Abstract
We conducted a study to evaluate the follow-up characteristics of patients with trigeminal neuralgia (TN) and to evaluate the factors affecting long-term outcome of microvascular decompression (MVD) in TN. Between 1983 and 2003, 156 patients with TN treated with MVD by 4 neurosurgeons at University Medical Centre Groningen/the Netherlands were evaluated. Baseline data from operative outcome were evaluated using univariate and multivariate analysis. The group consisted of 156 patients with TN: 90 females and 66 males with a median follow-up period of 9.7 years. The average age of initial symptoms was 51 years. The average duration of symptoms was 58 months. Postoperative 22 patients had a facial hyperpathia or hyperesthesia. Postoperatively, 137 patients had immediate relief. Postoperatively 1 year, 140 patients still had a good outcome of the operation. Twenty-seven patients with good immediate postoperative results had recurrent pain. From the group of patients with typical TN, 82% had good long-term results after operation. Patients with typical TN and immediate postoperative remission, in univariate analysis, had significantly more often an excellent/good postoperative outcome. Immediate postoperative remission is an independent predictive factor for a good long-term outcome. The long-term results of MVD in majority of patients were good with no mortalities and no major morbidities. Patients with typical TN had better long-term outcomes and less recurrence.
Keywords: Microvascular decompression, trigeminal neuralgia, typical trigeminal neuralgia, Jannetta, long-term follow-up
Trigeminal neuralgia (TN) is a disorder characterized by sudden sharp, shooting, lancinating pain attacks lasting several seconds to several minutes and localized to one or two branches of the trigeminal nerve. These attacks may begin spontaneously or they can be initiated by stimulation of the so-called trigger zones. The classic episodes of shooting pain are interrupted by pain-free intervals with remissions occasionally lasting for years.1
There are various treatment options for TN, but they all have their limitations. Therefore, the order and selection of treatment options are carefully chosen.
From the invasive treatment options for TN we can distinguish the percutaneous and surgical interventions. Surgical intervention means mainly microvascular decompression (MVD). Sometimes, the procedure of Dandy performed whereby the trigeminal nerve is (partially) cut.
The anatomical background of TN, a compression of the trigeminal nerve root by blood vessels, was described by Dandy as a cause of TN.1
Percutaneous techniques include glycerol injections, radiofrequency lesion, and balloon compression in or around the Gasserian ganglion.2 There are also noninvasive methods such as Gamma Knife and drug treatments. Carbamazepine is the gold standard of drug therapy for TN.
After development of MVD by Jannetta in the 1970s, MVD became one of the most important treatment options for TN.3,4
The results of this procedure are variable. Not all patients with TN who undergo MVD will improve, in some patient facial pain becomes even worse and a small number of patients are showing no response to MVD.5,6,7,8,9 Various studies showed that immediate postoperative pain relief is between 87 and 98%. After 1 to 2 years postoperative the percentage of pain-free patients is 78 to 80. After 8 to 10 years, this percentage is between 58 and 68%.5,6,10,11,12,13,14,15,16
In this study, 156 patients with TN were followed postoperatively. The aim of this study is to evaluate the preoperative characteristics of patients with TN and their postoperative long-term results. Prognostic factors are also evaluated for a good surgical outcome to be predicted.
EPIDEMIOLOGY
TN is the most common cranial neuralgia, and usually not difficult to distinguish from other cranial neuralgia, the age at which TN begins is usually above 50 years, with an average of 63 years.17 The annual incidence is 4.7/100,000.18 TN occurs more often in females (female to male ratio 1.8:1) and more often on the right facial side (60% right, 39% left, 1% bilateral). An important aspect is the relationship with multiple sclerosis (MS). Of the patients with MS, 2 to 4% has TN of which 18% is bilateral. Of the patient with MS, 1 to 5% develops TN.19,20,21,22,23,24,25,26,27
The pain is usually located in the area of the maxillary (second) and mandibular (third) branch. In many cases, the pain is located in both (>40%) branches, while presentation in the first branch is rare (only 2%). Spontaneous remissions happened and leads to a fluctuating trend. The patient may be in pain for weeks to several months, and then a comparable period of pain free. In time, the pain lasts for longer periods and with increasingly shorter remissions.21,23
ETIOLOGY
There is an association between TN and compression of the nerve by tumors, aneurysms, cysts, vascular anomalies, but also by normal arteries and veins. Compressions by arteries and veins are most common cause.1 It is shown that in about 96% of cases of typical TN, vascular compression is to be found.5,28 In only 3% of people without TN, there is a vascular contact with the trigeminal nerve.29
The pathological substrate is located in the building of the cranial nerves. In the brain stem the oligodendroglia are responsible for axonal isolation, and outside brain stem is Schwann cells. The transition zone “gaps” is called Redlich-Obersteiner's zone. Thus at this point, there is a transition from oligodendrocytes myelin to Schwann cell myelin. In this area a number of axons have a marginal or no isolation at all. Lack of isolation could leads to a strengthening effect on input (or output). This transmission, promoted by the vascular compression, leads to increased central stimulation, or to increased activity in the central neuron. Sensory neurons of the faces are often hyperexcited when damage occurs in the nerve and this leads to spontaneous generation of electrical impulses at the damaged spot.30,31,32,33,34 The chronic vascular compression of the trigeminal nerve has focal de-myelinization effect. This leads to dysfunction of local inhibitory interneurons and development of ectopic neuronal pacemakers. The combination of increased input by afferents ectopic pacemakers and the dysfunction of the intersegmental inhibitory neurons lead to hyperactivity of the nucleus of the trigeminal nerve. The result is TN attacks after stimulation of trigger points supplied by trigeminal nerve during washing, tooth brushing, eating, or even by touching the face.35,36,37,38
DIAGNOSIS AND DIFFERENTIAL DIAGNOSIS
The diagnosis of TN is primarily made on the basis of a careful history of presenting symptoms. The typical “lancinating” pain in one or more of the branches of the trigeminal nerve, a typical course in time, and one or more typical pain triggers are essential for diagnosing TN. In typical TN, on examination no neurological deficit is found in trigeminal nerve distribution. This distinguishes typical from atypical TN; the latter is described as a burning and continuous pain (neuropathy).
Sometimes there is a combination of both types of pain. This is especially common in a long-existing TN. In a longer existing TN a continuous underlying pain with a more continuous nature may exist between the attacks.
Only a very careful history taking shows that the pain started as atypical TN. Patients with atypical or combined type of TN are treated the same way as typical.1,2,38
However, there are conditions that may present with symptoms similar to TN (e.g., sinusitis, dental cavities, migraines, and diseases of the temporomandibular joint). With the help of a good history, physical examination of most of the above diagnosis could be easily excluded. Further diagnostic imaging (X-ray, computed tomography [CT] scan, or magnetic resonance imaging [MRI] scan) is therefore focused on other causes to be excluded.1,2,38 Other cranial neuralgia (glossopharyngeal neuralgia, the superior laryngeal neuralgia, and occipital neuralgia) are rare and can cause the same pain as in TN but with different localization of pain.1,2,38
TREATMENT
In principle, drug therapy is the first treatment option. The best medicine is so far (and used for almost 40 years) carbamazepine, in a gradually ascending dose. There is a long experience with this medication among neurologists and neurosurgeons. The side effects are mild. When the dose is not too quickly increased, it can be used in most patients with good effect to be achieved by a daily dose between 600 and 1200 mg. The slight nausea and drowsiness at the start of treatment are often seen, and the medication can be used for many years without significant toxicity. When there is absolutely no effect on the pain the diagnosed TN must be put to question. In other words, the use of carbamazepine is also a diagnostic.39,40,41,42 In intolerance (approximately 10%), relapse or inadequate effects to carbamazepine, you can choose from other medicines. It is about Diophantine and Baclofen. But these medicines have side effects.5,43,44,45,46 Of the new anticonvulsants, gabapentin (Neurontin) has a beneficial effect on neuropathic pain.47,48 When drug therapy fails or the patient wants a more permanent solution, a choice must be made from the other options: a percutaneous technique or surgical treatment such as the MVD. A number of factors are taken into account for this choice; factors concerning the method itself, known as results regarding effectiveness, side effects, and risks and factors regarding the patient, such as general health, age, and the experience of the surgeon with various treatments types also play a major role.
After providing good information and identification, the patient and doctor are expected to cooperate; they will have to come to a choice. At this stage it is important to do an MRI or CT scan, especially if surgical treatment is considered. This imaging is primarily intended to exclude other pathology, such as tumors, vascular malformations, or abnormalities in the brain stem.
The MRI should not, at least so far, be seen as a way to indicate the MVD. Depending on pathophysiology, Jannetta operation is the most logical procedure through which the important and most frequent cause removed without affecting the function of trigeminal nerve. It remains a delicate procedure which requires much experience.
MVD
MVD in the TN is an effective treatment method. The favorable long-term impact of MVD in TN ranges from 69%.10,13,49,50,51,52,53,54,55,56,57,58 The general morbidity, such as hearing loss, and sensory disorders in the trigeminal distribution varies between 0.2 and 4.5%.5,50,52,54,59,60,61 The aim of the operation is to remove the suspected compression of the nerve by a loop of an artery (sometimes a vein) near the brain stem, usually superior cerebellar artery (SCA). For the operation to be performed, the patient under general anesthesia in the supine position, with a wedge under the shoulder and with the head put aside so that the space behind the ear is freely accessible. The operation begins with a slightly curved incision just behind the ear, after which the muscle attachment is detached. An opening with a diameter of about 2 to 3 in is made in the cranial bone. The dura mater can now be seen. The opening is created so that the sinus transversus and sigmoideus become visible. They are the limits to which the dura can be opened with two triangular patches. If that is done, the cerebrospinal fluid drained, after which the visible cerebellum will be slightly sinking. The area is then obtained sufficient to meet through the magnification of the operating microscope between the cerebellum and the tentorium. The area around the brain stem is now visible as well as the exit spot of the trigeminal nerve. Usually the arachnoidea surrounds the facial and vestibulocochlear nerve; these cranial nerves should not be detached. The whole area around the trigeminal nerve is then inspected. Usually at the front of the nerve an artery loop is to be found—the SCA. This is mobilized and positioned in a new place and separated from trigeminal nerve by Teflon (DuPont, Wilmington, DE). This is done so that there is minimal or no contact with the nerve. Then the dura mater is waterproof closed, as far as possible, and covered with synthetic material (Spongostan [Ethicon, Somerville, NJ]). The bone flap is then placed back. The muscles and skin are then closed in layers.16,62,63 The area where the operation takes place is the cerebellopontine angle, an area located between the pons and cerebellum. In this area the important structures are feeding blood vessels and various nerves. In addition to the trigeminal nerve we will find the vestibulocochlear nerve (VIII) important for hearing and balance and the nerve that controls motor skills of the face, facial nerve (VII). The presence of these important structures in the area of operation constitutes a potential risk for damage.
As a complication hearing loss or reduced hearing may occur (usually transient), for example, because the cranial nerve VIII has been compromised by the release of the trigeminal nerve decompression. Other risks are bleeding and infection. Bleeding in this vital structures area means of course a serious complication. Cerebral spinal fluid leakage is also one of the possible complications.16,18
Patients and Methods
DESIGN OF STUDY
In the period from 1983 to 2003, there were 156 patients with TN operated by a total of 4 surgeons in University Medical Centre Groningen/The Netherlands (UMCG). This group is followed with regard to outcome after surgery. All patients had given verbal consent for participation in this study. All patients in this study had preoperative drug therapy and some had undergone other invasive interventions. There were 25 patients (16%) who had preoperative another invasive therapy with insufficient results. Preoperative CT head and/or MRI head for other cause of TN to be ruled out. Patients with MS were excluded in this study
DATA COLLECTION
The overall information included the baseline data, the preoperative data, follow-up data directly after surgery—at 3 months, 1 year, and long-term follow-up. Preoperatively every patient was seen by the operating neurosurgeon and the following information was recorded: age, gender, affected side, affected nerve root, preoperative pain period, preoperative treatment, hypertension, type of pain in TN (typical, atypical), and the severity of the pain. Direct postoperative complications were divided into local, general postoperative complications.
DEFINITIONS
The patients' complaints were subdivided into typical, atypical, and a mixed type TN. Typical pain was defined as pain shoots or as lightning flashes to be experienced. The pain shoots last for a few seconds to several minutes at most with typical trigger points such as cold, wind, talking, shaving, and teeth cleaning. An atypical pain was defined as a continuous pain, without the presence of trigger points.
A mix type was considered if there was a combination of both types. The severity of the pain was divided into serious and very serious. Severe pain is defined as spontaneous pain occurred during shaving, washing, and teeth cleaning. Very serious is considered if there was weight loss due to the inability to eat because of the pain. The patients were regarded as hypertensive if they were on antihypertensive drugs. In the preoperative stage, a neurological examination was made to see whether there were sensory or motor abnormalities in trigeminal nerve divisions. The results were considered as excellent or good if the patient had no pain without medication after the operation. These information were obtained from direct questions asked by neurosurgeon to the patients.
The result of operation was regarded as a failure if patients had pain despite medication use. The patients were followed from the operation until their death. The final date of the study and the end of the follow-up was 2003 or earlier if patient died before 2003. No final date could be obtained from one patient in connection with his emigration. The total follow-up time in this patient was considered as 1 year.
STATISTICS
Statistical analysis was performed with SPSS 11.5 (IBM Corp., Armonk, NY) for Windows. The results were double-checked. A p value of less than 0.05 was considered significant. Data were shown as averages, standard deviations, or percentiles. Differences at the time of the preoperative visit were tested between those who received a good and excellent operation result or had a bad result after long term. It was taken into account the gender, age, duration of symptoms, severity of pain, type of TN, affected nerve branch, affected facial side, sort of compression, preoperative treatment, hypertension, and the neurosurgeon. Continuous variables were tested with the “two-sample student's t-test.” For non-normal distributed data, a nonparametric test was applied. Categorical variables were tested with chi-square tests. Univariate analysis was done for the outcome of the operation. For determining the factors related to mortality, Cox proportional hazard analysis was used to calculate hazard ratios (HRs) with 95% confidence intervals.64,65 Results were shown as HRs. There was a predictive model that was used the following variables to see whether a significant predictive affected the outcome measure good or excellent result. A p value of 0.05 or less was considered significant (Table 1).
Table 1.
Baseline Patients' Characteristics and Long-Term Outcomes with Corresponding p Value
| Variables | Good Outcome | Poor Outcome | p Value | |
|---|---|---|---|---|
| Gender (% female) | 57.7 | 57.0 | 59.5 | 0.779 |
| Age in years (average) | 58.3 | 59.8 | 54.2 | 0.022 |
| Affected facial side (% right) | 65.5 | 64.9 | 64.3 | 0.942 |
| Affected trigeminal branch | ||||
| V1 | 0 | |||
| V2 | 43 (27.6%) | |||
| V3 | 26 (16.7%) | |||
| V1,2 | 12 (7.7%) | |||
| V1,3 | 9 (5.8%) | |||
| V2,3 | 66 (42.3%) | |||
| V1,3 | 0 | |||
| Duration preop pain in months | 87.0 | 87.8 | 85.3 | 0.850 |
| Neurological deficit preop (%) | 14.7 | 15.9 | 11.6 | 0.498 |
| Immediate postop pain relief | 87.8 | 95.6 | 67.4 | 0.001 |
| Glycerol injection (%) | 14.1 | 12.4 | 18.6 | 0.319 |
| Sweet coagulation (%) | 11.5 | 9.6 | 16.3 | 0.253 |
| Types of compression: | ||||
| Superior cerebellar artery | 103 (66%) | |||
| Venous compression | 14 (9%) | |||
| Arterial compression | 22 (14.1%) | |||
| Tumor | 3 (1.9%) | |||
| No compression | 4 (2.6%) | |||
| Hypertension (%) | 46.2 | 45.1 | 48.8 | 0.678 |
| Age onset of TN | 51.3 | 52.1 | 48.1 | 0.123 |
| Atypical pain (%) | 22.4 | 13.3 | 46.5 | 0.001 |
| Very severe preoperative pain (%) | 87.8 | 85.8 | 90.7 | 0.133 |
| Follow-up in years | 9.65 | 9.72 | 9.45 | 0.792 |
RESULTS
Baseline Data
In the period from 1983 to 2003, MVD was done for 156 patients with TN in UMCG and pursued over a median period of 9.65 years (with a range of 1 to 21 years).
It could not be ascertained what happened with five patients in the period after the last outpatient clinic visit. One patient had emigrated. Two patients were deceased in the follow-up period. Of 156 patients, 90 (58%) were females and 66 (42%) were males. The average age at surgery was 58 years with a minimum of 18 and a maximum age of 82. The average age when pain occurred first time was 51 years (range, 13 to 81 years). Of 156 patients, 120 patients (78%) had typical TN, 27 patients (17%) had atypical TN, and 8 patients (5%) had a mixture of TN.
The average preoperative duration of pain was 87 months (with a range of 4 to 456 months). In total, 137 patients (88%) had very severe pain and the other 19 (12%) had serious pain. In preoperative neurological examination hypesthesia was found in five patients, hyperpathia in two patients, seven patients with hearing loss, and two patients with ptosis. Seventy-two patients (46%) had hypertension. In 101 patients (65%) the right facial side was affected and in 55 patients the left side. In 66 patients (42%) the second (optic) and third (oculomotor) nerves were affected.
In only 12 patients (8%) the ophthalmic and maxillary branch was affected and in 9 patients (6%) the ophthalmic and mandibular nerve branch were affected. In 43 patients (28%) only the maxillary nerve branch was affected and in 26 (17%) only the mandibular branch. Of the 156 patients, 18 patients (12%) had sweet coagulation, 22 (14%) had glycerol injection, and 8 (5%) had a combination therapy before surgery. In addition, all patients had medical treatment.
During MVD compression caused by the SCA was seen in 103 patients (66%). In 22 patients (14%) TN was caused by arterial and venous compression. In 14 patients (9%) only a venous compression and in 3 patients (2%) compression was caused by a tumor. In 10 patients (6%) no compression was seen.
Results after 3 Months, 1 Year, and 9.7 Years
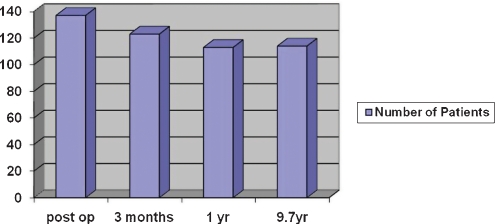
In the immediate postoperative period, 137 patients (88%) reported pain relief. After 3 months, 126 patients (81%) had good or excellent result. The remaining 30 patients still had pain despite drugs treatment. The number of relapses within 3 months was 18 (12%) and the number of relapses within 1 year was 29 (19%). In the long term, this number remained stable. After a year 114 patients (73%) remain pain free with medication.
Monitoring of patients continued until 2003 or until the death of the patient. The long-term results were similar to results obtained after 1 year. Of the group of patients with a typical pattern of complaints, 73% of patients had good/excellent results and 82% had a good long-term outcome (Fig. 1).
Figure 1.
Postoperative outcomes: immediate, 3 months, 1 year, and 9.7 years.
Postoperative Complications
Severe permanent postoperative complications were as follows: in one patient the facial nerve has been affected, one patient had the glossopharyngeal nerve paresis, one patient with vagus nerve damage, and one patient had vestibulocochlear nerve damage. Other permanent postoperative complications to the cranial nerves were: 4 patients (3%) had hearing loss and 20 patients (13%) hyperpathia and hyperesthesia. Of these 20 patients, 7 patients also had preoperative facial sensory impairment. Three patients (2%) had corneal anesthesia as a result of MVD (Table 2). Transient local compilations were as follows: in 44 patients (28.2%) there was postoperative dizziness, nausea, or headache. One patient had developed meningitis and one patient transient ischemic attack on admission. Both were fully recovered on discharge (Table 3). General surgery complication was as follows: urinary tract infection or urinary retention occurred in five patients. There were three patients with herpes labials, pneumonia in one patient, one patient with a pulmonary embolism, one patient with atrial fibrillation, and two with hallucination. All these patients were fully recovered and left the hospital in good condition (Table 4).
Table 2.
Postoperative Cranial Nerve Complications
| Cranial Nerve Complications | Number | Percentage |
|---|---|---|
| Mild hearing loss | 4 | 2.6 |
| Facial nerve palsy | 1 | 0.6 |
| Glossopharyngeal nerve paresis | 1 | 0.6 |
| Vagus nerve paresis | 1 | 0.6 |
| Severe hearing loss, n. VIII | 1 | 0.6 |
| Cornea anesthesia | 3 | 0.9 |
| Hyperpathy/hyperesthesia | 20 | 12.8 |
Table 3.
Temporary Complications
| Temporary Complications | Number | Percentage |
|---|---|---|
| Facial sensory loss | 6 | 3.8 |
| Mild hearing loss | 6 | 3.8 |
| Facial nerve palsy | 1 | 0.6 |
| Headache, dizziness, nausea | 44 | 28.2 |
| Meningitis | 1 | 0.6 |
| Brain infarctions | 1 | 0.6 |
Table 4.
General Postoperative Complications
| General Complications | Number | Percentage |
|---|---|---|
| Urinary tract infection | 5 | 3.8 |
| Pulmonary embolism | 1 | 0.6 |
| Herpes labialis | 3 | 1.9 |
| Pneumonia | 1 | 0.6 |
| Atrial fibrillation | 1 | 0.6 |
| Psychosis/hallucinations | 2 | 1.3 |
RESULTS OF UNIVARIATE ANALYSIS
Results of univariate analysis are divided into two categories, namely, good or excellent results and failure after long term (Table 1). Patients with good or excellent long-term results were older, had a more typical pattern of TN and had immediate pain relief postoperative. Patients with an atypical type of TN (29%) had significantly more relapses compared with patients with typical TN (15%) p = 0.021.
RESULTS OF MULTIVARIATE ANALYSIS
The factors associated with a good outcome of the MVD such as age, gender, affected facial side, affected branch, preoperative duration of pain, hypertension, type of compression, typical pattern of complaints, and the immediate postoperative pain relief have been studied by Cox proportional regression analysis. The different variables are shown in HRs. Only the immediate postoperative relieve of the symptoms had a significant positive predictive value with a HR of 2586 (CI 1305 to 5122, p = 0006) for a good outcome.
DISCUSSION
There are strong indications that the chronic vascular compression of the trigeminal nerve results in a focal de-myelinization. This leads to local dysfunction of inter inhibitory neurons and also to the development of ectopic neuronal pacemakers. The combination of increased input by afferent ectopic pacemakers and the failure of the intersegmental inhibitory neurons lead to hyperactivity of the core of the trigeminal nerve. The attack results are TN after stimulation of the footprint of trigeminal nerve such as washing, teeth cleaning, eating, or even by touching the face.35,36,37 The theory of vascular compression as the cause of TN is supported by clinical and anatomical evidence.6,29,50
TN usually begins as a relapsing disease with pain-free intervals, which sometimes can last for months or years. These pain-free intervals become shorter until they eventually disappear. With the disease progression, patients may have trouble in talking, eating, face washing, and teeth brushing because of pain caused by these activities. Current treatment usually begins with medications for example, carbamazepine, which fortunately gives an improvement of symptoms. But unfortunately, long-term effect is less effective. It is difficult to continue these drugs because of the many side effects they have, such as hyponatremia.40 In about half of patient with TN, surgical treatment is necessary.21 The average age of our patients was 58 years and that corresponds with the average age from other studies involving approximately 70% of the patients older than 50 years.21 The female to male ration in our study is 3:2. This is similar to other studies with a female to male ratio ranges from 2:1 to 4:3.3,66 Compression caused by the SCA was found in 103 patients (66%). In 101 patients (65%) the right side of the face was affected. In 66 patients (42%) the second and third branch of the trigeminal nerve was affected. These results are similar to previous studies.3,21,66,67
Immediate postoperative results were good to excellent in the 88% of patient. The long-term results (73%) were also excellent. Of the 88% of patients who were postoperative pain-free, 12% had a relapse within a month and 19% of patients, immediately postoperative, had a relapse after 1 year. This percentage is higher in comparison with other studies involving the relapse rate between 2 and 5%.6,50 Part of the difference found is explained by the fact that patients with atypical symptoms pattern had a significantly more often relapse. And in our patient group there are relatively many patients (17%) with atypical symptoms in comparison with other studies.6,50 It is described that MVD results for atypical TN had less good results.18 A relapse after MVD for TN suggests the presence in the trigeminal nerve of intrinsic abnormalities responsible for these recurrences.35,36,37 There was no mortality.
This corresponds with previous studies.8,59 The major complications are hyperpathia and hypesthesia. In 20 patients (13%) from this group, 7 patients had preoperative sensitivity disorders to the face. One patient had a postoperative facial paresis; a patient had vagus nerve paresis, a patient glossopharyngeal nerve paresis, and 1 patient had VIII nerve paresis. The surgery complications correspond with other studies.5,8,11 Preoperative treatments such as glycerol injection and sweet coagulation had no significant impact on the outcome of the operation on short or long term. This comes in contrast to other studies involving preoperative interventions chance of success of MVD in TN reduced by 43%.50,68 It is suggested that preoperative local interventions damage during MVD is more difficult to implement.50,68
Our research shows that MVD is a safe and effective treatment option in the treatment of TN in patients who no longer respond to medical treatment or to those who already have had surgery and still have pain.
There are a number of factors which are strongly correlated with a good outcome of TN after MVD. These factors include age, gender, preoperative pain longer than 8 years, and the type of compression. Venous compression correlates with a worse outcome.10,50,68,69,70
In our study, the multivariate analysis shows only the immediate postoperative remission as a significant independent predictor for a good outcome of the MVD. In univariate analysis patients who had good or excellent long-term results were older, had a more typical pattern of complaints, and were immediately relieved of postoperative complaints.
In a number of studies it was demonstrated a link between the severity of the compression by a blood vessel and postoperative outcome. If there is a serious compression, the results are better.13,66,69,71 The severity of the compression is not defined and not compared with our patients during the operation.
Retrospective determination of the compression severity is not accurate. The trigeminal affected branch had no significant impact on the operation outcome on long or short term and this is similar to the findings from other studies.50,72 Furthermore, there is no significant difference in outcome regarding surgery operators themselves. All operators are experienced neurosurgeons who all had comparable numbers of MVD operation.
In conclusion, MVD is a safe and effective treatment option in the treatment of patients with typical TN. In atypical TN, MVD is also an effective and safe treatment method with more recurrence rate.
RECOMMENDATIONS
Good patient selection is essential in treatment of TN. Patient with typical TN have better long-term outcomes after MVD and have less frequent recurrences. Preselection of patient with TN is essential through a well-taken history and examination. The diagnosis of TN is based on the history states. Imaging studies such as CT/MRI is important to exclude other pathology. Patient with typical TN can be selected to improve the results of operation.
References
- Dandy W E. Concering the cause of trigeminal neuralgia. Am J Surg. 1934;24:447–455. [Google Scholar]
- Nurmikko T J, Eldridge P R. Trigeminal neuralgia—pathophysiology, diagnosis and current treatment. Br J Anaesth. 2001;87(1):117–132. doi: 10.1093/bja/87.1.117. [DOI] [PubMed] [Google Scholar]
- Jannetta P J. Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia. J Neurosurg. 1967;26(1):159–162. doi: 10.3171/jns.1967.26.1part2.0159. [DOI] [PubMed] [Google Scholar]
- Jannetta P J. Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia. J Neurosurg. 1967;26(1):159–162. doi: 10.3171/jns.1967.26.1part2.0159. [DOI] [PubMed] [Google Scholar]
- Barker F G, II, Jannetta P J, Bissonette D J, Larkins M V, Jho H D. The long-term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med. 1996;334(17):1077–1083. doi: 10.1056/NEJM199604253341701. [DOI] [PubMed] [Google Scholar]
- Burchiel K J, Clarke H, Haglund M, Loeser J D. Long-term efficacy of microvascular decompression in trigeminal neuralgia. J Neurosurg. 1988;69(1):35–38. doi: 10.3171/jns.1988.69.1.0035. [DOI] [PubMed] [Google Scholar]
- Elias W J, Burchiel K J. Microvascular decompression. Clin J Pain. 2002;18(1):35–41. doi: 10.1097/00002508-200201000-00006. [DOI] [PubMed] [Google Scholar]
- Kondo A. Follow-up results of microvascular decompression in trigeminal neuralgia and hemifacial spasm. Neurosurgery. 1997;40(1):46–51. discussion 51–52. doi: 10.1097/00006123-199701000-00009. [DOI] [PubMed] [Google Scholar]
- Resnick D K, Levy E I, Jannetta P J. Microvascular decompression for pediatric onset trigeminal neuralgia. Neurosurgery. 1998;43(4):804–807. discussion 807–808. doi: 10.1097/00006123-199810000-00047. [DOI] [PubMed] [Google Scholar]
- Kolluri S, Heros R C. Microvascular decompression for trigeminal neuralgia. A five-year follow-up study. Surg Neurol. 1984;22(3):235–240. doi: 10.1016/0090-3019(84)90005-3. [DOI] [PubMed] [Google Scholar]
- Li S T, Wang X, Pan Q, et al. Studies on the operative outcomes and mechanisms of microvascular decompression in treating typical and atypical trigeminal neuralgia. Clin J Pain. 2005;21(4):311–316. doi: 10.1097/01.ajp.0000120790.69705.5b. [DOI] [PubMed] [Google Scholar]
- Lee K H, Chang J W, Park Y G, Chung S S. Microvascular decompression and percutaneous rhizotomy in trigeminal neuralgia. Stereotact Funct Neurosurg. 1997;68(1-4 Pt 1):196–199. doi: 10.1159/000099923. [DOI] [PubMed] [Google Scholar]
- Piatt J H, Jr, Wilkins R H. Treatment of tic douloureux and hemifacial spasm by posterior fossa exploration: therapeutic implications of various neurovascular relationships. Neurosurgery. 1984;14(4):462–471. doi: 10.1227/00006123-198404000-00012. [DOI] [PubMed] [Google Scholar]
- Slettebø H J, Eide P K. A prospective study of microvascular decompression for trigeminal neuralgia. Acta Neurochir (Wien) 1997;139(5):421–425. doi: 10.1007/BF01808878. [DOI] [PubMed] [Google Scholar]
- Taarnhøj P. Decompression of the posterior trigeminal root in trigeminal neuralgia. A 30-year follow-up review. J Neurosurg. 1982;57(1):14–17. doi: 10.3171/jns.1982.57.1.0014. [DOI] [PubMed] [Google Scholar]
- Jannetta P J. Microsurgical management of trigeminal neuralgia. Arch Neurol. 1985;42(8):800. doi: 10.1001/archneur.1985.04210090068018. [DOI] [PubMed] [Google Scholar]
- White J C, Sweet W H. Pain and the Neurosurgeon. Springfield: III. Chales C. Thomas; 1996. [Google Scholar]
- Bizzozero I, Ferrari F, Pozzoli S, Saetti M C, Spinnler H. Who is who: Italian norms for visual recognition and identification of celebrities. Neurol Sci. 2005;26(2):95–107. doi: 10.1007/s10072-005-0442-5. [DOI] [PubMed] [Google Scholar]
- Harris W. Rare forms of paroxysmal trigeminal neuralgia, and their relation to disseminated sclerosis. BMJ. 1950;2(4687):1015–1019. doi: 10.1136/bmj.2.4687.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen T S, Rasmussen P, Reske-Nielsen E. Association of trigeminal neuralgia with multiple sclerosis: clinical and pathological features. Acta Neurol Scand. 1982;65(3):182–189. doi: 10.1111/j.1600-0404.1982.tb03076.x. [DOI] [PubMed] [Google Scholar]
- Katusic S, Beard C M, Bergstralh E, Kurland L T. Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945-1984. Ann Neurol. 1990;27(1):89–95. doi: 10.1002/ana.410270114. [DOI] [PubMed] [Google Scholar]
- Rushton J G, Olafson R A. Trigeminal neuralgia associated with multiple sclerosis. A case report. Arch Neurol. 1965;13(4):383–386. doi: 10.1001/archneur.1965.00470040049007. [DOI] [PubMed] [Google Scholar]
- Clifford D B, Trotter J L. Pain in multiple sclerosis. Arch Neurol. 1984;41(12):1270–1272. doi: 10.1001/archneur.1984.04050230052017. [DOI] [PubMed] [Google Scholar]
- Hooge J P, Redekop W K. Trigeminal neuralgia in multiple sclerosis. Neurology. 1995;45(7):1294–1296. doi: 10.1212/wnl.45.7.1294. [DOI] [PubMed] [Google Scholar]
- Moulin D E, Foley K M, Ebers G C. Pain syndromes in multiple sclerosis. Neurology. 1988;38(12):1830–1834. doi: 10.1212/wnl.38.12.1830. [DOI] [PubMed] [Google Scholar]
- Stenager E, Knudsen L, Jensen K. Acute and chronic pain syndromes in multiple sclerosis. Acta Neurol Scand. 1991;84(3):197–200. doi: 10.1111/j.1600-0404.1991.tb04937.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen P. Facial pain. I. A prospective survey of 1052 patients with a view of: definition, delimitation, classification, general data, genetic factors, and previous diseases. Acta Neurochir (Wien) 1990;107(3-4):112–120. doi: 10.1007/BF01405789. [DOI] [PubMed] [Google Scholar]
- Meaney J FM, Eldridge P R, Dunn L T, Nixon T E, Whitehouse G H, Miles J B. Demonstration of neurovascular compression in trigeminal neuralgia with magnetic resonance imaging. Comparison with surgical findings in 52 consecutive operative cases. J Neurosurg. 1995;83(5):799–805. doi: 10.3171/jns.1995.83.5.0799. [DOI] [PubMed] [Google Scholar]
- Hamlyn P J, King T T. Neurovascular compression in trigeminal neuralgia: a clinical and anatomical study. J Neurosurg. 1992;76(6):948–954. doi: 10.3171/jns.1992.76.6.0948. [DOI] [PubMed] [Google Scholar]
- Namba S, Shimizu Y, Wani T, Fujiwara N. An experimental model of deafferented pain in the cat. Appl Neurophysiol. 1985;48(1-6):201–211. doi: 10.1159/000101128. [DOI] [PubMed] [Google Scholar]
- Devor M, Seltzer Z. Pathophysiology of damaged nerves in relation to chronic pain. In: Wall P D, Melzack R, editors. Textbook of Pain. 4th ed. London: Churchill Livingstone; 1999. pp. 129–164. [Google Scholar]
- Clavin W H, Howe J F, Loeser J D. Ectopic Repetitive Firing in Focally Demyelinated Axon and Some Implication for Trigeminal Region. Amsterdam: Elsevier/North-Holland; 1977. pp. 125–136. [Google Scholar]
- Burchiel K J. Abnormal impulse generation in focally demyelinated trigeminal roots. J Neurosurg. 1980;53(5):674–683. doi: 10.3171/jns.1980.53.5.0674. [DOI] [PubMed] [Google Scholar]
- Lewy F H, Grant F C. Physiopathologic and pathoanatomic aspects of major trigeminal neuralgia. Arch Neurol Psychiatry. 1938;40:1126–1134. [Google Scholar]
- Formm G H. Physiological rationale for the treatment of neuropathic pain. APS Journal. 1993;2:1–7. [Google Scholar]
- Fromm G H, Chattha A S, Terrence C F, Glass J D. Role of inhibitory mechanisms in trigeminal neuralgia. Neurology. 1981;31(6):683–687. doi: 10.1212/wnl.31.6.683. [DOI] [PubMed] [Google Scholar]
- Namba S, Shimizu Y, Wani T, Fujiwara N. An experimental model of deafferented pain in the cat. Appl Neurophysiol. 1985;48(1-6):201–211. doi: 10.1159/000101128. [DOI] [PubMed] [Google Scholar]
- Love S, Coakham H B. Trigeminal neuralgia: pathology and pathogenesis. Brain. 2001;124(Pt 12):2347–2360. doi: 10.1093/brain/124.12.2347. [DOI] [PubMed] [Google Scholar]
- Killian J M, Fromm G H. Carbamazepine in the treatment of neuralgia. Use of side effects. Arch Neurol. 1968;19(2):129–136. doi: 10.1001/archneur.1968.00480020015001. [DOI] [PubMed] [Google Scholar]
- Taylor J C, Brauer S, Espir M LE. Long-term treatment of trigeminal neuralgia with carbamazepine. Postgrad Med J. 1981;57(663):16–18. doi: 10.1136/pgmj.57.663.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom S. Trigeminal neuralgia: its treatment with a new anticonvulsant drug (G-32883) Lancet. 1962;1(7234):839–840. doi: 10.1016/s0140-6736(62)91847-0. [DOI] [PubMed] [Google Scholar]
- Blom S. Trigeminal neuralgia: its treatment with a new anticonvulsant drug (G-32883) Lancet. 1962;1(7234):839–840. doi: 10.1016/s0140-6736(62)91847-0. [DOI] [PubMed] [Google Scholar]
- Zakrzewska J M, Chaudhry Z, Nurmikko T J, Patton D W, Mullens E L. Lamotrigine (lamictal) in refractory trigeminal neuralgia: results from a double-blind placebo controlled crossover trial. Pain. 1997;73(2):223–230. doi: 10.1016/S0304-3959(97)00104-8. [DOI] [PubMed] [Google Scholar]
- Braham I, Saia A. Phenytoin in the treatment of trigeminal and other neuralgias. Lancet. 1960;2:892–893. [Google Scholar]
- Campbell F G, Graham J G, Zilkha K J. Clinical trial of carbazepine (tegretol) in trigeminal neuralgia. J Neurol Neurosurg Psychiatry. 1966;29(3):265–267. doi: 10.1136/jnnp.29.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol C F. A four year double-blind study of tegretol in facial pain. Headache. 1969;9(1):54–57. doi: 10.1111/j.1526-4610.1969.hed0901054.x. [DOI] [PubMed] [Google Scholar]
- Merren M D. Gabapentin for treatment of pain and tremor: a large case series. South Med J. 1998;91(8):739–744. doi: 10.1097/00007611-199808000-00007. [DOI] [PubMed] [Google Scholar]
- Valzania F, Strafella A P, Nassetti S A, et al. Gabapentin in idiopathic trigeminal neuralgia. (abstract) Neurology. 1998;50:A379. [Google Scholar]
- Apfelbaum R I. Surgical management of disorders of lower cranial nerve. In: Schmidek H H, Sweet W H, editors. Operative Neurosurgical Techniques. Indications, Methodes and Results. 2nd ed. Orlando: Grune & Stratton; 1988. pp. 1097–1109. [Google Scholar]
- Bederson J B, Wilson C B. Evaluation of microvascular decompression and partial sensory rhizotomy in 252 cases of trigeminal neuralgia. J Neurosurg. 1989;71(3):359–367. doi: 10.3171/jns.1989.71.3.0359. [DOI] [PubMed] [Google Scholar]
- Burchiel K J, Steege T D, Howe J F, Loeser J D. Comparison of percutaneous radiofrequency gangliolysis and microvascular decompression for the surgical management of tic douloureux. Neurosurgery. 1981;9(2):111–119. doi: 10.1227/00006123-198108000-00001. [DOI] [PubMed] [Google Scholar]
- Goya T, Wakisaka S, Kinoshita K. Microvascular decompression for trigeminal neuralgia with special reference to delayed recurrence. Neurol Med Chir (Tokyo) 1990;30(7):462–467. doi: 10.2176/nmc.30.462. [DOI] [PubMed] [Google Scholar]
- Jannetta P J. Microvascular decompression of the trigeminal nerve root entry zone. Theoretical considerations, operative anatomy, surgical technique, and results. In: Rovit R L, Murali R, Jannetta P J, editors. Trigeminal Neuralgia. Baltimore: Williams & Wilkins; 1990. pp. 201–222. [Google Scholar]
- Klun B. Microvascular decompression and partial sensory rhizotomy in the treatment of trigeminal neuralgia: personal experience with 220 patients. Neurosurgery. 1992;30(1):49–52. doi: 10.1227/00006123-199201000-00009. [DOI] [PubMed] [Google Scholar]
- Mori K, Morimoto M, Kurisaka M, Uchida Y, Eghwrudjakpor P. Analysis of microvascular decompression for the treatment of trigeminal neuralgia and hemifacial spasm. Nippon Geka Hokan. 1986;55(6):768–776. [PubMed] [Google Scholar]
- Richards P, Shawdon H, Illingworth R. Operative findings on microsurgical exploration of the cerebello-pontine angle in trigeminal neuralgia. J Neurol Neurosurg Psychiatry. 1983;46(12):1098–1101. doi: 10.1136/jnnp.46.12.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindou M, Amrani F, Mertens P. [Microsurgical vascular decompression in trigeminal neuralgia. Comparison of 2 technical modalities and physiopathologic deductions. A study of 120 cases] Neurochirurgie. 1990;36(1):16–25. discussion 25–26. [PubMed] [Google Scholar]
- Loveren H van, Tew J M, Jr, Keller J T, Nurre M A. a 10-year experience in the treatment of trigeminal neuralgia. Comparison of percutaneous stereotaxic rhizotomy and posterior fossa exploration. J Neurosurg. 1982;57(6):757–764. doi: 10.3171/jns.1982.57.6.0757. [DOI] [PubMed] [Google Scholar]
- Apfelbaum R. Neurovascular decompression: the procedure of choice? In: Grady S, editor. Clinical Neurosurgery. Vol 46, Baltimore: Lippincott Williams & Wilkins; 1998. pp. 473–498. [PubMed] [Google Scholar]
- McLaughlin M R, Jannetta P J, Clyde B L, Subach B R, Comey C H, Resnick D K. Microvascular decompression of cranial nerves: lessons learned after 4400 operations. J Neurosurg. 1999;90(1):1–8. doi: 10.3171/jns.1999.90.1.0001. [DOI] [PubMed] [Google Scholar]
- Barker F G, II, Jannetta P J, Bissonette D J, Jho H D. Trigeminal numbness and tic relief after microvascular decompression for typical trigeminal neuralgia. Neurosurgery. 1997;40(1):39–45. doi: 10.1097/00006123-199701000-00008. [DOI] [PubMed] [Google Scholar]
- Jannetta P J. Microsurgical approach to the trigeminal nerve of tic douloureux. Prog Neurol Surg. 1976;7:180–200. [Google Scholar]
- Jannetta P J. Neurovascular compression in cranial nerve and systemic disease. Ann Surg. 1980;192(4):518–525. doi: 10.1097/00000658-198010000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R F, Vermeulen S S, Grimm P, Blasko J, Posewitz A. Gamma Knife radiosurgery for treatment of trigeminal neuralgia: idiopathic and tumor related. Neurology. 1997;48(3):608–614. doi: 10.1212/wnl.48.3.608. [DOI] [PubMed] [Google Scholar]
- Cox D R. Regression models and life tables. J R Stat Soc [Ser B] 1972;34:187–220. [Google Scholar]
- Mendoza N, Illingworth R D. Trigeminal neuralgia treated by microvascular decompression: a long-term follow-up study. Br J Neurosurg. 1995;9(1):13–19. [PubMed] [Google Scholar]
- Lovely T J, Jannetta P J. Microvascular decompression for trigeminal neuralgia. Surgical technique and long-term results. Neurosurg Clin N Am. 1997;8(1):11–29. [PubMed] [Google Scholar]
- Barba D, Alksne J F. Success of microvascular decompression with and without prior surgical therapy for trigeminal neuralgia. J Neurosurg. 1984;60(1):104–107. doi: 10.3171/jns.1984.60.1.0104. [DOI] [PubMed] [Google Scholar]
- Apfelbaum R I. Surgery for tic douloureux. Clin Neurosurg. 1983;31:351–368. doi: 10.1093/neurosurgery/31.cn_suppl_1.351. [DOI] [PubMed] [Google Scholar]
- Puca A, Meglio M, Cioni B, Visocchi M, Vari R. Microvascular decompression for trigeminal neuralgia: prognostic factors. Acta Neurochir Suppl (Wien) 1993;58:165–167. doi: 10.1007/978-3-7091-9297-9_38. [DOI] [PubMed] [Google Scholar]
- Szapiro J JR, Jr, Sindou M, Szapiro J. Prognostic factors in microvascular decompression for trigeminal neuralgia. Neurosurgery. 1985;17(6):920–929. doi: 10.1227/00006123-198512000-00009. [DOI] [PubMed] [Google Scholar]
- Barker F G, II, Jannetta P J, Bissonette D J, Larkins M V, Jho H D. The long-term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med. 1996;334(17):1077–1083. doi: 10.1056/NEJM199604253341701. [DOI] [PubMed] [Google Scholar]