Abstract
The study was conducted to analyze outcomes following surgical management of large and giant vestibular schwannomas and management options for residual disease. This retrospective case note study includes patients who had undergone microsurgical resection of sporadic, large, or giant vestibular schwannomas from 1986 to 2008. Tumors are classified as large if the largest extracanalicular diameter was 3.5 cm or greater and giant if 4.5 cm or greater. The study included 45 patients (33 large, 12 giant tumors), mean tumor size 4.1 cm. Total excision was achieved in 14 cases (31.1%), near-total in 26 (57.8%), and subtotal in 5 (11.1%). Facial nerve outcome was House-Brackmann Grade I/II in 25 cases (55.6%), III/IV in 16 (35.6%), and V/VI in 4 (8.9%). No recurrence has been detected in those undergoing a complete resection. No residual tumor growth been observed in 15 of 26 who underwent near-total resection (57.7%). Of 11 patients, 10 received further treatment as their residual tumors showed growth. In the subtotal excision group, one patient died, three have demonstrated no growth, and one residual tumor has grown slightly but not required intervention. Optimal management for patients with large or giant vestibular schwannomas has yet to be determined. Management decisions must balance long term function with tumor control.
Keywords: Large vestibular schwannomas, management, residual disease, stereotactic radiotherapy
Over the last few decades there has been a significant progress in our understanding of the natural history and pathophysiology of vestibular schwannoma. This has prompted changes in our management strategies. As approximately two-thirds of vestibular schwannomas do not grow after initial diagnosis within the lifetime of the patient, many small tumors are managed by observation with serial imaging.1,2,3 Furthermore, a small proportion of tumors may even regress. Patients with small tumors that grow can be treated by either stereotactic radiotherapy or surgical resection.
The situation is much more difficult for patients with larger tumors that are closer to 3 cm or even larger in maximum diameter. Most surgeons would consider resection as the first-line treatment for patients with tumors of this magnitude, but several radiosurgeons are happy to treat patients with tumors that are 3 cm in maximal diameter and some even larger.4,5 There is consensus that some tumors are too large for radiation therapies and for these patients surgery is the only treatment option. For some there is a further dilemma about how extensive surgery should be, as potential morbidities have a significantly adverse effect on quality of life.
Unfortunately, little guidance is available from the medical literature. The problem is compounded by nonstandardized measurement of tumors despite internationally agreed criteria.6 What for one surgeon would be a 3-cm tumor is another's 4-cm tumor, if the latter includes the intracanalicular component. This factor alone confuses the interpretation of published outcome data. Furthermore, there is the problem of measuring the quantity of any residual disease as volumetric measurement software has yet to become universally available. Finally, if residual disease shows further growth, there is no consensus on optimal management at the present time.
The aim of this study was to review all patients with large or giant sporadic vestibular schwannomas treated in our skull base center over a 23-year period, to document the extent of resection, to evaluate their postoperative progress, and detail any interventions that became necessary subsequently.
MATERIALS AND METHODS
This was a retrospective case note study of patients who had undergone microsurgical resection of sporadic, large, or giant vestibular schwannomas at Kings College Hospital and the National Hospital for Neurology and Neurosurgery by the senior authors between 1986 and 2008. Patients with neurofibromatosis type 2 were excluded. For the purposes of this study, we considered tumors bigger than 3.5 cm in their greatest extracanalicular dimension as “large” and those bigger than 4.5 cm as “giant.” The rationale behind these size criteria was that most radiation oncologists would treat 3 cm tumors but few would irradiate tumors larger than 3.5 cm. Preoperative neurological deficits other than hearing loss were recorded and the extent of resection was classified into total (no tumor remaining), near-total (≥95% tumor removal), and subtotal (<95% tumor removal), based on postoperative imaging. To demonstrate this classification, examples are shown in Figs. 1–3. Major postoperative complications, final outcome of trigeminal and facial nerve function together with any further interventions were documented.
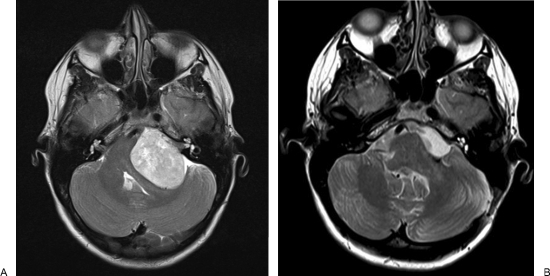
Figure 1.
(A, B) Pre- and postoperative magnetic resonance scans illustrating a totally resected tumor in a young woman.
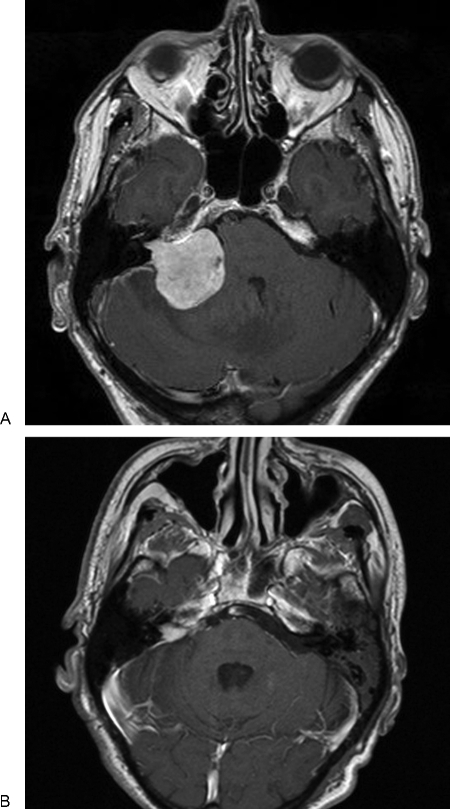
Figure 2.
(A, B) Pre- and postoperative magnetic resonance scans of a near-total resection undertaken in a 77-year-old man who had become confined to a wheel chair. He made a complete recovery and regained mobility.
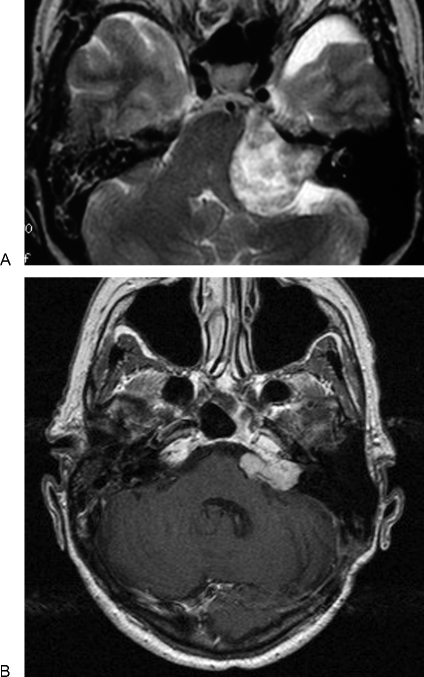
Figure 3.
(A, B) Pre- and postoperative magnetic resonance scans of a subtotal resection undertaken in a 74-year-old woman. At operation, it became apparent that a complete resection would inflict a permanent complete facial palsy.
RESULTS
Over a 23-year period between 1986 and 2008, 59 cases that met the inclusion criteria were identified from a series of 784 vestibular schwannoma resections. Of these, complete data were obtained in 45 cases (76.3%). There were approximately twice as many females as males (M:F = 16:29). The mean age of patients at presentation was 43 years (median 37 years; range, 16 to 84 years). Based on our size criteria, there were 33 patients with large tumors and 12 with giant tumors. The mean tumor size was 4.1 cm (median 4.0 cm; range, 3.5 to 5.5 cm). Two patients had received Gamma Knife radiotherapy prior to surgery. Preoperatively, 25 patients were ataxic, 23 had trigeminal deficits, and 3 had facial nerve weakness.
Total excision was achieved in 14 patients (31.1%), near-total in 26 patients (57.8%), and subtotal in 5 patients (11.1%). One patient died in the early postoperative period from brainstem infarction. One patient with a 4.5-cm tumor had staged surgeries. The initial procedure, attempted in the park bench position, had to be discontinued due to excessive hemorrhage. A second operation with the patient in the sitting position was successful and achieved a near-total resection. One patient had a cerebrospinal fluid (CSF) leak. The final facial nerve outcome was House-Brackmann (HB) grade I/II in 25 patients (55.6%), III/IV in 16 patients (35.6%), and V/VI in 4 patients (8.9%). Of 25, 6 patients with preexisting ataxia reported no improvement postoperatively and 3 patients developed ataxia following surgery. Six patients saw no improvement in their trigeminal deficits postoperatively and one patient developed a new deficit in all three divisions of the trigeminal nerve.
Total Excision Group
No recurrence has developed to date in any of the patients in whom a complete resection had been achieved, with a mean follow-up of 40.5 months (median 32.0 months; range, 12 to 112 months). Of 14 patients, 12 (85.7%) had large tumors by our criteria. One patient had received Gamma Knife radiotherapy prior to surgery. All patients had normal facial nerve function preoperatively. The final facial nerve outcome deteriorated to HB grade III/IV in six patients (42.8%). The patient who had received prior Gamma Knife radiotherapy achieved a grade IV result. Further three patients (21.4%) reported new ataxia postoperatively, but all described it as “mild.” No patient experienced new trigeminal deficits.
Near-Total Excision Group
No further growth in the residual tumor was observed in 15 out of 26 patients (57.7%) who had undergone near-total resection, including one patient in whom the residual tumor showed regression over 3 years following surgery. The mean follow-up period was 63.7 months (median 41.0 months; range 22 to 264 months). In this subgroup, 10 patients (66.7%) had large tumors and 5 had giant tumors (33.3%). In the remaining 11 patients (42.3%) who showed tumor regrowth, the proportions of large and giant tumors were similar (54.5 vs 45.5%). There were 23 patients (88.5%) who had normal facial nerve function before surgery. Of these, the final facial nerve outcome deteriorated to HB grade III or worse in 11 patients (47.8%), (7 to grade III/IV and 4 to grade V/VI). Of the three patients with preexisting facial nerve weakness, one improved slightly (grade IV to III), another remained the same (grade II), and the other deteriorated slightly (grade II to III).
Of 11 patients, 10 received further treatment as their residual tumors showed growth. The mean interval between the primary microsurgical resection and further intervention(s) was 59.2 months (median 66.0 months; range, 10 to 84 months). Six patients were treated with Gamma Knife radiotherapy, including one patient who received a second treatment. All have stable disease to date following treatment, with a mean follow-up of 87.3 months (median 91.5 months; range, 41 to 125 months). One patient received planned adjuvant Gamma Knife radiotherapy after revision surgery and has not shown further regrowth for 4 years. Three patients initially underwent revision surgery only of the growing residual disease, but two of these patients subsequently received interval Gamma Knife radiotherapy for control of further tumor growth 2 years and 6 years, respectively, after the second surgery. Apart from one patient who felt that his preexisting trigeminal deficits might have worsened following Gamma Knife radiotherapy, no other new or worsening neurological deficits were reported.
Subtotal Excision Group
All five patients in whom subtotal resection was only possible had large tumors, including one patient who had continuing growth of the tumor despite Gamma Knife radiotherapy 12 months previously. The residual tumor in one patient has continued to grow but only very slightly and over a period of 10 years. It has not required any further intervention to date. One patient who had a subtotal resection of a 3.5-cm tumor died in the early postoperative period from brainstem infarction. In the remaining three patients, no further growth has been observed over a mean follow-up period of 68.3 months (median 63.0 months; range, 46 to 96 months). All patients had normal facial nerve function preoperatively and had excellent facial nerve functions subsequently (three grade I and one grade II).
DISCUSSION
It is difficult to compare our figures to outcomes reported by other groups as most previous studies included smaller tumors and very few focused on tumors of this magnitude.7,8,9 It is evident that surgical morbidity increases with both the size of the tumor and the extent of its removal.9,10 Our data show that residual tumor may not grow and can be safely observed by serial scans. Furthermore, complete resection may not be possible without incurring significant permanent neurological deficits that have a huge impact on the patient's quality of life. While not disputing that complete resection should be the gold standard of surgery, careful consideration must be given to the balance between disease removal and functional preservation in this group of patients. After all, it has to be remembered that a vestibular schwannoma is a benign tumor albeit in a dangerous place.
In our cohort of patients, the overall regrowth rate of incompletely resected vestibular schwannomas was ~39%. It is likely that this proportion will increase over time with longer follow-up. It would seem logical to presume that the likelihood of clinically significant regrowth would parallel the volume of the disease left behind. This was indeed shown in a recent Japanese study.11 In contrast, Sughrue et al reported that there was no significant correlation between the extent of resection and tumor recurrence,12 which was also our observation. A possible explanation for this disparity may be different inherent tumor biology influencing the growth rate in tumors of different sizes. What is clear is that a significant proportion of incompletely resected tumors remain dormant without further growth for a long period of time. It is therefore appropriate to adopt the “watchful waiting” policy with serial imaging, if the patient is clinically well and free of symptoms from brainstem compression. Our experience suggests that patients should be monitored for several years, as the residual tumor can start growing again after a period of apparent stability.
It has been proposed that patients with recurrent or residual disease following failed primary microsurgery for large or giant tumors should be offered further surgery as the first-line treatment.8 However, it should be remembered that revision surgery is often significantly more difficult than primary surgery and is associated with higher complication rates.13 Other options should be considered. In our series, several patients were successfully managed with subsequent Gamma Knife radiotherapy, an experience shared by other groups.7,14,15,16 There has to be a case for both “upfront” adjuvant Gamma Knife radiotherapy for some patients and delayed treatment for others, given only if the residual tumor grows. The difficulty is to know who to treat and when. The existence of a reliable tumor marker which could predict further growth would make this decision very easy; however, none has yet been identified. The possibilities investigated include among others Ki-67, c-erbB 2 and 3, fibroblast growth factor receptors 1 and 4, estrogen receptors, and progesterone receptors.17,18 In our center, the decision is based on the patient's age, the speed of tumor regrowth since primary surgery, existing cranial nerve deficits and the potential implications of further growth on radiation fields, and any vital structures that may come to lie within them, such as the brainstem.
It has been suggested that changing the surgical approach in revision surgery may help preserve facial nerve function.13 It is hard to see how this would help if the tumor was adherent to the brainstem at the root entry zone of the facial nerve at primary surgery and complete excision without significant risk to the nerve was not possible at the first operation. Revision surgery still has its place as the first-line option in selected cases, for example, in those who have already acquired all the potential neural deficits, in which case further surgery may be considered a “free hit.” Another group who should be considered is those who would be at risk from critical enlargement of the residual tumor after radiotherapy.
CONCLUSION
In managing very large vestibular schwannomas, it is crucial to keep a clear vision of what needs to be achieved in terms of tumor removal and symptomatic improvement, and what can be offered as additional or repeated treatment if required. Better understanding of tumor biology and histological correlation of prognostic factors would help refine our management of this challenging disease entity.
References
- Nikolopoulos T P, Fortnum H, O'Donoghue G, Baguley D. Acoustic neuroma growth: a systematic review of the evidence. Otol Neurotol. 2010;31(3):478–485. doi: 10.1097/MAO.0b013e3181d279a3. [DOI] [PubMed] [Google Scholar]
- Verma S, Anthony R, Tsai V, Taplin M, Rutka J. Evaluation of cost effectiveness for conservative and active management strategies for acoustic neuroma. Clin Otolaryngol. 2009;34(5):438–446. doi: 10.1111/j.1749-4486.2009.02016.x. [DOI] [PubMed] [Google Scholar]
- Solares C A, Panizza B. Vestibular schwannoma: an understanding of growth should influence management decisions. Otol Neurotol. 2008;29(6):829–834. doi: 10.1097/MAO.0b013e318180a4c4. [DOI] [PubMed] [Google Scholar]
- Mandl E S, Meijer O W, Slotman B J, Vandertop W P, Peerdeman S M. Stereotactic radiation therapy for large vestibular schwannomas. Radiother Oncol. 2010;95(1):94–98. doi: 10.1016/j.radonc.2009.12.042. [DOI] [PubMed] [Google Scholar]
- Chung W Y, Pan D H, Lee C C, et al. Large vestibular schwannomas treated by Gamma Knife surgery: long-term outcomes. J Neurosurg. 2010;113(Suppl):112–121. doi: 10.3171/2010.8.GKS10954. [DOI] [PubMed] [Google Scholar]
- Kanzaki J, Tos M, Sanna M, Moffat D A, Monsell E M, Berliner K I. New and modified reporting systems from the consensus meeting on systems for reporting results in vestibular schwannoma. Otol Neurotol. 2003;24(4):642–648. discussion 648–649. doi: 10.1097/00129492-200307000-00019. [DOI] [PubMed] [Google Scholar]
- Godefroy W P, der Mey A G van, de Bruine F T, Hoekstra E R, Malessy M J. Surgery for large vestibular schwannoma: residual tumor and outcome. Otol Neurotol. 2009;30(5):629–634. doi: 10.1097/MAO.0b013e3181a8651f. [DOI] [PubMed] [Google Scholar]
- Ramina R, Coelho Neto M, Bordignon K C, Mattei T, Clemente R, Pires Aguiar P H. Treatment of large and giant residual and recurrent vestibular schwannomas. Skull Base. 2007;17(2):109–117. doi: 10.1055/s-2006-953510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C K, Jung H W, Kim J E, Son Y J, Paek S H, Kim D G. Therapeutic strategy for large vestibular schwannomas. J Neurooncol. 2006;77(2):167–171. doi: 10.1007/s11060-005-9015-y. [DOI] [PubMed] [Google Scholar]
- Bloch O, Sughrue M E, Kaur R, et al. Factors associated with preservation of facial nerve function after surgical resection of vestibular schwannoma. J Neurooncol. 2011;102(2):281–286. doi: 10.1007/s11060-010-0315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Oishi M, Hiraishi T, Natsumeda M, Fujii Y. Clinicopathological factors related to regrowth of vestibular schwannoma after incomplete resection. J Neurosurg. 2011;114(5):1224–1231. doi: 10.3171/2010.11.JNS101041. [DOI] [PubMed] [Google Scholar]
- Sughrue M E, Kaur R, Rutkowski M J, et al. Extent of resection and the long-term durability of vestibular schwannoma surgery. J Neurosurg. 2011;114(5):1218–1223. doi: 10.3171/2010.11.JNS10257. [DOI] [PubMed] [Google Scholar]
- Freeman S R, Ramsden R T, Saeed S R, et al. Revision surgery for residual or recurrent vestibular schwannoma. Otol Neurotol. 2007;28(8):1076–1082. doi: 10.1097/MAO.0b013e318159e76a. [DOI] [PubMed] [Google Scholar]
- Pollock B E, Link M J. Vestibular schwannoma radiosurgery after previous surgical resection or stereotactic radiosurgery. Prog Neurol Surg. 2008;21:163–168. doi: 10.1159/000156904. [DOI] [PubMed] [Google Scholar]
- Fuentes S, Arkha Y, Pech-Gourg G, Grisoli F, Dufour H, Régis J. Management of large vestibular schwannomas by combined surgical resection and gamma knife radiosurgery. Prog Neurol Surg. 2008;21:79–82. doi: 10.1159/000156709. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Yamanaka K, Ishiguro T. Surgery combined with radiosurgery of large acoustic neuromas. Surg Neurol. 2003;59(4):283–289. discussion 289–291. doi: 10.1016/s0090-3019(03)00025-9. [DOI] [PubMed] [Google Scholar]
- O'Reilly B F, Kishore A, Crowther J A, Smith C. Correlation of growth factor receptor expression with clinical growth in vestibular schwannomas. Otol Neurotol. 2004;25(5):791–796. doi: 10.1097/00129492-200409000-00024. [DOI] [PubMed] [Google Scholar]
- Cafer S, Bayramoglu I, Uzum N, Yilmaz M, Memis L, Uygur K. Expression and clinical significance of Ki-67, oestrogen and progesterone receptors in acoustic neuroma. J Laryngol Otol. 2008;122(2):125–127. doi: 10.1017/S0022215107000229. [DOI] [PubMed] [Google Scholar]