Abstract
We present a retrospective analysis of surgical outcome of sinonasal malignant tumors. Overall survival (OS), disease-specific survival (DSS), local control (LC), and disease-free survival (DFS) were calculated in 32 patients. Prognostic factors for survival and functional outcomes were investigated. The median follow-up period was 70 months. At 5 years, OS, DSS, LC, and DFS rates were 0.722, 0.745, 0.851, and 0.707, respectively. Prognostic factors for poor OS were involvement of the frontal sinus (p = 0.023), T classification (T4, p = 0.025), surgical complications (p = 0.029), chemotherapy (p = 0.035) postsurgical infection (p = 0.043), involvement of the orbit (p = 0.048), histology (squamous cell carcinoma, p = 0.049), and radiotherapy (p = 0.043). Prognostic factors for poor DSS were radiotherapy (p = 0.030), chemotherapy (p = 0.036), positive surgical margin (p = 0.034), and T classification (T4, p = 0.050). LC was adversely influenced by surgical procedure (combined frontotemporal resection, p = 0.035) and positive surgical margin (p = 0.049). DFS was adversely influenced by positive surgical margin (p = 0.001). Prognostic factors for poor functional outcome were postsurgical infection (p = 0.039), postsurgical complications (p = 0.040), tumor location (maxillary sinus, p = 0.042, orbit, p = 0.0002), number of sinuses involved (number of sinuses involved was inversely proportional to functional outcome, p = 0.027), T classification (T4 p = 0.007), pathology (squamous cell carcinoma, p = 0.023), and chemotherapy (p = 0.048). Craniofacial resection was an effective surgical option.
Keywords: Sinonasal malignant tumor, craniofacial resection, survival outcome, functional outcome, statistical analysis
The introduction of combined craniofacial resection has improved outcome for sinonasal malignant tumors extending to the anterior and middle skull base.1,2 This has led to this surgical technique being used for more aggressive tumors, hence patients with intracranial, intraorbital, and intracavernous sinus extension are undergoing surgery. In a previous report we presented the long-term survival and functional outcome of 13 consecutive patients with malignant sinonasal tumors extending to the anterior skull base who were treated with combined craniofacial resection between 1992 and 1998.3 In this study we performed statistical analysis of 32 patients treated between 1992 and 2009. Risk factors for survival and functional outcome are discussed.
PATIENTS AND METHODS
Patient Population
We studied 32 patients with sinonasal malignant tumors who underwent craniofacial resection at Chiba University Hospital between 1992 and 2009. These comprised 17 males and 15 females with a mean age at surgery of 57.5 ± 12.2 years (age range 17 to 76). Initial symptoms were nasal obstruction in 10 patients, epistaxis in 9, nasal discharge in 5, cheek swelling in 5, cheek pain in 5, diplopia in 4, proptosis in 2, and other symptoms in 4. The tumor origin, determined from the epicenter of the tumor, was the ethmoid sinus in 16 patients, maxillary sinus in 10, nasal cavity in 5, and epipharynx in 1. T classification according to the TNM stage of the International Union Against Cancer guidelines was T2 in 9 patients, T3 in 2, and T4 in 21. Tumors had advanced to the nasal cavity in 25 patients, frontal sinus in 5, ethmoid sinus in 24, sphenoid sinus in 8, maxillary sinus in 16, orbit in 17, pterygopalatine fossa in 7, pharynx in 1, and intracranial cavity in 7. Two patients who had undergone previous conventional sinus surgery had craniofacial resection as a salvage procedure for recurrence at the anterior skull base. The remaining 30 patients underwent primary cancer surgery. The histological diagnosis was squamous cell carcinoma in 12 patients, olfactory neuroblastoma in 4, adenocarcinoma in 3, adenoid cystic carcinoma in 2, chondrosarcoma in 2, and malignant melanoma in 2. Cylindrical cell carcinoma, fibrosarcoma, malignant glomus tumor, osteosarcoma, rhabdomyosarcoma, primitive neuroectodermal tumor, and teratocarcinosarcoma were each diagnosed in a single patient.
Treatment Strategies
All patients underwent combined transfacial and transcranial tumor removal as described previously.3 Surgical indications for the combined approach were tumor involvement of the anterior or middle skull base, or both, with the possibility of en-bloc resection. Contraindications were involvement of the cavernous sinus, bilateral orbit, or posterior wall of the sphenoid sinus, and distant metastasis. In cases of tumor invasion to the sphenoid sinus, surgery was indicated when the tumor could be removed without touching it. When the tumor invaded the pterygopalatine fossa, the posterior limit of resection included the anterior part of the middle skull base.4 When en-bloc resection was impossible, radiotherapy or chemotherapy, or both, were first administered and surgery was performed when tumor shrinkage was confirmed. In these circumstances, the resection margin was configured according to the initial tumor location. Thirteen patients underwent primary anterior skull base resection, and one underwent primary combined anterior and middle skull base resection. Twelve patients underwent anterior skull base resection after radiotherapy or chemotherapy, or both. Six patients underwent a combined anterior and middle skull base resection after radiotherapy or chemotherapy, or both. Complete tumor removal with a negative surgical margin on microscopic pathological analysis was achieved in 28 patients, whereas 4 patients were found to have microscopically positive surgical margins. In these four patients, two had postoperative radiotherapy, whereas two had already undergone presurgical radiotherapy. All patients with suspected cervical metastasis underwent curative neck resection.
Review of the Clinical Records
We conducted a retrospective analysis after reviewing the patients' medical records and radiological images. The follow-up period was 1 to 182 months with an average of 70.0 ± 59.8 months, and no patients were lost to follow-up. The cut-off date for follow-up was 31 December, 2009. Surgical complications, details of patient deaths, and prognostic factors were investigated. Prognostic factors analyzed were sex, age, time from onset to surgery, dural invasion, involvement of the middle skull base, postsurgical infection, postsurgical complications, tumor location, number of sinuses involved, T classification, status of the surgical margin, histological diagnosis, radiotherapy, and chemotherapy. The histological types were classified into three subgroups for statistical analysis. They were squamous cell carcinoma (n = 12), epithelial tumor other than squamous cell carcinoma (n = 6), and nonepithelial tumor (n = 14).
Statistical Analysis
Statistical analysis was performed using PASW Statistics 18® (IBM). Estimations of overall survival (OS), disease-specific survival (DSS), disease-free survival (DFS) and local control (LC) were performed based on the Kaplan-Meier method with univariate analysis by the log-rank test or Cox proportional hazard model. A multivariate Cox proportional hazard model was used to examine the relative impact of variables demonstrated to be statistically significant in univariate analysis. Before performing multivariate analyses, Spearman correlation coefficients were calculated. Functional outcome in terms of daily activities on discharge was assessed using Karnofsky performance status, with differences analyzed with the Mann-Whitney test. P values less than 0.05 were considered statistically significant.
RESULTS
Surgical Complications
Fifteen patients (47%) developed surgical complications after skull base resection. The most common complication was local infection, experienced by 12 patients (38%). Of these 12 patients, 2 needed removal of the bone flap applied for reconstruction of the skull base. One patient died during the immediate postoperative period from septic shock following local infection. Central nervous system (CNS) complications, other than simple cranial nerve disturbance, occurred in four patients. Three had frontal lobe contusion and one had late-onset meningitis with cerebrospinal fluid leakage. Multiple cranial nerve disturbances were observed. Olfactory nerve disturbance appeared in all patients, binocular vision defect in 2, dysphagia in 8, speech disturbance in 7, altered facial sensation in 11, and facial motor impairment in 7. These cranial nerve disturbances were not classified as CNS complications, because they were inevitable consequences of skull base resection.
Deaths
Eleven patients died during the follow-up period. Causes of death were local recurrence of the tumor (n = 4), remote metastasis (n = 5), postoperative infection (n = 1), and unknown (sudden death, n = 1). In four patients with local recurrence, this was detected at 6, 8, 44, and 132 months after surgery. Patients could therefore be classified into early recurrence (6 and 8 months) and late recurrence (44 and 132 months); early recurrence occurred only in squamous cell carcinoma whereas late recurrence occurred in olfactory neuroblastoma and adenocarcinoma. Remote metastasis occurred at 4, 7, 10, 26, and 74 months, with the latest metastasis detected in chondrosarcoma.
Survival Rates
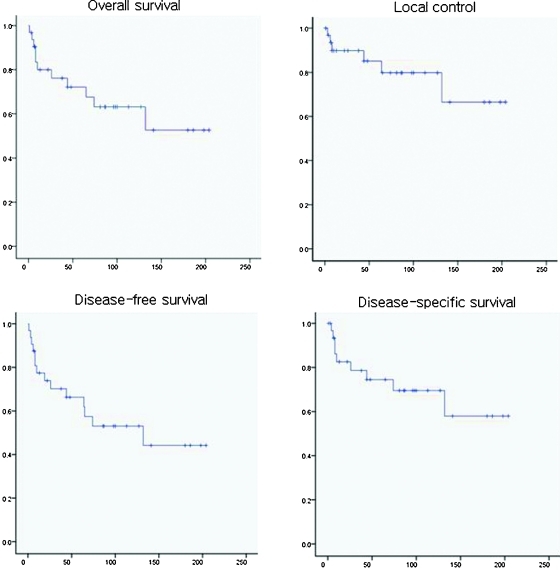
For the 32 patients, OS rate was 80% at 2 years, 72.2% at 5 years, and 63.1% at 10 years. DSS rate was 82.6%, 74.5%, and 69.5% at 2 years, 5 years, and 10 years, respectively. LC rate was 89.8%, 85.1%, and 79.8% at 2 years, 5 years, and 10 years, respectively (Fig. 1). DFS rate was 73.9%, 66.3%, and 53.0% at 2 years, 5 years, and 10 years, respectively. OS rates for each histological subgroup were 61.9% at 2 years and 49.5% at 5 years in squamous cell carcinoma, 100% at 2 years, 100% at 5 years, and 83.3% at 10 years in epithelial tumor other than squamous cell carcinoma, and 85.10% at 2 years, 76.6% at 5 years, and 67.0% at 10 years in nonepithelial tumor.
Figure 1.
Survival outcomes after craniofacial surgery for patients with sinonasal malignant tumor. The vertical axis shows cumulative proportion surviving and the horizontal axis shows follow-up interval after surgery (months).
Univariate Survival Analysis
The factors adversely influencing OS on univariate analysis, in order of significance, were involvement of the frontal sinus (p = 0.023), T classification (T4, p = 0.025), surgical complications (p = 0.029), chemotherapy (p = 0.035), postsurgical infection (p = 0.043), involvement of the orbit (p = 0.048), histology (squamous cell carcinoma, p = 0.049), and radiotherapy (p = 0.043). Prognostic factors for poor DSS, in order of significance, were radiotherapy (p = 0.03), chemotherapy (p = 0.036), positive surgical margin (p = 0.034), and T classification (T4, p = 0.05). LC was adversely influenced by surgical procedure (combined frontotemporal resection, p = 0.035) and positive surgical margin (p = 0.049). DFS was adversely influenced by positive surgical margins (p = 0.001).
Multivariate Survival Analysis
Multivariate analysis for OS was performed for surgical complications, chemotherapy, histology, T classification, and involvement of the frontal sinus. Since intraorbital invasion, radiotherapy, and postoperative infection appeared to be correlated with involvement of the frontal sinus or histology (squamous cell carcinoma), (Spearman correlation coefficient, p < 0.05), these factors were not included in the multivariate analysis. Involvement of the frontal sinus was the significant risk factor (p = 0.05) for poor OS; odds ratio was 10.64 with a 95% confidence interval from 1.00 to 111.1.
Univariate Functional Analysis
Karnofsky performance score on discharge was 90 in 13 patients, 80 in 6, 70 in 5, 60 in 2, 50 in 1, 40 in 1, 30 in 2, and 0 in 2. Factors adversely influencing functional outcome on univariate analysis, in order of significance, were orbital tumor (p = 0.0002), T classification (T4, p = 0.007), histology (squamous cell carcinoma, p = 0.023), number of sinuses involved (number of sinuses involved was inversely proportional to functional outcome, p = 0.027), postsurgical infection (p = 0.039), surgical complications (p = 0.040), maxillary sinus tumor (p = 0.042), and chemotherapy (p = 0.048).
Multivariate Functional Analysis
Multivariate analysis of functional outcome was evaluated by analysis of covariance. This revealed that the number of sinuses affected by the tumor was a risk factor for poor functional outcome (p = 0.024).
DISCUSSION
Survival Outcomes
Previously reported 5-year OS rates, DSS rates, DFS rates, and LC rates are 16 to 67%, 55 to 69%, 24 to 54%, and 41 to 65%, respectively (Table 1). Our outcomes were favorable in comparison, and we believe this resulted from our emphasis on en-bloc resection, single surgery, and patient selection. In the present study, 87.5% of patients had a negative surgical margin, which was better than the 60% reported by Bentz.5 Single surgery has also been reported as a factor positively influencing survival outcome.6 In our series, 94% of patients had single surgery, a higher proportion than in previous reports.
Table 1.
Reported 5-Year Survival (%) for Malignant Sinonasal Tumors Treated with Craniofacial Resection
| Author (ref) | n | OS | DSS | LC | DFS | Comment |
|---|---|---|---|---|---|---|
| Patel8 | 1307 | 54 | 60 | — | 53 | SC |
| Hoppe10 | 85 | 67 | 55 | 62 | — | SC |
| Suarez13 | 100 | 40 | — | — | — | SC |
| Bentz5 | 166 | 52 | 57 | 41 | — | SC |
| Bridger12 | 73 | 61 | 69 | — | — | SC |
| Cantu17 | 91 | 47 | — | — | 24 | SC |
| Salvan15 | 41 | 36 | — | — | — | SC |
| Guntinas-Lichius6 | 229 | 41 | 56 | 64 | 34 | INSC |
| Chen18 | 21 | 56 | — | — | — | INSC |
| McKay7 | 73 | 16 | — | — | — | INSC |
| Dirix16 | 127 | 54 | — | 53 | 37 | INSC |
| Chen21 | 127 | 52 | — | 62 | 54 | INSC |
| Blanco11 | 106 | 27 | — | 58 | 33 | INSC |
| Jansen22 | 73 | 60 | — | 65 | 53 | INSC |
| Katz23 | 78 | 50 | 56 | 60 | — | INSC |
| Present series | 32 | 72 | 75 | 85 | 71 | SC |
DFS, disease-free survival; DSS, disease-specific survival; INSC, included nonsurgical case; LC, local control; OS, overall survival; SC, surgical case.
Risk Factors for Poor Survival Outcome
Reported risk factors for poor survival outcome are increased age7; histology (malignant melanoma,5,8 undifferentiated carcinoma,9,10,11 and squamous cell carcinoma12); tumor extension to the sphenoid sinus,13 pterygopalatine fossa,6 orbit,6,13 dura,5,6,8,10,14,15 or brain13; UICC staging: T4,6,14,16,17 M1,6 and N16,11,16; positive surgical margin5,8,14; and incomplete removal.18 The present risk factors for survival outcome were consistent with these reports.
The present study is the first to find radiotherapy and chemotherapy to be risk factors for adverse survival outcome. In our series the principal role of radiotherapy and chemotherapy was in patients in whom en-bloc resection was impossible and in those with a positive surgical margin. These patients would be anticipated to have a worse survival outcome than those in whom en-bloc surgery was possible and surgical margins were free of the tumor. Even if tumor shrinkage was obtained by radiotherapy and chemotherapy, the surgical resection line would intersect the previous tumor boundary. Postsurgical radiotherapy as a salvage therapy for patients with a positive surgical margin was indeed effective. In our series four patients had positive surgical margins, and in two of them, radiotherapy prevented tumor recurrence. Postsurgical radiotherapy is therefore recommended as an adjuvant therapy when surgical margins are not tumor-free. In the present analysis, histological types of the tumors were classified into three subgroups for statistical analysis. As described previously, squamous cell carcinoma, undifferentiated carcinoma, and malignant melanoma were individual risk factors. In the report of Howard19 there was a difference in survival between epithelial and nonepithelial tumors, so we have classified the tumors into three subgroups as above. Squamous cell carcinoma had poorer survival than epithelial tumors other than squamous cell carcinoma and nonepithelial tumors. But, this classification could not fully consider the natural history of the individual histological type in the each subgroup. Analysis of larger number of the patients by the multi-institutional study will be necessary. Infection has not previously been reported as a factor adversely influencing survival outcome. Infection may reduce survival outcome because malignant cells are disseminated via pus containing malignant cells. In three cases of ethmoidal cancer with concomitant sphenoidal sinusitis we found tumor cells in the pus, and tumor recurred in two of these cases. Hence, particularly when the sinuses are obstructed by the tumor and infected, clinicians should be aware that the pus is likely to contain tumor cells. Then surgical management to the infected sinuses should be careful and we should not basically open the infected sinus when it should be removed. When en-bloc resection of the infected sinus is impossible, we should carefully manipulate the sinus. The sinus should be first opened without scattering the pus and infected surgical instruments should be carefully treated for prevention of dissemination of the tumor cells. Pus contained in the sinus should be cleared for prevention of residual tumor cell and postoperative infection.
Prevention of some of the above-mentioned risk factors should improve outcome. Risk factors which could be prevented in our series were postsurgical infection and surgical complications for OS, and positive surgical margins for DSS, LC, and DFS. Future studies should address active presurgical tumor shrinkage in patients considered for en-bloc resection.
Risk Factors for Poor Functional Outcome
The literature contains few reports about risk factors for functional outcome. Gil20 reported that age (older than 60 years) adversely influenced physical function, that tumor malignancy was correlated with specific neurological deficit, poor physical function and emotional health, and that radiotherapy negatively influenced specific neurological deficit and emotional health. In the present series tumor histology (squamous cell carcinoma), chemotherapy, tumor location (maxillary sinus or orbit), number of sinuses involved, and T classification (T4) were definite risk factors for poor functional outcome. These risk factors are therefore likely to reduce activities of daily living and neurological function of patients. Tumors located in functionally important areas that extend widely require large resections of these areas. Moreover, postsurgical complications require multiple surgery and removal of bony structures, which leads to poor functional outcome. Postsurgical infections and surgical complications were avoidable risk factors. From this analysis we propose to do the following to avoid complications: be careful in dealing with infected sinuses, do not use free bone graft, reduce third spaces such as epidural blood and fluid retention space by early removal of spinal drainage to enlarge the brain volume, suture the graft (pericranial flap) and dura, and use negative pressure subcutaneous drainage. Because we cannot avoid treating tumors in the maxillary sinus and orbit, and cannot change the number of sinuses involved, T classification (T4), histology (squamous cell carcinoma), or chemotherapy, we should be very careful when performing surgery in these cases.
References
- Smith R R, Klopp C T, Williams J M. Surgical treatment of cancer of the frontal sinus and adjacent areas. Cancer. 1954;7(5):991–994. doi: 10.1002/1097-0142(195409)7:5<991::aid-cncr2820070523>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Ketcham A S, Wilkins R H, Vanburen J M, Smith R R. A combined intracranial facial approach to the paranasal sinuses. Am J Surg. 1963;106:698–703. doi: 10.1016/0002-9610(63)90387-8. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Saeki N, Mine S, et al. Evaluation of outcome and QOL in patients with craniofacial resection for malignant tumors involving the anterior skull base. Neurol Res. 2000;22(6):545–550. doi: 10.1080/01616412.2000.11740716. [DOI] [PubMed] [Google Scholar]
- Kamata N. Middle fossa approach. In: Komatsuzaki A, Inuyama M, Honjo I, Moriyama H, editors. Atlas of otolaryngology, head and neck surgery. Vol. 2. Tokyo: Igaku-Shoin; 1999. pp. 337–344. [Google Scholar]
- Bentz B G, Bilsky M H, Shah J P, Kraus D. Anterior skull base surgery for malignant tumors: a multivariate analysis of 27 years of experience. Head Neck. 2003;25(7):515–520. doi: 10.1002/hed.10250. [DOI] [PubMed] [Google Scholar]
- Guntinas-Lichius O, Kreppel M P, Stuetzer H, Semrau R, Eckel H E, Mueller R P. Single modality and multimodality treatment of nasal and paranasal sinuses cancer: a single institution experience of 229 patients. Eur J Surg Oncol. 2007;33(2):222–228. doi: 10.1016/j.ejso.2006.10.033. [DOI] [PubMed] [Google Scholar]
- McKay S P, Shibuya T Y, Armstrong W B, et al. Cell carcinoma of the paranasal sinuses and skull base. Am J Otolaryngol. 2007;28(5):294–301. doi: 10.1016/j.amjoto.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Patel S G, Singh B, Polluri A, et al. Craniofacial surgery for malignant skull base tumors: report of an international collaborative study. Cancer. 2003;98(6):1179–1187. doi: 10.1002/cncr.11630. [DOI] [PubMed] [Google Scholar]
- Thompson L DR. Sinonasal carcinomas. Curr Diagn Pathol. 2006;12:40–53. [Google Scholar]
- Hoppe B S, Stegman L D, Zelefsky M J, et al. Treatment of nasal cavity and paranasal sinus cancer with modern radiotherapy techniques in the postoperative setting—the MSKCC experience. Int J Radiat Oncol Biol Phys. 2007;67(3):691–702. doi: 10.1016/j.ijrobp.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Blanco A I, Chao K SC, Ozyigit G, et al. Carcinoma of paranasal sinuses: long-term outcomes with radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59(1):51–58. doi: 10.1016/j.ijrobp.2003.09.101. [DOI] [PubMed] [Google Scholar]
- Bridger G P, Kwok B, Baldwin M, Williams J R, Smee R I. Craniofacial resection for paranasal sinus cancers. Head Neck. 2000;22(8):772–780. doi: 10.1002/1097-0347(200012)22:8<772::aid-hed5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Suarez C, Llorente J L, Fernandez De Leon R, Maseda E, Lopez A. Prognostic factors in sinonasal tumors involving the anterior skull base. Head Neck. 2004;26(2):136–144. doi: 10.1002/hed.10358. [DOI] [PubMed] [Google Scholar]
- Rutter M J, Furneaux C E, Morton R P. Craniofacial resection of anterior skull base tumours: factors contributing to success. Aust N Z J Surg. 1998;68(5):350–353. doi: 10.1111/j.1445-2197.1998.tb04770.x. [DOI] [PubMed] [Google Scholar]
- Salvan D, Julieron M, Marandas P, et al. Combined transfacial and neurosurgical approach to malignant tumours of the ethmoid sinus. J Laryngol Otol. 1998;112(5):446–450. doi: 10.1017/s0022215100140745. [DOI] [PubMed] [Google Scholar]
- Dirix P, Nuyts S, Geussens Y, et al. Malignancies of the nasal cavity and paranasal sinuses: long-term outcome with conventional or three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2007;69(4):1042–1050. doi: 10.1016/j.ijrobp.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Cantù G, Solero C L, Mariani L, et al. Anterior craniofacial resection for malignant ethmoid tumors—a series of 91 patients. Head Neck. 1999;21(3):185–191. doi: 10.1002/(sici)1097-0347(199905)21:3<185::aid-hed1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Chen A M, Daly M E, El-Sayed I, et al. Patterns of failure after combined-modality approaches incorporating radiotherapy for sinonasal undifferentiated carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2008;70(2):338–343. doi: 10.1016/j.ijrobp.2007.06.057. [DOI] [PubMed] [Google Scholar]
- Howard D J, Lund V J, Wei W I. Craniofacial resection for tumors of the nasal cavity and paranasal sinuses: a 25-year experience. Head Neck. 2006;28(10):867–873. doi: 10.1002/hed.20432. [DOI] [PubMed] [Google Scholar]
- Gil Z, Abergel A, Spektor S, Shabtai E, Khafif A, Fliss D M. Development of a cancer-specific anterior skull base quality-of-life questionnaire. J Neurosurg. 2004;100(5):813–819. doi: 10.3171/jns.2004.100.5.0813. [DOI] [PubMed] [Google Scholar]
- Chen A M, Daly M E, Bucci M K, et al. Carcinomas of the paranasal sinuses and nasal cavity treated with radiotherapy at a single institution over five decades: are we making improvement? Int J Radiat Oncol Biol Phys. 2007;69(1):141–147. doi: 10.1016/j.ijrobp.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Jansen E P, Keus R B, Hilgers F J, Haas R L, Tan I B, Bartelink H. Does the combination of radiotherapy and debulking surgery favor survival in paranasal sinus carcinoma? Int J Radiat Oncol Biol Phys. 2000;48(1):27–35. doi: 10.1016/s0360-3016(00)00594-0. [DOI] [PubMed] [Google Scholar]
- Katz T S, Mendenhall W M, Morris C G, Amdur R J, Hinerman R W, Villaret D B. Malignant tumors of the nasal cavity and paranasal sinuses. Head Neck. 2002;24(9):821–829. doi: 10.1002/hed.10143. [DOI] [PubMed] [Google Scholar]