VF testing was performed when preoperative deficits were identified and /or if patients had sustained visual complaints. Three of the 52 adenoma patients had recurrent tumors with prior transsphenoidal resections. Fourteen of the 52 (27%) adenomas were hormone-secreting tumors. All resected specimens underwent routine histological and immunofluorescence staining as indicated by a neuropathologist. Patients had a mean length of follow-up of 7.2 ± 0.5 months and a range of 12 days to 600 days.
High-Field iMRI Navigation System
Memorial Sloan-Kettering's BrainSuite consists of a 1.5-T iMRI scanner integrated with an image-guided neuronavigation system (BrainLab, Feldkirchen, Germany) that is installed within one of hospital's main operating room. The walls, floor, and ceiling contain radiofrequency shielding based on aluminum and copper mesh. The high-field-strength magnetic resonance scanner (Magnetom Espree; Siemens Healthcare, Erlangen, Germany) consists of a superconductive magnet with a length of 120 cm and an inner bore diameter of 70 cm. An elliptical line (8-m major and 5-m minor axis) is drawn around the scanner to mark the 5-G field limit for safety. The operating table is positioned parallel to the scanner during surgery so that the patient's head lies outside the 5-G line, allowing the use of standard operating equipment and instruments. The head holder has a bivalve shape, consisting of two separate parts, and containing an eight-channel receiver array. The upper part of the head holder contains 14 MRI-visible fiducial markers.
Imaging
Calculations of preoperative tumor dimension and extent of resection were based on analysis of MRI sequences and independent evaluation by neuroradiologists at our institution. Preoperatively, thin-slice (3 mm, 0 interslice gap) coronal precontrast and dynamic postcontrast T1- and T2-weighted imaging of the sellar region was obtained in addition to axial thin section (3 mm, 0 interslice gap) T1-weighted and three-dimensional spoiled gradient postcontrast sequences of the entire brain. These sequences were performed as a part of BrainLab protocol in addition to the routine imaging of the brain, which includes axial and sagittal T1-weighted, axial T2-eighted, fluid-attenuated inversion recovery, and diffusion-weighted sequences. The dynamic imaging protocol consists of a precontrast and four successive rapidly obtained T1-weighted images acquired 15 to 20 seconds after intravenous bolus injection of contrast in the coronal plane. This preoperative scan is then integrated into the BrainSuite MR neuronavigation system. Intraoperative scans typically consist of T1-weighted sequences in the axial and coronal planes without and with contrast. T2-weighted images, dynamic sequences, and three-dimensional reconstructions are obtained at the discretion of the surgeon. The intraoperative scan is then used for repeat registration if additional resection or exploration is to be performed. Postoperative scans are typically obtained 3 months postoperatively if a definitive intraoperative scan showing the final operative result was not obtained. Gadolinium diethylenetriaminepentaacetic acid (Magnevist, Berlex Laboratories, Wayne, NJ) 0.1 mmol/kg was used as the contrast agent for all scans, and the intraoperative contrast administration was limited to a single dose.
Operative Technique
All 59 patients underwent a preoperative thin-slice axial T1-weighted gadolinium-enhanced MRI for frameless stereotaxy. All procedures were performed under general endotracheal anesthesia. After intubation, two cottonoids soaked in 4% cocaine were placed in each nostril to promote vasoconstriction. Gauze was packed in the oropharynx to minimize drainage from the surgical site into the mouth and esophagus. The patients were placed on the MRI operating table in the supine position, and the head was rigidly fixed in an MRI-compatible frame suitable for frameless stereotaxy (BrainLab). We placed the head in a mildly extended configuration to facilitate access to the sphenoid sinus through the right nostril by the right-handed surgeon. Twenty-three patients (43.4%) had a lumbar drain placed at the beginning of the procedure to allow injection of saline solution, thus facilitating delivery of the suprasellar component of the tumor during resection. If an intraoperative leak was encountered, the lumbar drain was maintained for a minimum of 3 days postoperatively. Further vasoconstriction and nasal mucosa anesthesia were achieved by submucosal injection of 1% lidocaine with epinephrine solution. This was performed under the operating microscope (Leica, Wetzlar, Germany), which was used for the remainder of the procedure. The sphenoidal ostium was identified and the anterior wall of the sphenoid sinus rongeured using a variety of Kerrison instruments. The extent of resection of the anterior sphenoidal wall and vomer depended on tumor size and patient anatomy. The cartilaginous septum was displaced medially after incising the mucosa proximal to the vomer. The sellar anatomy and relative location of the cavernous sinuses and carotid arteries were confirmed with neuronavigation (BrainSUITE iMRI, BrainLab) and intracranial Doppler prior to opening the dura. In cases where the tumor invaded juxtasellar structures, such as the ethmoidal air cells, the clivus, or the lateral recesses of the sphenoid sinuses, the bony exposure was further extended as necessary. After incision of the dura, tumor resection was performed in a standard fashion. As mentioned above, a subset of patients had 10 to 50 mL normal saline injected through the lumbar drain to facilitate descent of suprasellar tumor into the sellar resection cavity. An equal volume of cerebrospinal fluid (CSF) was drained at the end of the resection, and the drain was removed unless an intraoperative CSF leak was identified.
When the surgeon felt that all resectable tumor had been evacuated, an iMRI was obtained. Preparation for imaging included a complete instrument and sponge/cottonoid counts and was often performed at the surgeon's indication while the surgical procedure was ongoing. A neuroradiologist was always available to analyze the iMRI images. Overall, there was minimal disruption of the usual workflow or violations of the sterile conditions. However, full instrument counts had to be performed prior to imaging each time an MRI had to be obtained, for safety purposes. Nursing staff received specific training for work within the iMRI suite. In the subset of patients who required more than one iMRI, no additional gadolinium was given after the first administration. Repeat registration for neuronavigation was performed using the intraoperative study.
After tumor resection was completed, the resection cavity was covered with Gelfoam (Pfizer, New York, NY) and Tisseal (Baxter, Deerfield, IL). CSF leaks were repaired with a fat graft or Duragen (Integra, Plainsboro, NJ) and Tisseal (Baxter), and a lumbar drain was placed. Nasal packing was used infrequently.
Statistics
All population statistics are presented as mean ± standard error of the mean.
RESULTS
Patient Population and Tumor Characteristics
The 52-patient cohort consisted of 25 males and 27 females, and 94% had macroadenomas. The most common diagnosis was a hormonally inactive adenoma (73%). One case presented with a cystic tumor and one with a hemorrhagic tumor. The mean tumor size was 2.3 ± 0.8 cm. Three of the patients had recurrences following previous resection. Seventeen percent of tumors were confined to the sella, 49% had suprasellar extensions without involvement of the cavernous sinus, and 34% had frank cavernous sinus invasion.
iMRI-Guided Resections
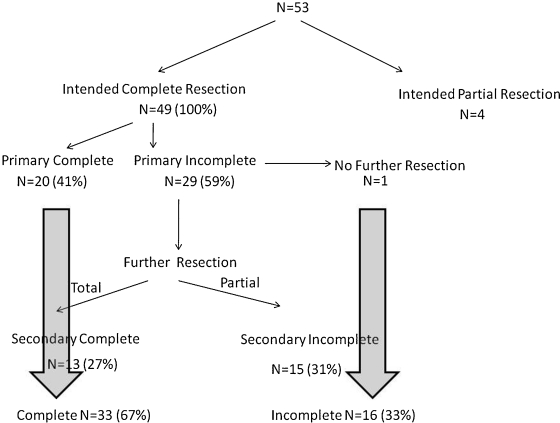
One surgery was aborted due to technical reasons; otherwise, the majority of patients (42; 80%) underwent a single set of iMRI, and 10 patients (19%) had additional intraoperative imaging after the initial resection. The information obtained from the iMRI led to more resection in 58% of cases. Of the 52 patients, three were intended for debulking or biopsy only based on clinical or radiographic features (Fig. 1). Twenty (41%) of the remaining forty-nine had a gross total resection at the time of the first MRI. In 29 patients (59%), the iMRI depicted residual tumor, which resulted in an extension of the resection in 28 patients, 13 of whom subsequently had a gross total resection. A subtotal resection was obtained in 15 patients (31%): in nine cases, there was definite invasion of the cavernous sinus and brisk venous bleeding upon attempted resection; in two patients, the tumor was very adherent to the optic apparatus, and in four patients, the residual tumor was very fibrous and adhered to normal tissue and could not be retrieved safely with standard instruments. Overall, 67% (n = 33) of patients taken for resection had gross total resections confirmed with either iMRI or postoperative MRI. A breakdown of complete radiographic resection rate by anatomic extension is shown in Table 1. The surgical time ranged from 1 hour to 4.5 hours, with an average of 166 minutes from the time of incision to the end of procedure, including all intraoperative imaging (Table 2). This is comparable to reported surgical times in the literature without any intraoperative imaging, which range from 35 to 90 minutes for some endoscopic transnasal cases to 130 to 175 minutes in another series.12 The timing of microscopic transsphenoidal surgeries was similarly variable. Our mean operating room time for 19 cases done before iMRI was 133 minutes. In this series, average operating room time was 176 minutes in the first 50% of patients and 151 minutes in the second half (p = 0.03), likely due to a learning curve for all staff involved. The added time compared with our pre-iMRI cases accounts for the 20 minutes of acquisition and interpretation of intraoperative imaging.
Figure 1.
Flow diagram depicting the resection algorithm with the intraoperative magnetic resonance imaging information.
Table 1.
Complete Radiographic Resection Rates
| Anatomic Location (n) | Complete Radiographic Resection Rate, n (%) |
|---|---|
| Sellar (9) | 8/9 (88%) |
| Sellar + suprasellar no cavernous invasion (25) | 17/25 (72%) |
| Cavernous sinus invasion (18) | 6/18 (33%) |
Table 2.
Demographic Information
| Mean ± SEM | |
|---|---|
| Age (y) | 49 ± 1.8 |
| Duration of procedure (min) | 166 ± 5 |
| Duration of hospitalization (d) | 3.6 ± 0.3 |
| Follow-up (mo) | 7.2 ± 0.5 |
SEM, standard error of the mean.
Endocrinologic Results
Overall, 13 patients presented with endocrine-related symptoms, four of whom presented with panhypopituitarism. There were 14 patients with oversecretion disorders: three patients with Cushing's disease (adrenocorticotropic hormone [ACTH]), five acromegalic patients (growth hormone/insulinlike growth factor-1 [IGF1]), and six with prolactinomas (prolactin). The remaining patients had a null secreting adenoma. All patients with preoperative panhypopituitarism continued to require full hormone replacement. Of the five patients with growth hormone-secreting tumors, three had a normal IGF1 level postoperatively: one had a significant reduction to near normal (from 852 ng/mL preoperatively to 227 ng/mL postoperatively; normal range is up to 200 ng/mL) requiring a low dose of sandostatin, one patient had radiographic resolution but IGF1 level dropped minimally from 852 to 768 ng/mL. Three of the patients with Cushing's disease had a complete endocrinologic response. Four of the prolactinoma patients had normal levels postoperatively. The remaining two patients had significantly decreased prolactin levels but had residual tumor in the cavernous sinus that could not be resected. The goal of the surgery was to achieve decompression, which resulted in significant improvement in VFs in one patient and dramatic decrease in prolactin level in the other. The indications for surgical intervention in the patients with prolactinomas included resistance to dopamine agonists (three patients), significant intolerance of the drug as determined by their endocrinologist (two patients), and large hemorrhagic prolactinoma with visual compromise and concomitant metastatic lung cancer requiring bevacizumab therapy (one patient). There was concern about further hemorrhage using the chemotherapeutic agent with further visual compromise.
Complications
Intraoperative CSF leaks were identified in 17 cases (32%); 12 of the 17 had a lumbar drain left in postoperatively. Drainage of CSF was performed at 10 mL/h with minor variations. The rate was decreased if patients experienced low-pressure headaches. As a rule, all patients were drained for 3 days, clamped for 12 hours, and discharged 12 hours later if they had no evidence of CSF leaks. This approach is consistent with other groups' methodology for effective prevention of complications of CSF leaks in this type of surgery.13 There were no CSF leaks reported after discharge. Transient diabetes insipidus was noted in five patients (9%). A single patient developed permanent diabetes insipidus requiring desmopressin acetate upon discharge; this patient had a large adherent prolactinoma that was medication-resistant and highly adherent to parasellar structures. Other complications included one vancomycin-induced skin rash and one narcotic-induced bradycardia in the acute postoperative period. One patient developed a subdural collection that resolved spontaneously 2 months later. This patient had a macroadenoma with a large suprasellar component; he had a lumbar drain placed intraoperatively, which was removed at the end of the procedure. Two patients received blood patches for positional headaches after removal of lumbar drain catheters. There were no vascular, optic, or neurological injuries.
ILLUSTRATIVE CASES
Patient 1: Microadenoma
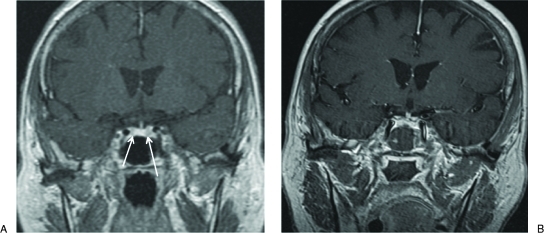
This was a 61-year-old woman with definitive endocrine and clinical features of Cushing's disease. The preoperative MRI showed two small hypoenhancing areas within the sella measuring 2 and 4 mm and located in the right upper quadrant and the left lower quadrant, respectively. The left-sided lesion was convincing for adenoma, and the right sided abnormality was considered equivocal by two independent neuroradiologists (Fig. 2A). Bilateral inferior petrosal sinus sampling showed a significant ACTH gradient between each inferior petrosal sinus sampling and the peripheral vein sample before and after corticotrophin-releasing hormone stimulation (730 pg/mL centrally and 28 pg/mL peripherally). However, there was significant cross-filling, and the test did not identify laterality. Neuronavigation based on preoperative MRI was utilized, and the areas pointed out by the navigation system were explored starting with the more convincing left-sided lesion. However, a nodule could not be visualized intraoperatively, and intraoperative frozen sections were nondiagnostic. We therefore proceeded to excise the areas at risk as predicted by the preoperative imaging and neuronavigation, despite the absence of direct identification. Two sets of iMRI studies were performed that eventually confirmed resection of these lesions. The procedure was then stopped and no further gland exploration was performed, thus preserving normal pituitary function (Fig. 2B). The patient experienced significant clinical improvement postoperatively with normalization of serum, salivary, and urinary cortisol levels as well as blood glucose levels. Her endocrine studies, including a full pituitary endocrine profile, remain normal postoperatively.
Figure 2.
Examples of intraoperative magnetic resonance imaging–aided sellar tumor resections of microadenoma. Coronal T1-weighted postgadolinium magnetic resonance images obtained preoperatively (A) and intraoperatively (B) in a patient with Cushing's disease. Two suspected microadenomas are visible on either side of the sella (arrows). Intraoperative scans confirmed resection of both lesions. The patient made a complete endocrinologic recovery after the procedure with resolution of her preoperative clinical symptoms.
Patient 2: Localization of Normal Gland
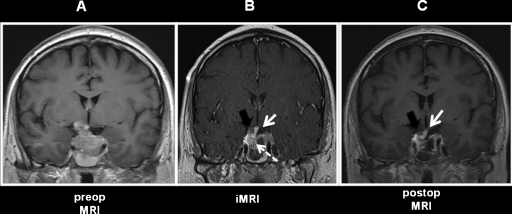
The patient was a 48-year-old man who presented with Cushingoid features, elevated urine and salivary cortisol levels, and an elevated serum ACTH. His brain MRI showed a macroadenoma with a significant suprasellar component and suspicion of cavernous sinus involvement. Normal gland could not be identified even on dynamic imaging (Fig. 3A). The patient was taken to the operating room with the goal of complete resection of the macroadenoma and preservation of normal pituitary function. After most of the tumor had been removed, the diaphragma sellae had descended and partially obscured the surgical view. Tissue remaining within the sella was suspicious for normal gland but it could not be readily distinguished from tumor. Because this was an ACTH-secreting tumor, it was crucial to distinguish tumor from normal, if highly compressed, gland. Navigation was not helpful as the anatomy within the sella was completely modified by the tumor resection and normal tissue could not be identified on preoperative scans. iMRI, including dynamic sequences, was performed and showed the descent of the diaphragma sellae, deviation of the pituitary stalk, and normal gland to the right of tumor remnant in the right aspect of the sella (Fig. 3B). The iMRI sequences were then used for surgical navigation, allowing the resection of the residual tumor and preservation of normal gland tissue. The patient had a complete symptomatic recovery and normal urinary cortisol levels postoperatively. The remainder of his endocrine profile was also completely normal. Thus the information obtained with the iMRI led to additional resection, with preservation of the pituitary stalk and gland.
Figure 3.
Example of use of intraoperative magnetic resonance imaging (iMRI) to identify normal gland intraoperatively. (A) Preoperative magnetic resonance coronal T1-weighted postgadolinium injection in a patient with a large macroadenoma with no discernable normal gland. Once the majority of the resection was complete, iMRI (B) was used to distinguish remaining tumor (dashed white arrow) from normal gland (black arrow). White solid arrow identifies the pituitary stalk with right inferior normal enhancing gland as well as visualization of the optic chiasm, all of which allows safer tumor resection. Postoperative scan (C) shows the patient had a gross total resection with an intact stalk (white arrow) and normal gland (black arrow). The patient had normal pituitary function postoperatively.
Other situations whereby imaging was helpful include: visualizing pneumocephaly, loss of neuronavigation remedied by a new T1-weighted scan with repeat intraoperative registration, and delayed arousal from anesthesia with concern about hemorrhage or ischemia in a case with an extensive suprasellar component leading to an intraoperative scan with diffusion and gradient echo sequences.
DISCUSSION
iMRI
The utilization of iMRI is gaining increased recognition as more institutions have access to this technology.14,15,16,17 Current iMRI data include mostly craniotomies.13 There have been few reports of transsphenoidal procedures for resection of pituitary adenomas. One of the first reports of using this modality came from Bohinski et al in 2001 from Cincinnati using a low-field intraoperative magnet (0.25 T).14 They prospectively examined the results of 30 consecutive patients who underwent transsphenoidal surgery with iMRI. They found that the iMRI identified residual tumor and led to further resection in 66% of cases. After this initial series, no other U.S. series of transsphenoidal procedures performed with iMRI guidance has been published. Published data on transsphenoidal surgeries with high-field magnets is limited to one center in Germany (University of Erlangen-Nuremberg) and suggests improved visualization of residual tumor and an increased extent of resection.17,18 In 2001, the group published a prospective series of 44 patients operated on in a low-field (0.2 T) MRI with similar results to the U.S. data.15 They then went on to publish work with a high-field iMRI (1.5 T). In 2005, Fahlbusch et al published a retrospective look at the role of iMRI in the surgical management of growth hormone-secreting tumors.18 They reviewed 23 patients with such tumors and found that iMRI led to an increase in the rate of endocrine normalization from 33 to 44%, with an additional 17% experiencing near normalization. It was concluded that this technology was beneficial but was still unable to detect all significant tumor remnants. In the case of nonsecreting pituitary tumors, they found iMRI to play a more substantial role, leading to an increase in total resection rates from 58 to 82%.
Others have looked at different low-field MRI designs all with similar results. Gerlach et al compared ultra-low-field (0.15 T) intraoperative imaging with pre- and postoperative high-field (1.5 T) imaging in 40 patients with macroadenomas and found that ultra-low field was adequate for sellar and most suprasellar tumors in identifying tumor remnants with 89% and 86% sensitivity, respectively.16
Groups like Schwartz et al and Theodosopoulos et al examined their experiences using endoscopic techniques in the iMRI setting.19,20 Schwartz et al retrospectively described 15 patients who underwent endoscopic transsphenoidal surgery with the use of low-field (0.12 T) iMRI. They found iMRI helpful and usable with endoscopic technology with minor alterations to account for the magnetic field interference.19 Theodosopoulus et al looked at their series of 27 patients and found similar results.20
High-field MRI scanners offer standard imaging capabilities including dynamic imaging, thus enhancing the accuracy of intraoperative imaging and obviating the need for additional postoperative studies.17,21
High-field intraoperative MRI is slowly gaining increased utilization in tertiary centers across the world. Emerging data suggest improved rates of gross total resections of a variety of brain tumors due to the ability to visualize residual tumor intraoperatively.13,22 Other advantages include the availability of repeat registration following partial resections, thus accounting for brain shift and leading to more accurate neuronavigation. Resection of pituitary tumors shares similar concerns but has its separate set of challenges that can be assisted by iMRI.
Although neuronavigation based on preoperative MRI greatly facilitates the anatomic localization of surgical targets, it does have limitations mostly related to the absence of real-time data. In particular, intracranial structures tend to shift their spatial location after the cranium and dura are open and spinal fluid is lost. Other factors that impact neuronavigation include alterations in spatial location due to ongoing resection or to positional effects whereby the patient's position intraoperatively is different from the normally supine position used for preoperative MRI acquisition. Ongoing surgical events such as the onset of ischemic change or intracranial air are also obviously not detected without live imaging. Ultrasound has been traditionally used for real-time imaging during brain tumor resection; however, it also suffers from drawbacks: It depends on differences in echogenic texture between the tumor and the gland, which is not always predictably present, and, specifically in the case of intrasellar use, it does suffer from suboptimal spatial resolution, and its use is complicated by anatomic constraints.
In the United States, most reports of iMRI for transsphenoidal procedures involved a low-field (0.15-T) scanners.23 However, high-field scanners offer a significantly improved signal-to-noise ratio and higher spatial and temporal resolution. This is particularly relevant in the case of sellar tumors, especially microadenomas, and obviates the need for an additional postoperative scan. Imaging times are also two- to threefold shorter with high-field magnets.24
Based on our experience with the iMRI-assisted resection of sellar, suprasellar, and juxtasellar tumors, we can identify the following advantages of high-field iMRI in our series:
The extent of resection is increased with use of iMRI. In our case, up to 58% of patients underwent additional resection for residual tumor following the first iMRI. This number probably overestimates the contribution of iMRI to complete resection because it ignores an element of dependence by the surgeon on imaging availability, leading to the performance of iMRI somewhat prematurely, knowing that the surgical bed probably harbors residual tumor. Nonetheless, intraoperative imaging has revealed unexpected findings or has suggested a different strategy to reach suspected residual tumor on multiple occasions. The data presented here are too preliminary but it will be important to monitor rates of endocrinologic control with the use of iMRI in cases of acromegaly and Cushing's disease.
In the case of poorly defined microadenomas, iMRI can provide real-time confirmation of the resection of the tumor, thus obviating the need for exploration and preventing unnecessary injury to the normal pituitary gland. Although our data are preliminary and suffer from short-term follow-up, they do suggest lower rates of hypopituitarism when compared with current literature.
Anatomic shifts during surgery may result in positional changes of important structures, such as the pituitary stalk, neurohypophysis, and diaphragma sellae. The adenohypophysis is not infrequently difficult to distinguish on preoperative images of large sellar tumors. Intraoperatively, the normal gland is often recognizable in view of its firmer texture and reddish coloration. However, it is sometimes difficult to distinguish from fibrous tumors or in case of severe compression, despite magnification and adequate exposure. Identifying the posterior lobe faces similar challenges at times. Upon tumor debulking, we have often been able to identify the gland on intraoperative imaging, thus guiding our resection and facilitating preservation of compressed gland tissue. This is further enhanced by the ability to perform dynamic imaging.
iMRI can also be helpful in diagnosing unexpected problems, such as pneumocephalus, hemorrhage, or infarcts. More commonly, we have reimaged patients when we lost registration during a procedure where navigation was required. Loss of registration during the procedure can be due to multiple technical problems (movement of the reference star or software problems). The design of the iMRI suite with a rotating operating table allows the utilization of other operative adjuncts including endoscopy and ultrasound. However, fluoroscopy remains unauthorized in the iMRI suite to date, mostly due to interference with the portable viewing monitors.
Disadvantages of iMRI
Potential disadvantages of high-field iMRI technology are mostly related to cost, the need for ongoing maintenance and upgrading of software programs, as well as the need for significant training of personnel. Some institutions such as ours establish a core of specifically trained staff to function as safety personnel, thus increasing the ratio of staff per procedure and the overall cost of iMRI interventions. Radiology personnel are needed to run the imaging sequences and monitor timing of contrast administration. They spend a maximum of 20 to 30 minutes in the operating room per imaging set. Radiologists are available upon request and will view intraoperative images via remote-access computers. In view of the elevated cost of maintenance of this technology and the fact that the high-field magnets must remain on at all times, institutions will often make efforts at widening utilization across different surgical services but with limited success to date. Discussions about utilizing the iMRI for routine outpatient imaging has also been entertained and abandoned for logistical reasons, namely providing routine access to a sterile area. We did, however, use the images obtained intraoperatively as postoperative baseline scans if no further resection was performed.
The logistics of operating in an iMRI suite are not very complex because standard instruments are used. The suite is designed so that the operating table, surgeon, scrub, and instruments are outside the 5-G line at all times. When imaging is needed, the operating table and patient are rotated into the gantry and the high magnetic field, thus the need for safety measures that ensure that no metallic objects have remained within the patient or on the table. Therefore, a count of all instruments is performed immediately prior to imaging. Minor changes were needed for the anesthesia setup, such as longer plastic tubes that connect the endotracheal tube to the ventilator and equipment for wireless monitoring, which allow continuous visualization of the patient's vital signs during imaging when all personnel has to leave the room.
iMRI will increase the length of surgical procedures. Once the operative team is beyond the training and acquaintance phase, the extension is limited to ∼20 minutes. This obviously depends on the number of sequences to be performed and whether contrast is administered. The instrument count and patient preparation takes ∼20 minutes but is now infolded into the last 15 minutes of the procedure, upon a sign from the surgeon. Interpretation of imaging occurs while the patient is removed from the scanner, who is then partially redraped and the new scan is entered into the navigation console for reregistration. This enfolds most of the additional time into the surgical case and in our series compared with our pre-MRI data and the literature added only ∼20 minutes to the actual case time. Although multiple imaging sets can be acquired intraoperatively including dynamic sequences, one should bear in mind that a second dose of gadolinium contrast within an hour or 2 of the initial dose is not recommended. This can be a limiting factor, and after he or she has acquired enough experience in the utilization of the scanner, the surgeon should take this limitation into consideration and plan timing for image acquisition accordingly. For example, we have routinely acquired preoperative imaging with a dynamic gadolinium contrast administration for neuronavigation 1 or 2 days prior to surgery. This allows us to bypass the need for a neuronavigation preoperative iMRI study as well as to obtain an intraoperative study with contrast during the procedure. We have also observed retention of contrast in subsequent studies if images were performed at short intervals. It should be noted that blood or bloody cottonoids will sometimes appear bright on the T1-weighted images, simulating enhancement with subsequent difficulties in interpretation. Fat packing is also bright on the T1-weighted images, and presence of fat can be confirmed by performing a fat-suppressing pulse sequence if necessary.
CONCLUSION
The utilization of iMRI is increasing steadily. Current data suggest improvement in resection rates of primary brain tumors. Data for resection of pituitary adenoma using high-field iMRI originate from essentially a single center. Here we add our experience to the current literature. In a group of 59 consecutive patients, we demonstrate a fairly rapid adaptation and learning curve for the surgical team and suggestions of potential advantages such as complete resection, identification of normal tissue, and diagnosis of unexpected events. Additional data and longer follow-up will be required prior to determining whether iMRI will have a significant impact on clinical outcomes in terms of alleviating oversecretion syndromes and/or reducing complications.
ACKNOWLEDGMENTS
We declare that there is no conflict of interest that might be construed to influence the results or interpretation of this manuscript. We have no financial conflicts with the production or publication of this manuscript.
References
- Yeh P J, Chen J W. Pituitary tumors: surgical and medical management. Surg Oncol. 1997;6:67–92. doi: 10.1016/s0960-7404(97)00008-x. [DOI] [PubMed] [Google Scholar]
- Kaltsas G A, Evanson J, Chrisoulidou A, Grossman A B. The diagnosis and management of parasellar tumours of the pituitary. Endocr Relat Cancer. 2008;15:885–903. doi: 10.1677/ERC-08-0170. [DOI] [PubMed] [Google Scholar]
- De Tommasi C, Vance M L, Okonkwo D O, Diallo A, Laws E R., Jr Surgical management of adrenocorticotropic hormone-secreting macroadenomas: outcome and challenges in patients with Cushing's disease or Nelson's syndrome. J Neurosurg. 2005;103:825–830. doi: 10.3171/jns.2005.103.5.0825. [DOI] [PubMed] [Google Scholar]
- Jagannathan J, Sheehan J P, Pouratian N, Laws E R, Jr, Steiner L, Vance M L. Gamma knife radiosurgery for acromegaly: outcomes after failed transsphenoidal surgery. Neurosurgery. 2008;62:1262–1269. discussion 1269–1270. doi: 10.1227/01.neu.0000333297.41813.3d. [DOI] [PubMed] [Google Scholar]
- Cohen-Gadol A A, Liu J K, Laws E R., Jr Cushing's first case of transsphenoidal surgery: the launch of the pituitary surgery era. J Neurosurg. 2005;103:570–574. doi: 10.3171/jns.2005.103.3.0570. [DOI] [PubMed] [Google Scholar]
- Liu J K, Cohen-Gadol A A, Laws E R, Jr, Cole C D, Kan P, Couldwell W T. Harvey Cushing and Oskar Hirsch: early forefathers of modern transsphenoidal surgery. J Neurosurg. 2005;103:1096–1104. doi: 10.3171/jns.2005.103.6.1096. [DOI] [PubMed] [Google Scholar]
- Jane J A, Jr, Han J, Prevedello D M, Jagannathan J, Dumont A S, Laws E R., Jr Perspectives on endoscopic transsphenoidal surgery. Neurosurg Focus. 2005;19:E2. doi: 10.3171/foc.2005.19.6.3. [DOI] [PubMed] [Google Scholar]
- Dehdashti A R, Ganna A, Witterick I, Gentili F. Expanded endoscopic endonasal approach for anterior cranial base and suprasellar lesions: indications and limitations. Neurosurgery. 2009;64:677–687. discussion 687–689. doi: 10.1227/01.NEU.0000339121.20101.85. [DOI] [PubMed] [Google Scholar]
- Erdoğan N, Tucer B, Mavili E, Menkü A, Kurtsoy A. Ultrasound guidance in intracranial tumor resection: correlation with postoperative magnetic resonance findings. Acta Radiol. 2005;46:743–749. doi: 10.1080/02841850500223208. [DOI] [PubMed] [Google Scholar]
- Fox W C, Wawrzyniak S, Chandler W F. Intraoperative acquisition of three-dimensional imaging for frameless stereotactic guidance during transsphenoidal pituitary surgery using the Arcadis Orbic System. J Neurosurg. 2008;108:746–750. doi: 10.3171/JNS/2008/108/4/0746. [DOI] [PubMed] [Google Scholar]
- Jagannathan J, Prevedello D M, Ayer V S, Dumont A S, Jane J A, Jr, Laws E R. Computer-assisted frameless stereotaxy in transsphenoidal surgery at a single institution: review of 176 cases. Neurosurg Focus. 2006;20:E9. doi: 10.3171/foc.2006.20.2.10. [DOI] [PubMed] [Google Scholar]
- Koc K, Anik I, Ozdamar D, Cabuk B, Keskin G, Ceylan S. The learning curve in endoscopic pituitary surgery and our experience. Neurosurg Rev. 2006;29:298–305. discussion 305. doi: 10.1007/s10143-006-0033-9. [DOI] [PubMed] [Google Scholar]
- Rabadán A T, Hernández D, Ruggeri C S. Pituitary tumors: our experience in the prevention of postoperative cerebrospinal fluid leaks after transsphenoidal surgery. J Neurooncol. 2009;93:127–131. doi: 10.1007/s11060-009-9858-8. [DOI] [PubMed] [Google Scholar]
- Bohinski R J, Warnick R E, Gaskill-Shipley M F, et al. Intraoperative magnetic resonance imaging to determine the extent of resection of pituitary macroadenomas during transsphenoidal microsurgery. Neurosurgery. 2001;49:1133–1143. discussion 1143–1144. doi: 10.1097/00006123-200111000-00023. [DOI] [PubMed] [Google Scholar]
- Fahlbusch R, Ganslandt O, Buchfelder M, Schott W, Nimsky C. Intraoperative magnetic resonance imaging during transsphenoidal surgery. J Neurosurg. 2001;95:381–390. doi: 10.3171/jns.2001.95.3.0381. [DOI] [PubMed] [Google Scholar]
- Gerlach R, du Mesnil de Rochemont R, Gasser T, et al. Feasibility of Polestar N20, an ultra-low-field intraoperative magnetic resonance imaging system in resection control of pituitary macroadenomas: lessons learned from the first 40 cases. Neurosurgery. 2008;63:272–284. discussion 284–285. doi: 10.1227/01.NEU.0000312362.63693.78. [DOI] [PubMed] [Google Scholar]
- Nimsky C, von Keller B, Ganslandt O, Fahlbusch R. Intraoperative high-field magnetic resonance imaging in transsphenoidal surgery of hormonally inactive pituitary macroadenomas. Neurosurgery. 2006;59:105–114. discussion 105–114. doi: 10.1227/01.NEU.0000219198.38423.1E. [DOI] [PubMed] [Google Scholar]
- Fahlbusch R, Keller B, Ganslandt O, Kreutzer J, Nimsky C. Transsphenoidal surgery in acromegaly investigated by intraoperative high-field magnetic resonance imaging. Eur J Endocrinol. 2005;153:239–248. doi: 10.1530/eje.1.01970. [DOI] [PubMed] [Google Scholar]
- Schwartz T H, Stieg P E, Anand V K. Endoscopic transsphenoidal pituitary surgery with intraoperative magnetic resonance imaging. Neurosurgery. 2006;58(1 Suppl):ONS44–ONS51. discussion ONS44–ONS51. doi: 10.1227/01.neu.0000193927.49862.b6. [DOI] [PubMed] [Google Scholar]
- Theodosopoulos P V, Leach J, Kerr R G, et al. Maximizing the extent of tumor resection during TSS for pituitary mastoadenomas: can endoscopy replace iMRI? J Neurosurg. 2010;112:736–743. doi: 10.3171/2009.6.JNS08916. [DOI] [PubMed] [Google Scholar]
- Nimsky C, Ganslandt O, von Keller B, Fahlbusch R. Intraoperative high-field MRI: anatomical and functional imaging. Acta Neurochir Suppl (Wien) 2006;98:87–95. doi: 10.1007/978-3-211-33303-7_12. [DOI] [PubMed] [Google Scholar]
- Schulder M, Sernas T J, Carmel P W. Cranial surgery and navigation with a compact intraoperative MRI system. Acta Neurochir Suppl (Wien) 2003;85:79–86. doi: 10.1007/978-3-7091-6043-5_11. [DOI] [PubMed] [Google Scholar]
- Wu J S, Shou X F, Yao C J, et al. Transsphenoidal pituitary macroadenomas resection guided by PoleStar N20 low-field intraoperative magnetic resonance imaging: comparison with early postoperative high-field magnetic resonance imaging. Neurosurgery. 2009;65:63–70. discussion 70–71. doi: 10.1227/01.NEU.0000348549.26832.51. [DOI] [PubMed] [Google Scholar]
- O'Malley B W, Jr, Grady M S, Gabel B C, et al. Comparison of endoscopic and microscopic removal of pituitary adenomas: single-surgeon experience and the learning curve. Neurosurg Focus. 2008;25:E10. doi: 10.3171/FOC.2008.25.12.E10. [DOI] [PubMed] [Google Scholar]