Abstract
Trigeminal schwannomas (TS), though the second most common intracranial schwannomas, represent only 0.8 to 8% of all Schwannomas. Advancement in imaging and microsurgical techniques has led to a remarkable improvement in the outcome of these benign tumors. Multicompartmental TS, though extensive, have an excellent outcome after surgery. In this article, we present our experience in the management of multicompartmental TS (types middle/posterior [MP], middle/extracranial [ME], and middle/posterior and extracranial [MPE]) and outcome in this rather uncommon group of tumors. This retrospective study included all the cases of multicompartmental TS operated at our institute from 1999 to 2009. The medical data were analyzed retrospectively. The demographic profile, clinical features, radiological findings, management strategies, postoperative complications, length of hospitalization, and outcome were noted. Follow-up data were collected from outpatient department records. The range and average duration of follow-up were noted. There were a total of 43 patients with TS operated over this period. Among them, 4 were type B, 5 type C, 11 type D, 18 type E, and 5 type F. The study included 26 patients (4 type B, 18 type E, and 4 type B). A variety of approaches were used to approach the tumor. Of 26, 23 patients had a gross total or near-total excision while 2 patients were lost to follow-up. Among the three patients who had a near-total excision and follow-up magnetic resonance imaging showed a small residual tumor, two are on close follow-up with no increase in the size of the tumor over a follow-up period of 3 years, the other patient is a 5-year-old boy who is too young for radiosurgery and is on follow-up. There was no mortality while four patients have had fresh permanent postoperative deficits. Multicompartmental TS are a rare, complex but eminently treatable group of tumors. A variety of surgical approaches can be used to excise the tumor. The choice of approach needs to be individualized with total excision providing excellent results.
Keywords: Trigeminal schwannomas, multicompartmental, review
Trigeminal schwannomas (TS), though the second most common intracranial schwannomas represent only 0.8 to 8% of all schwannomas.1 Advancement in imaging and microsurgical techniques has led to a remarkable improvement in the outcome of these benign tumors. Described first by Smith in 1849, schwannomas can arise from any segment of the trigeminal complex (the trigeminal nerve root, Gasserian ganglion, or one of the three divisions of the nerve or a combination of the above). Based on the origin they can be unicompartmental or multicompartmental.2 The tumors almost always arise from one of the sensory fascicles of the nerve or root; therefore, some type of facial numbness is invariably present. These tumors usually displace the carotid artery and the cranial nerves (CNs) inside the cavernous sinus or the petrous bone, rather than encasing them. Thus, preservation of these structures during surgery is possible in most cases.2 However, Goel et al hypothesized that TS usually arise in the point where the CNV enters the Meckel cave.3 In the middle fossa, the tumor is located interdurally while it is intradural in the posterior fossa. Initially classified by Jefferson and later modified into types A, B, C, and D, there have been other classifications4 notably by Ramina et al and Yoshida and Kawase (Tables 1 and 2). In this article we present our experience in the management of TS types B, E, and F of Ramina et al and middle and posterior fossae (MP), middle fossa and extracranial space (ME), and middle and posterior fossae and extracranial space (MPE) types of Yoshida and Kawase, that is, the multicompartmental TS.
Table 1.
Modified Classification (Ramina et al)
| A—Predominantly extracranial with small extension in middle fossa |
| B—Intracranial predominantly in middle fossa with extracranial extension |
| C—Middle fossa |
| D—Posterior fossa |
| E—Middle and posterior fossa |
| F—Middle, posterior, and extracranial extension |
Table 2.
Classification Given by Yoshida/Kawase
| M—Middle fossa |
| P—Posterior fossa |
| E—Extracranial space |
| MP—Middle/posterior |
| ME—Middle/extracranial |
| MPE—middle and posterior fossae and extracranial space |
MATERIALS AND METHODS
This retrospective study included all the cases of multicompartmental TS operated at our institute from 1999 to 2009. The medical data were analyzed retrospectively. The demographic profile, clinical features, radiological findings, management strategies, postoperative complications, length of hospitalization, and outcome were noted. The pathology of the tumors was reconfirmed via reexamination of the histological slides. Follow-up data were collected from outpatient department records. The range and average duration of follow-up were noted.
RESULTS
There were a total of 43 patients with TS operated over this period. Among them, 4 were type B, 5 type C, 11 type D, 18 type E, and 5 type F. Of the 27, types B, E, and F, the records of one patient were not available for follow-up and were therefore excluded from this study. There were four cases of type F, which is the least common of all the types with only four being recorded in available literature until 2007.5
Clinical and Demographic Features
There were a total of 26 cases operated over a period of 10 years (Figs. 1 to 6).The male to female ratio was 1.7:1. The average age of presentation was 32 years with a range of 5 to 70 years. The duration of symptoms ranged from 2 months to 3 years with an average duration of 8 months. The clinical features are elaborated in Table 3. It is important to note that the motor division of CNV was involved in 90% of cases. Three patients presented with pathological laughter, which subsided after surgery. One patient presented with history of acute deterioration, the radiology was suggestive of intratumoral hemorrhage. A relatively large number of patients (63%) had long tract signs which may point to the rather delayed presentation of our patients, which is fairly common in India due to a variety of socioeconomic factors. This also probably explains the relatively large number of patients who required a ventriculoperitoneal shunt preoperatively.
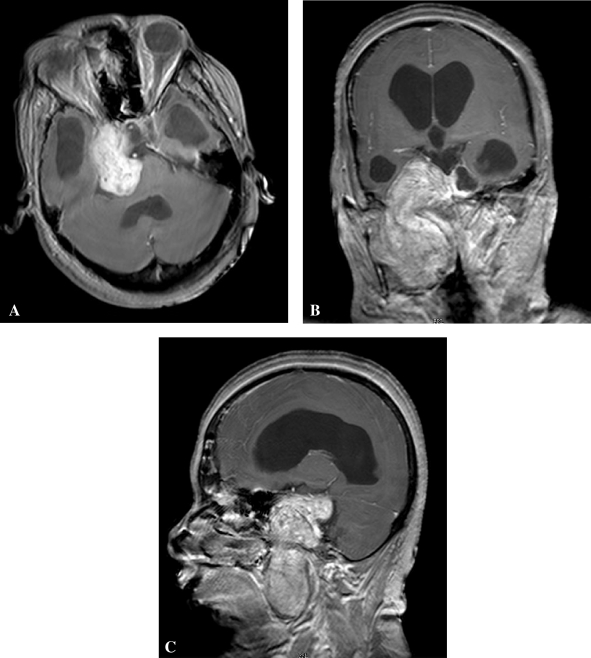
Figure 1.
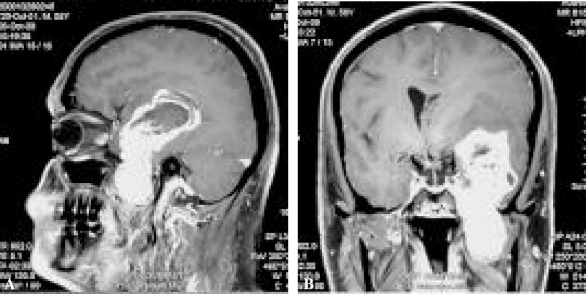
Preoperative magnetic resonance imaging (postcontrast—axial [A], sagittal [B], coronal [C]) showing a large type F (MPE) trigeminal schwannoma.
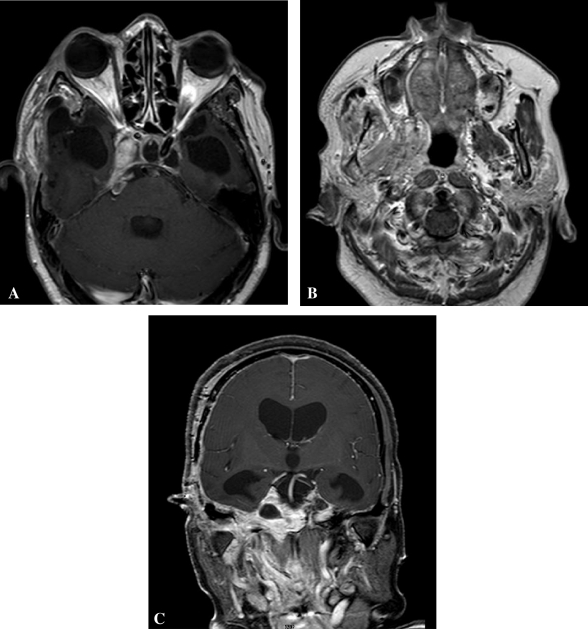
Figure 2.
Postoperative magnetic resonance imaging (Postcontrast—axial [A], axial [B], coronal [C]) showing near-total excision of the tumor with a small residual lesion in the area of the cavernous sinus.
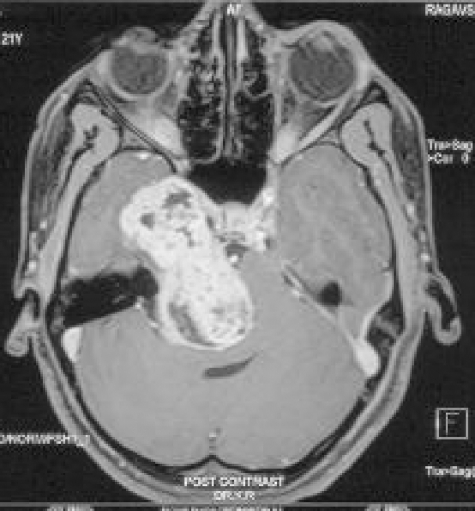
Figure 3.
Preoperative magnetic resonance imaging (Postcontrast—axial) showing a large type E (MP) trigeminal schwannoma.
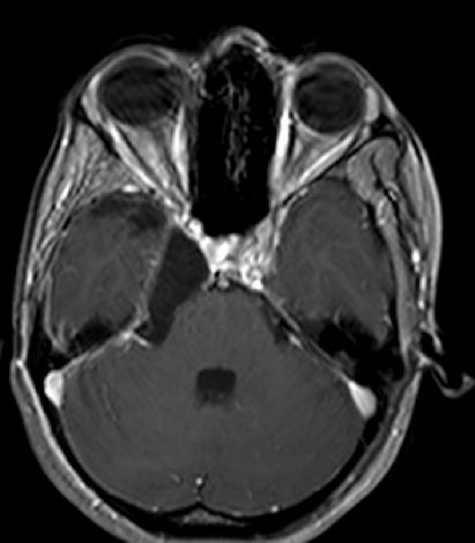
Figure 4.
Postoperative magnetic resonance imaging scan (Postcontrast—axial) showing gross total excision of the tumor.
Figure 5.
Preoperative magnetic resonance imaging (Postcontrast—sagittal (5), coronal (5) showing a large type B (ME) trigeminal schwannoma.
Figure 6.
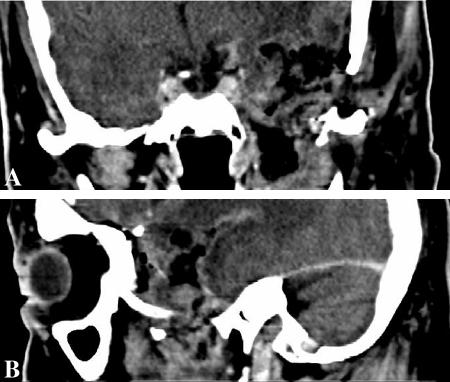
Postoperative computed tomography scan (Postcontrast—coronal (6), sagittal (6) showing near-total excision of the tumor.
Table 3.
Clinical Features at Presentation (Includes Both Symptoms and Signs)
| Clinical Features | No. of Patients | Percentage |
|---|---|---|
| CNV—Sensory | 26 | 100 |
| CNV—Motor | 24 | 90 |
| CNVI | 6 | 34 |
| CNVII | 16 | 74 |
| CNVIII | 16 | 74 |
| Lower cranial nerves | 9 | 42 |
| Long tract signs | 13 | 63 |
| Cerebellar signs | 13 | 63 |
| Pathological laughter | 3 | 15 |
CN, cranial nerve.
Radiology
There were a total of 26 patients (4 type B, 18 type E, and 4 type F tumors). Cystic changes were rather uncommon and seen in only three patients. The petrous apex was eroded in all patients signifying the rather large size of the tumor at presentation. In two patients the tumor had extended into the cavernous sinus. One patient presented with features of intratumoral bleed.
Surgical Strategies
The tumors were approached by a variety of strategies. The distribution is given in Table 4. All the type B tumors were approached through a frontotemporal craniotomy, orbitozygotomy, and excision. However, there was a difference in the approach to the type E and F tumors. We compared the strategies for type E and F tumors by broadly dividing the approaches into a single-stage and a two-stage approach. In the two-stage approach the posterior fossa component was excised followed by the middle fossa component at a later stage. The intraoperative comparison is given in Table 5. The two-stage approach was primarily used in those patients with a large posterior fossa component and a relatively small middle fossa component with clinical evidence of brainstem compression. This approach, however, has the disadvantage that it is difficult to remove the middle fossa component completely through this route, and often necessitates a second stage. This also has a disadvantage in India, in that the patient can be lost to follow-up, as it happened in two of our patients.
Table 4.
Surgical Approaches to Type E and F Tumors
| Single Stage (n = 10) | |
| Frontotemporal craniotomy/GTE, NTE | 2 |
| FTOZ + GTE/NTE | 5 |
| FTOZ + mandibular swing + NTE | 1 |
| FT + petrosectomy + GTE | 1 |
| Combined middle/posterior fossa + STE | 1 |
| Staged Surgeries (n = 9) | |
| NTE/GTE | 6 |
| Awaiting surgery | 1 |
| Lost to follow-up (after first stage) | 2 |
Table 5.
Single Versus Staged Surgery: Operative Comparison
| Single Stage | Two Stage | |
|---|---|---|
| Duration | 9 h | 13 h |
| Blood loss | 1100 mL | 1700 mL |
| Crystalloids | 3300 mL | 7000 mL |
| Colloids | 125 mL | − |
| Blood units given | 2 units | 2 units |
| Intraoperative events | − | − |
A petrosectomy is rarely required, due to the petrous apex erosion by the tumor; in our series only one patient required it. There was no mortality. Seven patients developed complications. One patient developed meningitis, chest infection, and cerebrospinal fluid (CSF) leak which subsided with antibiotics and temporary CSF drainage. Six patients developed fresh CN deficits, which remained persistent in three. These included two CNVII and one CNIII deficits. One patient developed ipsilateral superior cerebellar artery (SCA) infarction, following a middle fossa approach, to a type E tumor. He required posterior fossa decompression but recovered with ipsilateral cerebellar signs. The follow-up period ranged from 1 month to 8 years with an average of 4.3 years. Among the 22 patients with type E/F tumors, follow-up magnetic resonance imaging (MRI) revealed that 16 patients had no residual tumors, while 3 patients had a small residue. These patients are on regular follow-up and the tumor size has been stable for 3 years in two. One of these three patients is a 5-year-old boy who is on close follow-up as we felt he was too young for radiosurgery. Two patients have been lost to follow-up, while one patient has a residual infratemporal component and is awaiting surgery. We were able to achieve gross total excision and near-total excision in all the four patients with type B TS. Our results are comparable to major series on multicompartmental TS2,3,4,6,7 (Table 6).
Table 6.
Literature Review
| Series | Years | Cases | NTE/GTE | Mort/Morb | Follow-Up |
|---|---|---|---|---|---|
| Samii et al (1995)4 | 10 | 6 | 6 (100%) | Nil | 12–60 mo |
| Yoshida and Kawase (1999)2 | 25 | 13 | 12 (92%) | − | |
| Goel et al (2003)3 | 12 | 37 | (89%) | 1.8%/4.5% | 6–120 mo |
| Al-Mefty (1996)7 | 11 | 19 | 19/19 (100%) | 0/3 | 3–134 mo |
| Zhou et al (2007)6 | 25 | 57 | 44 (77%) | 1/9 | 2–20 y |
| Current study (2009) | 10 | 26 | 25/26 (92%) | 0/4 | 1–96 mo |
GTE, gross total excision; Morb, morbidity; Mort, mortality; NTE, near-total excision.
DISCUSSION
Meckel cave named after Johann Friedrich Meckel is a dural cleft extending from the posterior fossa to the posteromedial middle fossa. The TS are said to usually arise from the trigeminal nerve in the Meckel cave.3 Thus they expand the cave and provide a natural route to the posterior fossa when being approached from the middle cranial fossa. This along with the erosion of the petrous apex provides excellent access to the posterior fossa component making a petrosectomy usually unnecessary. The trigeminal nerve, which is intradural in the posterior fossa, becomes interdural in this cave thus allowing an interdural approach. The clinical features depend on the size and extent of the tumor. However, CNV is invariably involved. Rare symptoms, which have occurred, are intratumoral hemorrhage and pathological laughter. Imaging in form of an MRI and CT are a necessity along with either a CT angiogram or magnetic resonance angiogram. This is to delineate the exact extent of the tumor, its relations, the extent of erosion of the petrous bone, the course and relation of the internal carotid artery all of which help in the surgical planning.
In our series a variety of approaches have been used. We preferred a middle fossa interdural approach for tumors with a large middle fossa component. A standard frontotemporal craniotomy along with an orbitozygotomy is used. Like mentioned above, the approach to the posterior fossa is often without any petrosectomy. However, coagulation of the superior petrosal sinus and sectioning of the dura can aid the approach. As the tumor arises from the rootlets the preservation of the branches of CNV is almost always possible. The use of Cavitron suction aspiration greatly aids the decompression. Advances in microsurgery and a better understanding of the microsurgical anatomy have helped increase the number of gross total and near-total excisions (Table 6). One of the main risks of approaching the posterior fossa component through the middle fossa is the risk of injury to the SCA, which happened in one of our patients. The goal is gross total or near-total excision, which can be invariably achieved. Small residual tumors (<3 cm) in diameter can be treated by Gamma Knife radiosurgery.
CONCLUSION
Multicompartmental TS are a rare, complex but eminently treatable group of tumors. A variety of surgical approaches can be used to excise the tumor with minimal morbidity. The choice of approach needs to be individualized with total excision providing excellent results.
References
- Ramina R, Neto M C, Fernandes Y B, Leal A G, da Silva E B. The surgical management of trigeminal schwannomas. In: Ramina R, Aguiar H P, Tatagiba M, editors. Samii's Essentials in Neurosurgery. New York: Springer; 2008. pp. 155–164. [Google Scholar]
- Yoshida K, Kawase T. Trigeminal neurinomas extending into multiple fossae: surgical methods and review of the literature. J Neurosurg. 1999;91(2):202–211. doi: 10.3171/jns.1999.91.2.0202. [DOI] [PubMed] [Google Scholar]
- Goel A, Muzumdar D, Raman C. Trigeminal neuroma: analysis of surgical experience with 73 cases. Neurosurgery. 2003;52(4):783–790. discussion 790. doi: 10.1227/01.neu.0000053365.05795.03. [DOI] [PubMed] [Google Scholar]
- Samii M, Migliori M M, Tatagiba M, Babu R. Surgical treatment of trigeminal schwannomas. J Neurosurg. 1995;82(5):711–718. doi: 10.3171/jns.1995.82.5.0711. [DOI] [PubMed] [Google Scholar]
- Kouyialis A T, Stranjalis G, Papadogiorgakis N, et al. Giant dumbbell-shaped middle cranial fossa trigeminal schwannoma with extension to the infratemporal and posterior fossae. Acta Neurochir (Wien) 2007;149(9):959–963. discussion 964. doi: 10.1007/s00701-007-1173-6. [DOI] [PubMed] [Google Scholar]
- Zhou L F, Mao Y, Zhang R. Surgical treatment of dumbbell-shaped neurinomas: report of an experience with 57 cases in a single hospital. Surg Neurol. 2007;68(6):594–602. doi: 10.1016/j.surneu.2006.12.065. [DOI] [PubMed] [Google Scholar]
- Al-Mefty O, Ayoubi S, Gaber E. Trigeminal schwannomas: removal of dumbbell-shaped tumors through the expanded Meckel cave and outcomes of cranial nerve function. J Neurosurg. 2002;96(3):453–463. doi: 10.3171/jns.2002.96.3.0453. [DOI] [PubMed] [Google Scholar]