Abstract
Complex intracranial aneurysms (CIAs) include those classified as giant, those located in brain regions of technically difficult access, or that involve arterial trunks/branches, and/or have complicated wall structure. We reviewed retrospectively our management of such lesions in a 12-year period. From 1997 to 2009, 192 patients were admitted with CIAs (133 females, 59 males; average age 55 years); 128 presented with subarachnoid hemorrhage (SAH) and 64 with unruptured, symptomatic CIAs. The SAH group had 73 anterior- and 55 posterior-circulation aneurysms. Most frequent location was middle cerebral artery. Treatment strategies included clipping (65.6%), coiling/stenting (28.1%), bypass (3.1%), no treatment (3.1%). Coiling/stenting was exclusively used for posterior-circulation aneurysms. Outcomes were good (modified Rankin Scale [mRS] 0 to 2) in 54 patients (42.2%), fair (mRS = 3 to 4) in 38 (29.7%), and poor (mRS = 5 to 6) in 36 (28.1%). Among unruptured CIAs, there were 47 anterior- and 17 posterior-circulation aneurysms. Most frequent location was ophthalmic. Thirty (46.9%) were clipped, 19 (29.7%) coiled, 6 (9.4%) by-passed, 2 (3.1%) wrapped, and 7 (10.9%) had no treatment. Outcomes were good in 57 patients (89%) and fair in 7 (11%). Good outcomes were obtained in unruptured CIAs using a multidisciplinary approach. Ruptured CIAs carry a significantly worse prognosis than overall SAH patients.
Keywords: Cerebral aneurysm, clipping, coiling, cerebral revascularization
Complex intracranial aneurysms (CIAs) rank high among the most technically demanding neurosurgical pathologies. Although extensive work and effort has been directed to the treatment of these challenging vascular lesions, no formal definition exists on what complex aneurysms are, and it is rather subjective to individual interpretation that an aneurysm receives such label.1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22 However, labeling an aneurysm as complex implicitly means increased risk of a worse outcome in terms of natural history and/or therapy, and increased need for therapeutic skill and expertise for its treatment. Recently, Hanel and Spetzler6 suggested a definition to include the following attributes to consider an aneurysm as complex: (1) diameter greater than 25 mm; (2) location; (3) previous treatments; (4) presence or absence of collateral circulation; (5) intraluminal thrombus; and (6) calcification of the aneurysmal wall.6 We have included additional features that warrant the classification of an aneurysm as complex, as depicted in Figure 1 and presented in Table 1.
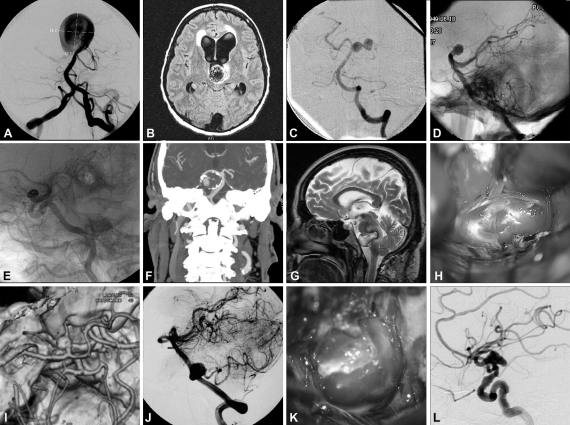
Figure 1.
Intraoperative and angiographic images of cerebral aneurysms with complex features, including examples of giant size (A), intraluminal thrombosis (B), complex configuration (C), difficult access location (D), previous treatments (E), calcification of the aneurysm wall (F), embedding on surrounding tissues (G), blister-like aneurysm (H), aneurysm involving parent artery (I), branch arising from aneurysm (J), broad neck (K), and fusiform aneurysm (L). (Reprinted with permission of Mayfield Clinic.)
Table 1.
Features Commonly Recognized in Association with Complex Aneurysms
| Giant (≥25 mm diameter) |
| Location of difficult or morbid access |
| Configuration |
| Broad neck |
| Branches arising from aneurysm itself |
| Parent artery part of the aneurysm itself |
| Wall structure (blister-like, dissecting) |
| Calcification of the aneurysmal wall |
| Intraluminal thrombus |
| Absence of collateral circulation |
| Embedding on surrounding brain, brainstem, cranial nerves |
| Previous treatments |
The development of improved strategies for the treatment of CIAs includes skull base approaches, revascularization procedures, improved surgical instrumentation, and anesthetic/postoperative care. Additionally, the more recent introduction of endovascular techniques has endowed us with the ability to push the boundaries of operability of most of these previously inoperable lesions.2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24 Using the criteria set forth in Table 1 for patient selection, we conducted a retrospective review of prospectively collected clinical data on our management strategies in a 12-year period for patients harboring CIAs at the University of Cincinnati.
METHODS
Using the criteria set forth in Table 1, we retrospectively reviewed prospectively collected data following our 12-year experience of multimodality approach for the treatment of 1332 patients with cerebral aneurysms at the University of Cincinnati. In this series, 1006 patients presented with aneurysmal subarachnoid hemorrhage (SAH) and 326 with unruptured aneurysms from 1997 to 2009. A cohort of 192 patients, representing 9.68% of our total population fit our criteria of CIAs. This group included 131 females and 61 males whose ages averaged 55 years (range 16 to 85 years). In this study population of 192 patients, 128 presented with SAH, representing 12.72% of all SAH patients, and 64 with unruptured, symptomatic CIAs, totaling 19.63% of all unruptured aneurysm patients. Using our database, we compared treatment strategies, treatment-related complications, and outcomes at discharge in both CIAs subgroups (i.e., ruptured and unruptured) respective to those in their subgroups from the total population.
Treatment Planning
For the treatment of complex cerebral aneurysms, this stage was, in our opinion, as important as the treatment procedure itself. At this juncture, ancillary imaging modalities added valuable information for a successful procedure. Unenhanced computed tomography (CT) was used when necessary to assess for calcium deposition on the aneurysm or parent arteries, bone erosion, or pneumatization of the anterior clinoid process. Magnetic resonance imaging (MRI) provided valuable information regarding the relationship of the aneurysm with the surrounding anatomy, the presence of intraluminal thrombus, intrawall hemorrhage, perilesional edema, embolism and its possible time frame, and the occurrence of subarachnoid hemorrhage (SAH) in cases when minor aneurysmal leakage was suspected. CT angiography (CTA) provided superior three-dimensional (3-D) resolution, and allowed for image manipulation to almost replicate surgical views. The same applied to rotational digital subtraction angiography with 3-D reconstruction, allowing for clear visualization of the aneurysm, its neck, the parent artery, and the origin and trajectory of nearby arterial branches.
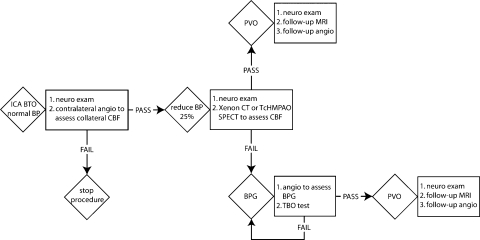
Strategies for which treatment (or part of it) contemplated therapeutic parent artery occlusion a balloon occlusion test, with or without cerebral blood flow (CBF) studies, was always employed to ascertain the blood reserve through collateral flow, and thus the need for bypass surgery. At the University of Cincinnati, a test protocol for temporary balloon occlusion (TBO) has been developed for patients with anterior-circulation aneurysms that may require carotid ligation due to the anticipated complexity of the lesion that would preclude parent vessel salvage or remodeling by direct clipping (Fig. 2). Evaluation of collateral flow was performed by selective angiography. After pharmacologic reduction of the mean arterial pressure by 25 to 30%, the patient then underwent continuous clinical neurologic testing. Technetium hexylmethylpropylene amineoxine (TcHMPAO), single-photon emission CT (SPECT) imaging, or xenon-enhanced CT was performed to assess asymmetry in CBF during TBO. An extracranial-intracranial (EC-IC) arterial bypass was recommended for patients in whom TBO fails, those with asymmetry on the SPECT scan, or those with CBF less than 30 cc/100 g/min.25
Figure 2.
Temporary balloon occlusion (TBO) test protocol for treatment of large and giant unclippable aneurysms. BP, blood pressure; BPG, bypass graft; CBF, cerebral blood flow; CT, computed tomography; ICA, internal carotid artery; MRI, magnetic resonance imaging; PBO, permanent balloon occlusion; PVO, parent vessel occlusion; SPECT, single-photon emission computed tomography; TcHMPAO, technetium hexylmethylpropylene amineoxine. (Reprinted with permission of Mayfield Clinic.)
Once all necessary testing was complete, the results were analyzed by a multidisciplinary neurovascular team. Recently published data suggest that this approach is associated with better outcomes.2,5,8,9,10,11,24,26,27,28,29
Therapeutic Aspects
As with any other cerebrovascular lesion prone to rupture or that has ruptured already, the goal of therapy is exclusion of the aneurysm from the cerebral circulation with preservation of the blood flow in the neural tissues supplied by the parent vessel and its branches, without disrupting the adjacent brain parenchyma. To accomplish this goal, all possible approaches should be considered, either as a sole or a combined modality strategy, which may require one or multiple stages. The availability of a multispecialty team, which includes such resources as neuro-otology, specialized neuroanesthesia, vascular surgery, and interventional neuroradiology are key to plan a treatment without logistic restrictions. The same applies to equipment and surgical instrumentation. Finally, specialized neurocritical care and rehabilitation units are equally as important in the outcome.
For those patients in whom a surgical intervention was planned, the basic principles of aneurysm surgery were relentlessly respected. These principles included adequate exposure with minimal brain dissection and retraction, appropriate proximal and distal vessel control, circumferential dissection of the aneurysmal neck, and dissection and preservation of perforating vessels and vascular branches. We frequently resorted to skull base techniques that provide a comfortable operating field. Approaches were individually tailored to expose what was necessary and to preserve cosmesis. Later in this series, we introduced the use of endoscopes to aid in the assessment of clip placement as well as indocyanine green (ICG) video angiography for assessment of parent vessel and branches patency after clipping; the latter reduced our use of intraoperative digital subtraction angiography (DSA) by 90% since its adoption. Vessel trapping with and without blood flow restoration (bypass) occupied a valuable, increasing role in our armamentarium. We used the radial artery, whenever available, as our conduit of choice.30 Bypass patency was verified intraoperatively by intraoperative DSA earlier in the series; and later by ICG video angiography, as it was adopted. Once available, an ultrasonic perivascular flow probe (i.e., Charbel probe, Transonic Systems Inc., Ithaca, NY) was used to quantify flow at the level of the anastomosis. Other techniques reported by others, such as intraoperative hypothermia and adenosine-induced transient cardiac standstill, have not been used by our group.6,12 Endovascular techniques were performed by our neurointerventionalist partners whose array of procedures included coiling, stenting, and use of embolic agents.
RESULTS
In the CIA-SAH group (78 females, 50 males), there were 73 anterior- and 55 posterior- circulation aneurysms, representing a 1.33:1 ratio, in marked contrast with a 5.66:1 anterior- circulation: posterior-circulation ratio in the overall SAH population. The most frequent location was middle cerebral artery. Twelve aneurysms were giant (>25 mm), 111 were 10 to 25 mm, and five were <10 mm. Location of the aneurysms is summarized in Fig. 3. When compared with the overall SAH population, location distribution was different at the expense of a larger number of anterior communicating aneurysms (the most frequent), followed by middle cerebral artery (the second most frequent), and a higher frequency of posterior communicating aneurysms. Hunt-Hess and Fisher grades in the CIA-SAH group did not differ from the overall distribution in the SAH cohort.
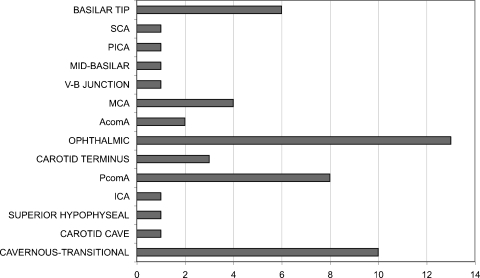
Figure 3.
Bar graphic depicting aneurysm location distribution in 128 patients with complex intracranial aneurysms (CIAs) who presented with subarachnoid hemorrhage (SAH). (Reprinted with permission of Mayfield Clinic.)
Treatment strategies included direct clipping through a skull base approach in 84 patients (65.6%), endovascular treatment in 36 (28.1%), extra-intracranial bypass followed by vessel ligation in 4 (3.1%), and no treatment in 4 (3.1%). The skull base approaches employed included fronto-temporal-orbito-zygomatic (FTOZ) in 39 patients, orbitopterional in 17 patients, extended far lateral in 14 patients, and transpetrosal in 14 patients. Additional skull base techniques utilized included 23 anterior clinoidectomies (15 extradural, 8 intradural). Endovascular therapy was exclusively used for posterior-circulation aneurysms. At discharge, outcomes were good (modified Rankin Scale [mRS] 0 to 2) in 54 patients (42.2%), fair (mRS = 3 to 4) in 38 (29.7%), and poor (mRS = 5 to 6) in 36 (28.1%). Outcomes at discharge in the CIA-SAH population were worse than the overall SAH population, in which outcomes were good in 47.5%, fair in 23.8%, and poor in 28.7%. Of 128 patients, symptomatic vasospasm occurred in 28.9% (37 patients) and negatively impacted the outcomes of 25 patients (19.5%). Surgery-related complications included eight perforator infarcts, two postoperative branch occlusions, and one bypass occlusion. Among patients who underwent endovascular treatment, complications observed included four intracranial vessel dissections, one cervical carotid dissection, two intracranial vessel ruptures, one femoral pseudoaneurysm, one retroperitoneal hemorrhage, and three embolic strokes. When compared with the general SAH population, coiling and clipping had similar rates of procedural complications.
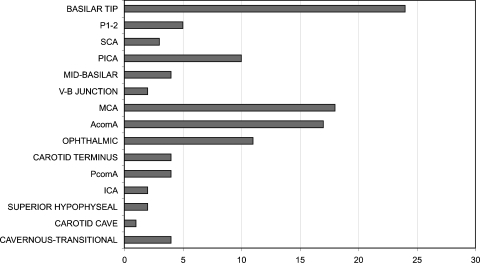
Among unruptured CIAs (53 females, 11 males), there were 47 anterior- and 17 posterior-circulation aneurysms, representing a 2.75:1 ratio, compared with a 6.69:1 anterior-circulation: posterior-circulation ratio in the general unruptured population. The most frequent location was the ophthalmic segment of the internal carotid artery in both the CIA and the general unruptured aneurysm population. Basilar tip was the most frequent location for posterior-circulation unruptured aneurysms in both the CIA and overall unruptured cohorts. Location of the aneurysms is presented in Fig. 4. Nineteen aneurysms were giant, 42 were 10 to 25 mm, and 3 were <10 mm. Presenting symptoms included vision loss,16 cranial nerve palsy,14 embolic stroke,11 mass effect,9 quadriparesis,5 vertebrobasilar insufficiency,5 seizure,2 and ataxia.2
Figure 4.
Bar graphic depicting aneurysm location distribution in 64 patients who presented with unruptured complex intracranial aneurysms (CIAs). (Reprinted with permission of Mayfield Clinic.)
Treatment strategies included 30 aneurysms clipped (46%), 19 coiled (29.7%), 6 (9.4%) had extra-intracranial bypass followed by parent vessel occlusion, 2 (3.1%) were wrapped, and 7 (10.9%) had no treatment. Skull base approaches employed included FTOZ in 16 patients, orbitopterional in 2 patients, transpetrosal in 7 patients, and extended far lateral in 1 patient. Additional skull base techniques employed included 14 anterior (12 extradural, 2 intradural) and 2 posterior clinoidectomies. Outcomes at discharge were good in 57 (89%) patients and fair in 7 (11%), and did not differ significantly from those in the overall unruptured aneurysm population (which had 89% good outcomes and 19% fair outcomes), except for mortality. There were no deaths in the CIA subgroup, but two patients (0.6%) in the overall population of unruptured aneurysm died (one after endovascular coiling, one after surgical clipping). Surgical complications included four perforator infarcts, two vessels occlusions, one vocal cord paralysis, and one postoperative blindness. Coiling complications included one infected groin hematoma, two postprocedural blindness (one required decompression), two perforator infarcts, and three vessel dissections. In this group, 10 aneurysms were treated in a staged fashion that included first surgical clipping or bypass followed by coiling. In our series, the first patient in this subgroup suffered an embolic complication after EC-IC bypass and another patient previously treated by EC-IC bypass experienced a postprocedural retroperitoneal hematoma after coil occlusion of the parent artery and aneurysm. When compared with the overall unruptured population, there were no significant differences in the incidence of complications for coiling. However, surgical treatment had a higher incidence of postoperative ischemic complications (4 perforator infarcts and 2 vessel occlusions in 38 patients; 16% incidence) when compared with the general unruptured population treated by surgery, which had a 2% incidence of such complications.
An aneurysm site-specific comparison of clipping to coiling as method of treatment was not pursued given the multiplicity of subgroups with reduced numbers and unmatched patients that would significantly affect any analysis (e.g., there were no coiling procedures for anterior-circulation SAH-CIA). Furthermore, such comparison would be beyond the scope of this article and the philosophy of our multidisciplinary approach as an evolving concept of a partnership between two treatment methods that, rather as competing, we prefer to see as complementary. In other words, every patient in this series of CIAs, whether ruptured or not, received the treatment that a multidisciplinary group felt was the most appropriate for the aneurysm and the context of clinical presentation, as a result of consensus.
DISCUSSION
In this retrospective review of our multimodality approach to the treatment of ICAs as defined by our proposed classification during a 12-year period, we have concluded that good outcomes can be attained in patients with complex unruptured aneurysms using a multidisciplinary approach. However, the outcomes for our SAH patients with complex aneurysms, while worse than in the general SAH population, were more difficult to analyze due to unmatched populations and the impact of variables such as severity of SAH, vasospasm, and medical complications.
In our unruptured aneurysm population, the CIA group was composed of aneurysms that are typically associated with worse outcomes (i.e., large or giant, posterior-circulation aneurysms). Therefore, it could be speculated that the lack of significant difference in outcomes at discharge when compared with the general unruptured population represents actually a better result than expected. However, this speculation should be tested in a controlled setting. An interesting finding in our unruptured CIA patient population is that of a high incidence of ophthalmic segment and cavernous/transitional aneurysms, which could be explained not only by the tendency of these aneurysms to present with other symptoms than rupture, but also by the long-standing availability of expertise and resources to treat such aneurysms that have made us a regional referral center.
An analysis of the SAH patients was more complicated due to the heterogeneity of groups and the impact of variables that usually affect the outcomes of aneurysmal SAH, such as vasospasm and medical complications. The multimodality approach strategy did not carry a higher rate of procedural complications than those observed in the overall SAH population. Complex ruptured aneurysm patients fared worse than the general SAH patient population. These results, which are not surprising given the features of the aneurysms in this group, likely speak for the complexity of the ruptured aneurysms being treated in such an adverse context as SAH.
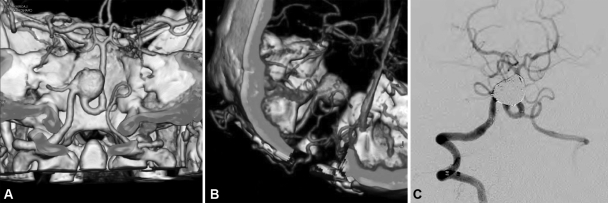
During this particularly prolific period in terms of technological advances, several technologies and techniques were introduced and matured.2,4,6,9,12,21,31,32 On the endovascular side, improved coils and stents provide better options for the treatment of complex aneurysms for which a high morbidity would be expected relative to a surgical approach, or for those patients with high surgical risk or severe SAH that would increase surgical complexity. That is reflected in our selection of endovascular procedures for the treatment of ruptured posterior-circulation aneurysms. Surgical improvements have not only included new instrumentation, such as endoscopic technologies and improved intraoperative flow assessment (i.e., ICG video angiography), but also refinement of skull base approaches. These modern approaches focus to provide an effective, targeted surgical field rather than a “just as big as possible” craniotomy; these improvements provide our patients treatment in a more focused, practical, faster, and even cosmetically better fashion.31,32,33,34 During this time frame, the most significant breakthrough was the complementary interaction of endovascular and surgical technologies with the waning of the coil versus clip controversy. We believe that logical evolution keeps in mind patient outcomes as the main goal of treatment as depicted in Fig. 5.
Figure 5.
Large aneurysm arising from the vertebral artery involving the origin of the posterior inferior cerebellar artery (PICA), which originates from the aneurysm itself, as depicted in CT angiography (CTA) (A) in a 46-year-old woman with history of remote subarachnoid hemorrhage (SAH). An occipital-PICA bypass graft as seen on CTA (B) was performed in anticipation for coil occlusion of the vertebral artery 3 months following the bypass procedure. The patient has remained clinically intact and the bypass patent for the past 2 years. (Reprinted with permission of Mayfield Clinic.)
There is paucity in the literature regarding features that would merit classification of an aneurysm as complex. Whereas there is implicit intricacy and undeniable subjectivity in calling an aneurysm “complex,” a definition is necessary to better rate expected treatment needs, outcomes, and complications. The most recent attempt to define complex aneurysms was by Hanel and Spetzler, as detailed above.6 No recent series describing our study methodology were found in the indexed English literature. We believe that it is particularly important to present series with contemporary data to reflect the impact of recent changes in technology and therapeutic techniques (both surgical and endovascular). Unfortunately, in that respect, most recent publications consist of endovascular series, and site-specific series comparing coiling versus clipping for cerebral aneurysms.2,4,5,7,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,27,31,32,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57 While these comparisons between competing techniques are obviously expected to occur, we feel that, as endovascular treatment for aneurysms continues to evolve and find its definite place in the therapeutic armamentarium, its proven benefits are best weighed in a multidisciplinary, complementary approach. We have experienced the benefits of this therapeutic philosophy as the natural evolution of a collaborative approach to these complex lesions from all possible therapeutic approaches to improve our patient outcomes. Finally, this report should be seen as an observational study on outcomes of a strategy that involves a collaboration of all available modern resources to treat the most challenging cerebral aneurysms.
In this article, we attempted to summarize a series of technically demanding cerebral aneurysms using a consistent set of attributes previously associated in the literature with the undefined label of “complex cerebral aneurysm,” as detailed in Table 1. We conclude that, for these complicated lesions, a multidisciplinary team approach has helped significantly in the planning and execution of a treatment for complex aneurysms, as well as with the management of possible complications. The availability of multiple avenues for the approach of these aneurysms has allowed us to treat lesions that we would have otherwise considered untreatable.
CONCLUSION
CIAs constitute a poorly defined group of technically demanding lesions that are usually associated with worse outcomes because of the therapeutic difficulties posed by their own nature and the need for increased skill and use of resources. In our 12-year series, good outcomes were obtained in unruptured CIAs using a multidisciplinary approach. Ruptured CIAs carried a worse prognosis than overall SAH patients. Patients who underwent the complement of surgical and endovascular treatment fared better as our experience on multidisciplinary team approach strengthened.
References
- Albanese E, Russo A, Ulm A J. Fenestrated vertebrobasilar junction aneurysm: diagnostic and therapeutic considerations. J Neurosurg. 2009;110(3):525–529. doi: 10.3171/2008.9.JNS08170. [DOI] [PubMed] [Google Scholar]
- Alexander B L, Riina H A. The combined approach to intracranial aneurysm treatment. Surg Neurol. 2009;72(6):596–606. discussion 606. doi: 10.1016/j.surneu.2009.06.027. [DOI] [PubMed] [Google Scholar]
- Barrow D L, Cawley C M. Surgical management of complex intracranial aneurysms. Neurol India. 2004;52(2):156–162. [PubMed] [Google Scholar]
- Chen L, Kato Y, Sano H, et al. Management of complex, surgically intractable intracranial aneurysms: the option for intentional reconstruction of aneurysm neck followed by endovascular coiling. Cerebrovasc Dis. 2007;23(5-6):381–387. doi: 10.1159/000101460. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey L, Connolly E S, Jr, Mayer S A, Young W L, Pile-Spellman J, Solomon R A. Complex intracranial aneurysms: combined operative and endovascular approaches. Neurosurgery. 1998;43(6):1304–1312. discussion 1312–1313. doi: 10.1097/00006123-199812000-00020. [DOI] [PubMed] [Google Scholar]
- Hanel R A, Spetzler R F. Surgical treatment of complex intracranial aneurysms. Neurosurgery. 2008;62(6, Suppl 3):1289–1297. discussion 1297–1299. doi: 10.1227/01.neu.0000333794.13844.d9. [DOI] [PubMed] [Google Scholar]
- Hauck E F, Wohlfeld B, Welch B G, White J A, Samson D. Clipping of very large or giant unruptured intracranial aneurysms in the anterior circulation: an outcome study. J Neurosurg. 2008;109(6):1012–1018. doi: 10.3171/JNS.2008.109.12.1012. [DOI] [PubMed] [Google Scholar]
- Hoh B L, Carter B S, Putman C M, Ogilvy C S. Important factors for a combined neurovascular team to consider in selecting a treatment modality for patients with previously clipped residual and recurrent intracranial aneurysms. Neurosurgery. 2003;52(4):732–738. discussion 738–739. doi: 10.1227/01.neu.0000053209.61909.f2. [DOI] [PubMed] [Google Scholar]
- Hoh B L, Putman C M, Budzik R F, Carter B S, Ogilvy C S. Combined surgical and endovascular techniques of flow alteration to treat fusiform and complex wide-necked intracranial aneurysms that are unsuitable for clipping or coil embolization. J Neurosurg. 2001;95(1):24–35. doi: 10.3171/jns.2001.95.1.0024. [DOI] [PubMed] [Google Scholar]
- Jin S C, Kwon H, Song Y, Kim H J, Ahn J S, Kwun B D. Multimodal treatment for complex intracranial aneurysms: clinical research. J Korean Neurosurg Soc. 2008;44(5):314–319. doi: 10.3340/jkns.2008.44.5.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton M T, Quinones-Hinojosa A, Sanai N, Malek J Y, Dowd C F. Combined microsurgical and endovascular management of complex intracranial aneurysms. Neurosurgery. 2008;62(6, Suppl 3):1503–1515. doi: 10.1227/01.neu.0000333814.02649.a0. [DOI] [PubMed] [Google Scholar]
- Mack W J, Ducruet A F, Angevine P D, et al. Deep hypothermic circulatory arrest for complex cerebral aneurysms: lessons learned. Neurosurgery. 2007;60(5):815–827. discussion 815–827. doi: 10.1227/01.NEU.0000255452.20602.C9. [DOI] [PubMed] [Google Scholar]
- Mohit A A, Sekhar L N, Natarajan S K, Britz G W, Ghodke B. High-flow bypass grafts in the management of complex intracranial aneurysms. Neurosurgery. 2007;60(2, Suppl 1):ONS105–ONS122. discussion ONS122–ONS123. doi: 10.1227/01.NEU.0000249243.25429.EE. [DOI] [PubMed] [Google Scholar]
- Murakami K, Shimizu H, Matsumoto Y, Tominaga T. Acute ischemic complications after therapeutic parent artery occlusion with revascularization for complex internal carotid artery aneurysms. Surg Neurol. 2009;71(4):434–441. discussion 441. doi: 10.1016/j.surneu.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Park E K, Ahn J S, Kwon H, Kwun B D. Result of extracranial-intracranial bypass surgery in the treatment of complex intracranial aneurysms : outcomes in 15 cases. J Korean Neurosurg Soc. 2008;44(4):228–233. doi: 10.3340/jkns.2008.44.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñones-Hinojosa A, Lawton M T. In situ bypass in the management of complex intracranial aneurysms: technique application in 13 patients. Neurosurgery. 2008;62(6, Suppl 3):1442–1449. doi: 10.1227/01.neu.0000333808.64530.dd. [DOI] [PubMed] [Google Scholar]
- Sanai N, Zador Z, Lawton M T. Bypass surgery for complex brain aneurysms: an assessment of intracranial-intracranial bypass. Neurosurgery. 2009;65(4):670–683. discussion 683. doi: 10.1227/01.NEU.0000348557.11968.F1. [DOI] [PubMed] [Google Scholar]
- Scamoni C, Dario A, Castelli P, Caronno R, Picano M, Tomei G. Extracranial-intracranial bypass for giant aneurysms and complex vascular lesions: a clinical series of 10 patients. J Neurosurg Sci. 2008;52(1):1–9. discussion 9–10. [PubMed] [Google Scholar]
- Seo B R, Kim T S, Joo S P, et al. Surgical strategies using cerebral revascularization in complex middle cerebral artery aneurysms. Clin Neurol Neurosurg. 2009;111(8):670–675. doi: 10.1016/j.clineuro.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Surdell D L, Hage Z A, Eddleman C S, Gupta D K, Bendok B R, Batjer H H. Revascularization for complex intracranial aneurysms. Neurosurg Focus. 2008;24(2):E21. doi: 10.3171/FOC.2008.25.2.E21. [DOI] [PubMed] [Google Scholar]
- Doormaal T P van, der Zwan A van, Verweij B H, Langer D J, Tulleken C A. Treatment of giant and large internal carotid artery aneurysms with a high-flow replacement bypass using the excimer laser-assisted nonocclusive anastomosis technique. Neurosurgery. 2008;62(6, Suppl 3):1411–1418. doi: 10.1227/01.neu.0000333804.74832.e5. [DOI] [PubMed] [Google Scholar]
- Yang I, Lawton M T. Clipping of complex aneurysms with fenestration tubes: application and assessment of three types of clip techniques. Neurosurgery. 2008;62(5, Suppl 2):ONS371–ONS378. discussion 378–379. doi: 10.1227/01.neu.0000326021.14810.0c. [DOI] [PubMed] [Google Scholar]
- Beretta F, Andaluz N, Zuccarello M. Aneurysms of the ophthalmic (C6) segment of the internal carotid artery: treatment options and strategies based on a clinical series. J Neurosurg Sci. 2004;48(4):149–156. [PubMed] [Google Scholar]
- Proust F, Debono B, Hannequin D, et al. Treatment of anterior communicating artery aneurysms: complementary aspects of microsurgical and endovascular procedures. J Neurosurg. 2003;99(1):3–14. doi: 10.3171/jns.2003.99.1.0003. [DOI] [PubMed] [Google Scholar]
- Andaluz N, Beretta F, Keller J T, Zuccarello M. Aneurysms of the ophthalmic (C6) segment of the internal carotid artery: clinical experience, treatment options, and strategies (Part 2) Neurosurg Q. 2005;15:91–102. [PubMed] [Google Scholar]
- Andaluz N, Zuccarello M. Recent trends in the treatment of cerebral aneurysms: analysis of a nationwide inpatient database. J Neurosurg. 2008;108(6):1163–1169. doi: 10.3171/JNS/2008/108/6/1163. [DOI] [PubMed] [Google Scholar]
- Cowan J A, Jr, Ziewacz J, Dimick J B, Upchurch G R, Jr, Thompson B G. Use of endovascular coil embolization and surgical clip occlusion for cerebral artery aneurysms. J Neurosurg. 2007;107(3):530–535. doi: 10.3171/JNS-07/09/0530. [DOI] [PubMed] [Google Scholar]
- Johnston S C. Effect of endovascular services and hospital volume on cerebral aneurysm treatment outcomes. Stroke. 2000;31(1):111–117. doi: 10.1161/01.str.31.1.111. [DOI] [PubMed] [Google Scholar]
- Qureshi A I, Suri M F, Nasar A, et al. Trends in hospitalization and mortality for subarachnoid hemorrhage and unruptured aneurysms in the United States. Neurosurgery. 2005;57(1):1–8. discussion 1–8. doi: 10.1227/01.neu.0000163081.55025.cd. [DOI] [PubMed] [Google Scholar]
- Kocaeli H, Andaluz N, Choutka O, Zuccarello M. Use of radial artery grafts in extracranial-intracranial revascularization procedures. Neurosurg Focus. 2008;24(2):E5. doi: 10.3171/FOC/2008/24/2/E5. [DOI] [PubMed] [Google Scholar]
- Abdel Aziz K M, Andaluz N, Zuccarello M. Basilar bifurcation aneurysms: Strategies for surgical approach selection. Neurosurg Q. 2007;17:101–112. [Google Scholar]
- Andaluz N, Loveren H R Van, Keller J T, Zuccarello M. Anatomic and clinical study of the orbitopterional approach to anterior communicating artery aneurysms. Neurosurgery. 2003;52(5):1140–1148. discussion 1148–1149. [PubMed] [Google Scholar]
- Andaluz N, Romano A, Reddy L V, Zuccarello M. Eyelid approach to the anterior cranial base. J Neurosurg. 2008;109(2):341–346. doi: 10.3171/JNS/2008/109/8/0341. [DOI] [PubMed] [Google Scholar]
- Beretta F, Andaluz N, Chalaala C, Bernucci C, Salud L, Zuccarello M. Image-guided anatomical and morphometric study of supraorbital and transorbital minicraniotomies to the sellar and perisellar regions: comparison with standard techniques. J Neurosurg. 2010;113(5):975–981. doi: 10.3171/2009.10.JNS09435. [DOI] [PubMed] [Google Scholar]
- Ponce F A, Spetzler R F, Han P P, et al. Cardiac standstill for cerebral aneurysms in 103 patients: an update on the experience at the Barrow Neurological Institute. Clinical article. J Neurosurg. 2011;114(3):877–884. doi: 10.3171/2010.9.JNS091178. [DOI] [PubMed] [Google Scholar]
- Vendrell J F, Costalat V, Brunel H, Riquelme C, Bonafe A. Stent-assisted coiling of complex middle cerebral artery aneurysms: initial and midterm results. AJNR Am J Neuroradiol. 2011;32(2):259–263. doi: 10.3174/ajnr.A2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B N, Sun Z H, Romani R, et al. Microsurgical management of large and giant paraclinoid aneurysms. World Neurosurg. 2010;73(3):137–146. discussion e17, e19. doi: 10.1016/j.surneu.2009.07.042. [DOI] [PubMed] [Google Scholar]
- Cunha e Sá M. The use of deep hypothermia and cardiac arrest in the surgical treatment of large and complex intracranial circulation aneurysms. Acta Neurochir (Wien) 2010;152(6):1089–1090. doi: 10.1007/s00701-010-0619-4. [DOI] [PubMed] [Google Scholar]
- Patel H C, Teo M, Higgins N, Kirkpatrick P J. High flow extra-cranial to intra-cranial bypass for complex internal carotid aneurysms. Br J Neurosurg. 2010;24(2):173–178. doi: 10.3109/02688690903531075. [DOI] [PubMed] [Google Scholar]
- Schebesch K M, Proescholdt M, Ullrich O W, et al. Circulatory arrest and deep hypothermia for the treatment of complex intracranial aneurysms—results from a single European center. Acta Neurochir (Wien) 2010;152(5):783–792. doi: 10.1007/s00701-009-0594-9. [DOI] [PubMed] [Google Scholar]
- Sano H. Treatment of complex intracranial aneurysms of anterior circulation using multiple clips. Acta Neurochir Suppl (Wien) 2010;107:27–31. doi: 10.1007/978-3-211-99373-6_4. [DOI] [PubMed] [Google Scholar]
- Connolly E S, Jr, Hoh B L, Selden N R, et al. Clipping versus coiling for ruptured intracranial aneurysms: integrated medical learning at CNS 2007. Neurosurgery. 2010;66(1):19–34. discussion 34. doi: 10.1227/01.NEU.0000362005.93515.5B. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Matsumoto Y, Tominaga T. Parent artery occlusion with bypass surgery for the treatment of internal carotid artery aneurysms: clinical and hemodynamic results. Clin Neurol Neurosurg. 2010;112(1):32–39. doi: 10.1016/j.clineuro.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Xu F, Qin X, Tian Y, Gu Y, Leng B, Song D. Endovascular treatment of complex intracranial aneurysms using intra/extra-aneurysmal stent. Acta Neurochir (Wien) 2011;153(4):923–930. doi: 10.1007/s00701-010-0934-9. [DOI] [PubMed] [Google Scholar]
- Albuquerque F C, Gonzalez L F, Hu Y C, Newman C B, McDougall C G. Transcirculation endovascular treatment of complex cerebral aneurysms: technical considerations and preliminary results. Neurosurgery. 2011;68(3):820–829. discussion 829–830. doi: 10.1227/NEU.0b013e3182077f17. [DOI] [PubMed] [Google Scholar]
- Saatci I, Geyik S, Yavuz K, Cekirge S. X–Configured Stent-Assisted Coiling in the Endovascular Treatment of Complex Anterior Communicating Artery Aneurysms: A Novel Reconstructive Technique. November 24 (Epub ahead of print] AJNR Am J Neuroradiol. 2010 doi: 10.3174/ajnr.A2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv X, Jiang C, Li Y, Wu Z. Clinical outcomes of ruptured and unruptured vertebral artery-posterior inferior cerebellar artery complex dissecting aneurysms after endovascular embolization. AJNR Am J Neuroradiol. 2010;31(7):1232–1235. doi: 10.3174/ajnr.A2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lot G, Houdart E, Cophignon J, Casasco A, George B. Combined management of intracranial aneurysms by surgical and endovascular treatment. Modalities and results from a series of 395 cases. Acta Neurochir (Wien) 1999;141(6):557–562. doi: 10.1007/s007010050343. [DOI] [PubMed] [Google Scholar]
- Natarajan S K, Sekhar L N, Ghodke B, Britz G W, Bhagawati D, Temkin N. Outcomes of ruptured intracranial aneurysms treated by microsurgical clipping and endovascular coiling in a high-volume center. AJNR Am J Neuroradiol. 2008;29(4):753–759. doi: 10.3174/ajnr.A0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoh B L, Chi Y Y, Dermott M A, Lipori P J, Lewis S B. The effect of coiling versus clipping of ruptured and unruptured cerebral aneurysms on length of stay, hospital cost, hospital reimbursement, and surgeon reimbursement at the university of Florida. Neurosurgery. 2009;64(4):614–619. discussion 619–621. doi: 10.1227/01.NEU.0000340784.75352.A4. [DOI] [PubMed] [Google Scholar]
- Jahromi B S, Mocco J, Bang J A, et al. Clinical and angiographic outcome after endovascular management of giant intracranial aneurysms. Neurosurgery. 2008;63(4):662–674. discussion 674–675. doi: 10.1227/01.NEU.0000325497.79690.4C. [DOI] [PubMed] [Google Scholar]
- Krisht A F, Krayenbühl N, Sercl D, Bikmaz K, Kadri P A. Results of microsurgical clipping of 50 high complexity basilar apex aneurysms. Neurosurgery. 2007;60(2):242–250. discussion 250–252. doi: 10.1227/01.NEU.0000249265.88203.DF. [DOI] [PubMed] [Google Scholar]
- Collice M, Arena O, D'Aliberti G, et al. Aneurysms of the vertebro-basilar junction area: preliminary experience in endovascular and surgical management. Acta Neurochir (Wien) 1997;139(2):124–133. doi: 10.1007/BF02747192. [DOI] [PubMed] [Google Scholar]
- de Oliveira J G, Borba L A, Rassi-Neto A, et al. Intracranial aneurysms presenting with mass effect over the anterior optic pathways: neurosurgical management and outcomes. Neurosurg Focus. 2009;26(5):E3. doi: 10.3171/2009.3.FOCUS0924. [DOI] [PubMed] [Google Scholar]
- Vendrell J F, Menjot N, Costalat V, et al. Endovascular treatment of 174 middle cerebral artery aneurysms: clinical outcome and radiologic results at long-term follow-up. Radiology. 2009;253(1):191–198. doi: 10.1148/radiol.2531082092. [DOI] [PubMed] [Google Scholar]
- Sedat J, Chau Y, Mondot L, Vargas J, Szapiro J, Lonjon M. Endovascular occlusion of intracranial wide-necked aneurysms with stenting (Neuroform) and coiling: mid-term and long-term results. Neuroradiology. 2009;51(6):401–409. doi: 10.1007/s00234-009-0502-2. [DOI] [PubMed] [Google Scholar]
- Porter P J, Mazighi M, Rodesch G, et al. Endovascular and Surgical Management of Multiple Intradural Aneurysms. Review of 122 Patients Managed between 1993 and 1999. Interv Neuroradiol. 2001;7(4):291–302. doi: 10.1177/159101990100700403. [DOI] [PMC free article] [PubMed] [Google Scholar]