Abstract
The objective of this study was to assess the long-term survival of patients with a paraganglioma of the head and neck compared with the survival of the general Dutch population. This historic cohort study was conducted using nationwide historical data of paraganglioma patients. We retrieved a cohort of 86 patients diagnosed with a paraganglioma of the head and neck between 1945 and 1960 in the Netherlands. Dates of death were retrieved from the national bureau of genealogy. Survival after diagnosis was compared with age and sex adjusted survival in the general population, by means of Wilcoxon signed rank test and Kaplan-Meier actuarial survival curves. Although surgery had more complications in the studied era than today and the death of five patients with carotid body tumors caused immediate excess mortality, the survival of the followed cohort was not significantly reduced if compared with the general population. Paragangliomas of the head and neck do not reduce life expectancy.
Keywords: Paraganglioma, carotid body tumor, glomus tumor, survival
Paragangliomas are neuroendocrine tumors originating from paraganglia of the autonomic nervous system. Head and neck paragangliomas are parasympathetic paragangliomas that mostly occur at the carotid bifurcation (carotid body tumors) but also along the vagal nerve and in the temporal bone. Malignant degeneration is rare and cannot be predicted histologically; hence metastasis is regarded as the only true sign of malignancy.1 Paragangliomas usually grow extremely slowly and the primary cause of morbidity in patients with paragangliomas is cranial nerve impairment caused by compression or infiltration of the nerves.2 Unfortunately, surgery of paragangliomas often leads to similar palsies, which makes the management of these tumors a difficult choice between the natural course of the disease and the expected benefits of treatment.3,4,5
The survival of paraganglioma patients has not extensively been studied. One would expect paragangliomas to have a negative effect on survival. Aspiration pneumonia is a common complication after loss of lower cranial nerves and intracranial extension of tumor may cause lethal compression of the brainstem. Surgery of paragangliomas is dangerous: review of more than 4000 American carotid body tumor cases proved a mortality of 2% that increased to 8.8% in the 855 cases where an endarterectomy was performed.6 Malignancy is reported in variable frequency but may be more than 5%.7,8 Finally, concomitant pheochromocytomas may cause cardiac arrest in patients with hereditary paragangliomas.9,10 However, these are long-term risks and most patient series quote high 5- and 10-year survival rates of head and neck paragangliomas. In the only published controlled series Nora et al reported equivalent survival of 55 patients after carotid body surgery compared with matched controls.11
To evaluate the survival of Dutch patients with paragangliomas we studied the cohort of 86 patients diagnosed with head and neck paraganglioma between 1945 and 1960 and published by the Dutch surgeon R.A.R. Elders in his 1962 PhD thesis, allowing us to evaluate more than 50 years of follow-up.12
PATIENTS, MATERIALS, AND METHODS
In 1960, Elders performed a nationwide enquiry among general surgeons, ear, nose, and throat surgeons, neurosurgeons, and pathologists for histologically proven paragangliomas diagnosed between 1945 and 1960. For the present study we included patients with a head and neck paraganglioma and recorded demographic data, the chosen therapy, and the clinical state of the patient at the last contact with their physicians. A positive family history, genealogically proven relationship with ascertained paraganglioma families, and the occurrence of multiple paraganglioma, were considered to be proof of a hereditary cause.
For all patients we sent an inquiry to the Central Bureau of Genealogy that receives and publishes the records of all deaths in The Netherlands 2 years after they have been accounted for by the local authorities. If no death certificate was issued, patients were censored at the last recorded date alive.
The age of diagnosis was considered to be the reference date for survival analysis. From the online database of the Dutch Central Bureau of Statistics (www.cbs.nl) we retrieved the life expectancy of the general Dutch population for which the cases were matched for age and sex, stratified to year of birth. We therewith could compare the actual age the patients reached with their statistically expected life span at the time of diagnosis.
Comparative analysis of survival was performed with Wilcoxon rank sum test which compares paired continuous variables. In addition to analysis of the total group, the patients with carotid body tumors and those with temporal bone paragangliomas were compared separately. Second a Kaplan-Meier actuarial survival curve was constructed for patients and controls to depict the actual survival in years.
All statistical tests were applied two-sided, and p values less than 0.05 were considered statistically significant. Statistical analysis was performed with SPSS statistical software (version 14.0 for Windows).
RESULTS
The 86 patients accounted for 49 carotid body tumors, 36 temporal paragangliomas, and 1 vagal body tumor (this patient also had a tumor of the carotid body and was included in that group without separate analysis). Six patients had multiple tumors. Eight patients had a positive family history and another 16 could be included in a pedigree of known paraganglioma patients. Together 28 patients (33%) were considered hereditary cases.
The median age at diagnosis was 43 years (mean 42.25 years; range, 16 to 77 years). Women were more frequently affected than men, 51 (59%) versus 35 (41%) (Table 1).
Table 1.
Results of Paraganglioma Patients
| Total | TBP | CBT | |
|---|---|---|---|
| No. of patients | 86 | 36 | 50 |
| Sex | |||
| Male | 35 (41%) | 14 (39%) | 21 (42%) |
| Female | 51 (59%) | 22 (61%) | 29 (58%) |
| Mean age at diagnosis | 42.25 (16–77) | 44 (18–77) | 40.24 (16–63) |
| Mean survival* | 26.40 (0–56) | 29.56 (3–50) | 25.27 (0–56) |
| Mean expected survival | 28.02 (5.8–52.2) | 26.72 (5.8–52.2) | 29.65(12.4–51.3) |
| Mean difference from expected | −1.59 (−42–21) | 2.84 (−27–20) | −4.33 (−42–21) |
CBT, carotid body tumor; TBP, temporal bone paraganglioma.
Patients with a confirmed date of death (n = 69).
All patients with a carotid body tumor were treated surgically, although in four patients only a biopsy was taken. Five patients died as a complication of surgery within 7 days postoperatively. Sixteen patients (32%) had central neurological symptoms (aphasia, hemiplegia) after surgery, only four patients recovered without remaining symptoms. Cranial nerve deficit before or after surgery was not documented unequivocally.
Patients with a paraganglioma of the temporal bone where treated surgically (n = 9), with radiotherapy (n = 10), or a combination of both (n = 14). Three tumors were not treated. There was no surgical mortality in these series. Morbidity in this group was mainly due to cranial nerve deficit. Fourteen patients did not have cranial nerve impairment, the other 12 patients all had 1 or more cranial nerves affected. Whether cranial nerve deficit was induced by treatment or by the natural course of the tumor could not be established.
In 17 out of 86 patients, a date of death could not be found. Three patients were confirmed to be alive after 39 years, 48 years, and 50 years follow-up, respectively. Two patients moved abroad and hence do not have a record in the Dutch registry, the fate of the remaining 12 patients remains unknown. Together this accounts for 16% missing data.
The mean survival of the complete group was 26.40 years after diagnosis, whereas the mean expected survival was 28.02 years. The average loss of life expectancy is therefore 1.62 years. Separately analyzed, patients with carotid body tumors lost 4.33 years, which is explained by the surgical mortalities. Wilcoxon signed rank test showed these figures not to be of significant difference (p = 0.998).
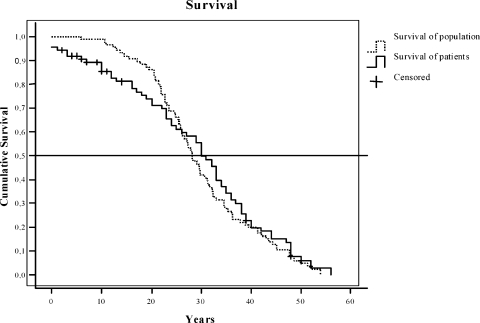
Fig. 1 shows the Kaplan-Meier survival curves of the paraganglioma patients and the survival in the general population. The median cumulative survival of the patients is 30 years compared with 29.55 in the general population (mean 28.87 vs 31.70 years). The difference between the two curves in the first 20 years can largely be explained by the postoperative mortality in the carotid body tumor group.
Figure 1.
Kaplan-Meier survival curve: survival of patients (solid line) and survival of the general population (dotted line) in years (n = 86).
DISCUSSION
In the present study we describe the survival of patients with a paraganglioma of the head and neck compared with the general population where we did not find a significant difference. The unexpected deaths that did occur in the study group were due to surgical complications, accounting to a 10% mortality rate in carotid body tumor surgery. Today surgical skills and techniques together with improved postoperative care have reduced this mortality to an approximate 2% and probably lower in skilled hands.8 This is in accordance to the study by Nora et al who, with a much shorter follow-up, had similar results in 55 surgically treated carotid body tumor patients. This also confirms the generally excellent, 5- and 10-year survival rates quoted by surgeons and radiotherapists.
These results are nonetheless remarkable. Surgical mortality, morbidity, and metastases are the serious dangers that threaten these patients. Apparently these dangers are not so grave that they decrease the life expectancy of the entire patient group.
As this series is the best recorded effort to acquire a nationwide cohort of paraganglioma patients we must consider the figures as the most reliable available, but it can be questioned whether the current study group was a favorable selection. As only histologically proven cases were included, large tumors deemed inoperable might have been excluded. Based on our estimate of the incidence of paraganglioma (0.15/100,000/y), the number of included patients is not more than 50% of the patients that must have been present between 1945 and 1960. The series, however, contains a large number of tumors that only have been biopsied or irradically removed accounting for inoperable tumors. Our experience is that especially patients with little or no symptoms refrained from seeking medical intention in that time.13 Moreover our data suggest that the untreated patients had at least a similar outcome compared with those patients treated surgically. Elders already addressed the absence of more vagal body tumors in this series in his thesis. Based on the described extension four cases of alleged carotid body tumors could actually have been vagal body tumors.
It is also possible that the 12 cases in our series, whose fate is unknown, are confounding the results of the present study, but, given the comprehensiveness of the civil registration, it is more likely that we have excluded living patients. Kaplan-Meyer analysis takes missing cases into account and still yields a strikingly similar curve for patients and controls.
Because the majority of the Dutch cases of paraganglioma caused by inherited mutations in the SDHD gene, it is very well possible that sporadic paragangliomas, similar to paragangliomas caused by SDHB mutations, have a different, more aggressive natural course, although this is not supported by the findings of Nora et al.
It is of importance to note that, even though no decreased residual life span can be attributed to head and neck paragangliomas, the quality of life of these patients is reduced by the disease.14 This is illustrated by the high percentage of cerebrovascular injury after surgery of the carotid body tumors in this study (32%). The figures have improved in the last decades but are still a matter of concern.15 Recently we measured a substantially decreased quality of life in our patient group, partially attributed to cranial nerve injury but also caused by general symptoms such as fatigue that may be related to altered ventilatory regulation.16
We conclude that head and neck paragangliomas have no important effects on survival. Treatment of a head and neck paraganglioma should focus on preservation of the quality of life rather than on cure of the disease.
References
- Warshawski S J, de Souza F M. The carotid body tumor. J Otolaryngol. 1989;18(6):306–310. [PubMed] [Google Scholar]
- Jansen J C, den Berg R van, Kuiper A, der Mey A G van, Zwinderman A H, Cornelisse C J. Estimation of growth rate in patients with head and neck paragangliomas influences the treatment proposal. Cancer. 2000;88(12):2811–2816. [PubMed] [Google Scholar]
- Hallett J W, Jr, Nora J D, Hollier L H, Cherry K J, Jr, Pairolero P C. Trends in neurovascular complications of surgical management for carotid body and cervical paragangliomas: a fifty-year experience with 153 tumors. J Vasc Surg. 1988;7(2):284–291. [PubMed] [Google Scholar]
- Gjuric M, Rüdiger Wolf S, Wigand M E, Weidenbecher M. Cranial nerve and hearing function after combined-approach surgery for glomus jugulare tumors. Ann Otol Rhinol Laryngol. 1996;105(12):949–954. doi: 10.1177/000348949610501204. [DOI] [PubMed] [Google Scholar]
- Sajid M S, Hamilton G, Baker D M, Joint Vascular Research Group A multicenter review of carotid body tumour management. Eur J Vasc Endovasc Surg. 2007;34(2):127–130. doi: 10.1016/j.ejvs.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Maxwell J G, Jones S W, Wilson E, et al. Carotid body tumor excisions: adverse outcomes of adding carotid endarterectomy. J Am Coll Surg. 2004;198(1):36–41. doi: 10.1016/j.jamcollsurg.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Havekes B, Corssmit E P, Jansen J C, der Mey A G van, Vriends A H, Romijn J A. Malignant paragangliomas associated with mutations in the succinate dehydrogenase D gene. J Clin Endocrinol Metab. 2007;92(4):1245–1248. doi: 10.1210/jc.2006-1993. [DOI] [PubMed] [Google Scholar]
- Boedeker C C, Neumann H P, Maier W, Bausch B, Schipper J, Ridder G J. Malignant head and neck paragangliomas in SDHB mutation carriers. Otolaryngol Head Neck Surg. 2007;137(1):126–129. doi: 10.1016/j.otohns.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Maxwell J G, Jones S W, Wilson E, et al. Carotid body tumor excisions: adverse outcomes of adding carotid endarterectomy. J Am Coll Surg. 2004;198(1):36–41. doi: 10.1016/j.jamcollsurg.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Noshiro T, Shimizu K, Watanabe T, et al. Changes in clinical features and long-term prognosis in patients with pheochromocytoma. Am J Hypertens. 2000;13(1 Pt 1):35–43. doi: 10.1016/s0895-7061(99)00139-9. [DOI] [PubMed] [Google Scholar]
- Nora J D, Hallett J W, Jr, O'Brien P C, Naessens J M, Cherry K J, Jr, Pairolero P C. Surgical resection of carotid body tumors: long-term survival, recurrence, and metastasis. Mayo Clin Proc. 1988;63(4):348–352. doi: 10.1016/s0025-6196(12)64856-3. [DOI] [PubMed] [Google Scholar]
- Elders R AR. Paraganglioma. [Ph.D. dissertation] Groningen: University of Groningen; 1962. [Google Scholar]
- der Mey A G van, Frijns J H, Cornelisse C J, et al. Does intervention improve the natural course of glomus tumors? A series of 108 patients seen in a 32-year period. Ann Otol Rhinol Laryngol. 1992;101(8):635–642. doi: 10.1177/000348949210100802. [DOI] [PubMed] [Google Scholar]
- Netterville J L, Civantos F J. Rehabilitation of cranial nerve deficits after neurotologic skull base surgery. Laryngoscope. 1993;103(11 Pt 2, Suppl 60):45–54. doi: 10.1002/lary.1993.103.s60.45. [DOI] [PubMed] [Google Scholar]
- Westerband A, Hunter G C, Cintora I, et al. Current trends in the detection and management of carotid body tumors. J Vasc Surg. 1998;28(1):84–92. discussion 92–93. doi: 10.1016/s0741-5214(98)70203-4. [DOI] [PubMed] [Google Scholar]
- Havekes B, der Klaauw A A van, Hoftijzer H C, et al. Reduced quality of life in patients with head-and-neck paragangliomas. Eur J Endocrinol. 2008;158(2):247–253. doi: 10.1530/EJE-07-0464. [DOI] [PubMed] [Google Scholar]