Abstract
We conducted a retrospective observational study to assess the consequences of conservative management of vestibular schwannoma (VS). Data were collected from tertiary neuro-otological referral units in United Kingdom. The study included 59 patients who were managed conservatively with radiological diagnosis of VS. The main outcome measures were growth rate and rate of failure of conservative management. Multivariate analysis sought correlation between tumor growth and (i) demographic features, (ii) tumor characteristics. The mean tumor growth was 0.66 mm/y. 11 patients (19%) required intervention. Mean time to intervention was 37 months with two notable late “failures” occurring at 75 and 84 months. Tumors extending into the cerebellopontine angle (CPA) grew significantly faster than intracanalicular tumors (p = 0.0045). No association was found between growth rate and age, sex, tumor laterality, facial nerve function, and grade of hearing loss. Conservative management is acceptable for a subset of patients. Tumors extending into the CPA at diagnosis grow significantly faster than intracanalicular tumors. No growth within 5 years of surveillance does not guarantee a continued indolent growth pattern; surveillance must therefore continue.
Keywords: Vestibular schwannoma, conservative, surveillance
Vestibular schwannoma (VS) is a benign tumor of Schwann cells insulating the vestibulocochlear nerve. Three approaches to management are employed: surgical resection or debulking, radiotherapy, or conservative management (via serial magnetic resonance imaging [MRI] surveillance). The advent and greater availability of MRI has increased the detection of much smaller and sometimes asymptomatic tumors. This contrasts with a previous era in which VS were characteristically large at diagnosis and necessitated urgent surgical resection. Consequently, increasing numbers of patients are being managed according to a “wait-rescan” protocol. This provides an opportunity to gain insights into the natural history of the disease; promising better decision-making with regard to time and type of intervention and improved patient counseling.
Interestingly, incidence of VS appears to be increasing.1,2,3 Data from Denmark (based upon an established comprehensive VS tumor registry) suggest an increase from 7.8 to 12.4 cases per million from 1976 to 1995.4 As incidence appears to be increasing, tumor size at time of diagnosis is falling, while median age at diagnosis remains relatively static.5 In addition, there has been a marked decrease in the incidence of “giant” tumors from 28 to 1%. These findings probably reflect better diagnostic methods rather than a genuine increase in VS incidence. Intriguingly, significantly lower numbers of patients are presenting in need of treatment than these incidence figures suggest ought be necessary.6 It is therefore likely that the vast majority do not grow to a clinically relevant extent and therefore never require treatment.
MANAGEMENT STRATEGIES FOR VS
The choice of management strategy for VS is multifactorial; influenced by tumor size, patient age, and general health (providing an idea of their longevity and stratifying the risks of surgery), extent of hearing loss and other neurological signs, and the patient's own preference. The fundamental benefit of successful surgery is capacity to achieve complete tumor resection. Although surgical resection provides an opportunity to cure, it also involves potential significant morbidity.7,8 Radiation therapy, such as surgery, is becoming more refined as delivery techniques improve and radiation doses fall. Although it is generally well tolerated,9 concerns remain regarding long-term tumor control and the potential for malignant transformation.10,11 Moreover, the need for surveillance persists even after this intervention. In addition, there is debate as to whether radiosurgery makes subsequent surgery more difficult.12 The risks attributable to surgery and radiosurgery are arguably inappropriate in the context of an histologically benign and often minimally symptomatic lesion. As a result, patients with small tumors, advanced age, or with comorbidities precluding safe anesthetic are candidates for a conservative approach. In addition, some patients chose themselves to watch and wait.
GROWTH PATTERNS AND NATURAL HISTORY OF VS
Growth rate of VS is variable and difficult to predict. Tumor size either remains static, increases, or (in a small minority) regresses. When growth occurs, it is typically quite slow. Some VS, however, will behave differently and may cause life-threatening neurological signs due to growth and brain stem compression. There is a need to identify those tumors that pose a threat from those that will behave indolently. This study aims to add and improve the quality of existing data assessing the efficacy of a conservative approach to VS management. In due course, this may inform the results of more robust meta-analysis.
METHODS
We conducted a retrospective observational study of 453 consecutive cases of VS treated at two neuro-otological tertiary referral units (Department of Neurosurgery, Royal Free Hospital, London; and Department of Neuro-otology, University College London Ear Institute, Royal National Throat, Nose and Ear Hospital, London) from 1988 to 2010. These cases were filtered to include only those patients who had followed a conservative “wait-rescan” management plan and adhered to the following criteria:
Inclusion Criteria
Patients aged 16 years and above with a radiological diagnosis of VS managed from the outset with a conservative radiological surveillance plan.
Exclusion Criteria
Patents with cerebellopontine angle (CPA) lesions deemed radiologically to not represent VS. Patients with neurofibromatosis type 2 were also excluded, as were patients with recurrent VS who were being observed with regular MRI surveillance following a previous intervention (in the form of surgery or stereotactic radiosurgery). The following data were collected for each case:
Patient age (in years) at time of diagnosis
Patient sex
Initial tumor size and size at serial follow-up(s)
Laterality of tumor
Gross tumor location (categorized into tumors confined to the internal auditory canal (IAC) and those extending from IAC into CPA)
Presenting symptoms at time of diagnosis
Clinical signs at time of diagnosis including cranial nerve palsies
Reason(s) for choice of conservative management
Whether (and why) conservative management failed and what form of intervention was required
Where possible, a calculation of tumor growth rate was ascertained, based on tumor size measurements at interval imaging
The tumor growth pattern was also subjectively characterized based on patient records (see categorization below), for those cases that lacked sufficient objective data from which to measure growth from available imaging or radiology reports
Duration of total follow-up and duration of successful conservative management was calculated
Outcome Measures
The primary end-point was whether or not intervention occurred in a patient who, at diagnosis, had embarked on a conservative treatment pathway. Secondary end-points were the rationale and justification for intervention (when required), duration of successful conservative management, tumor growth rate, and nature of intervention. To identify factors associated with tumor growth, a multivariate analysis was performed investigating for any correlations with tumor growth.
When possible, tumor size was measured according to the Committee on Hearing and Equilibrium guidelines (written by the American Academy of Otolaryngology - Head and Neck Surgery).13 Tumor growth patterns were additionally categorized descriptively according to the following criteria:
No growth/insignificant growth, defined as <2 mm increase in size during follow-up period
Continuous growth, defined as >2 mm increase during early follow-up
No growth followed by significant growth
Regression
Growth followed by regression
The Mann-Whitney U test was used to compare growth rates in different patient groups (as the data are nonparametric). Kaplan-Meier survival curves were adapted to illustrate the probability of continuing successful conservative management over time. MedCalc software (Brockstraat, Mariakerke, Belgium) was used for statistical analysis (http://www.medcalc.be/). Calculations were deemed to be statistically significant when p < 0.05.
RESULTS
Of 453 patients, 46 underwent radiotherapy, 336 underwent surgery, and 71 were managed conservatively (diagnosed between 1988 and 2010). One further patient was excluded due to a diagnosis of neurofibromatosis type 2. Two patients were excluded as their CPA lesion was considered radiographically more likely to represent a meningioma. Nine other patients were excluded from our analysis as they left our catchment area and their follow-up was managed elsewhere. Fifty-nine suitable patients are therefore included in this analysis, having been radiographically diagnosed with unilateral VS between 1988 and 2007.
Patient Demographics, Symptoms, and Signs
Of the cohort, 25 (42%) were females and 34 (58%) were males. Mean patient age at diagnosis was 53.5 years (range, 16 to 79 years). The breakdown of presenting symptoms at time of diagnosis is shown in Table 1. In the majority of cases hearing loss was accompanied by another symptom, with the combination of hearing loss and tinnitus most frequent.
Table 1.
Percent Rate of Occurrence of Selected Presenting Symptoms among the Cohort
| Presenting Symptoms | % Occurrence |
|---|---|
| Hearing loss | 88 |
| Tinnitus | 50 |
| Vertigo | 16 |
| Otalgia | 3 |
| Altered taste sensation | 3 |
For 41 of the 59 patients, it was possible to ascertain facial nerve function and the extent of hearing loss at the time of diagnosis. All of the 41 patients were House-Brackmann grade I. Of 41 patients, 28 were assessed to be Gardner-Robertson grade 1 (good/excellent hearing) at diagnosis while the remaining 13 of the 41 patients were assessed as Gardner-Robertson grade 2 (serviceable hearing).
Rationale for Conservative Management
The primary reason for opting for a conservative management strategy was identified in 55 of the 59 patients and is illustrated in Table 2. Small tumor size (84% of cases) dominated as the primary reason cited for choosing a “wait-rescan” approach. One patient was managed conservatively as she was pregnant at the time of her diagnosis. After delivery, it was apparent that significant tumor growth had occurred and she subsequently underwent surgical resection.
Table 2.
Reasons for Choice of Conservative Management
| Primary Reason for Conservative Management | Number of Patients |
|---|---|
| Small tumor size | 46 |
| Advanced age | 5 |
| Significant comorbidity | 1 |
| Patient choice | 2 |
| Pregnant at time of diagnosis | 1 |
Tumor Characteristics
Table 3 illustrates tumor anatomical characteristics; specifically with regards to laterality, gross tumor location (with respect to the IAC and CPA), and tumor grade (according to the Tokyo Consensus Meeting on Systems for Reporting Results in Vestibular Schwannoma14).
Table 3.
Gross Anatomical Features of VS
| Tumor laterality | n = 59 |
| Right | 32 |
| Left | 27 |
| Tumor location | n = 59 |
| IAC | 34 |
| IAC + CPA | 24 |
| Intracochlear | 1 |
| Tumor grade | n = 58* |
| Intrameatal | 34 |
| Grade 1 (1–10 mm IC) | 14 |
| Grade 2 (11–20 mm IC) | 10 |
| Grade 3 (21–30 mm IC) | 1 |
| Grade 4 (31–40 mm IC) | 0 |
| Grade 5 (>40 mm IC) | 0 |
CPA, cerebellopontine angle; IAC, internal auditory canal; IC, intracranial extent (largest diameter).
The single intracochlear VS has been excluded from grading.
Tumor Growth Rates and Failure Rate of Conservative Management
Of 59 patients who were enrolled on a conservative treatment pathway, 11 (18.6%) subsequently underwent intervention. A breakdown of reasons for, and type of, intervention is shown in Tables 4 and 5.
Table 4.
Reasons for Failure of Conservative Management
| Reason for Stopping Conservative Management | n |
|---|---|
| Significant tumor growth | 9 |
| Patient choice | 1 |
| Deterioration of symptoms | 1 |
Table 5.
Nature of Intervention Performed when Conservative Management Failed
| Type of Intervention | n |
|---|---|
| Stereotactic radiosurgery | 6 |
| Surgical resection/debulking | 3 |
| Ventriculoperitoneal shunt | 1 |
| Yet to be decided at MDT meeting | 1 |
MDT, multidisciplinary team.
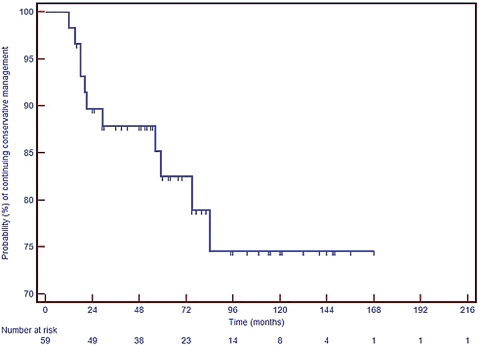
Overall mean duration of successful conservative management (as of January 2010) was 68 months (range, 11 to 156 months, median 60 months). In those patients who “failed” conservative management, the mean time to intervention was 37 months (range, 11 to 84 months). Fig. 1 illustrates the probability of ongoing successful conservative management for all cases with respect to time, in the form of a Kaplan-Meier survival curve.
Figure 1.
Kaplan-Meier survival curve adapted to illustrate probability of ongoing successful conservative management.
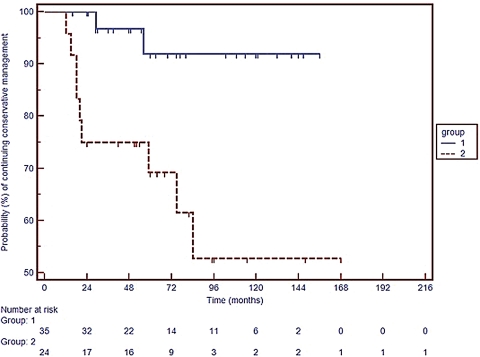
Fig. 2 is a second Kaplan-Meier plot illustrating the probability of continuing conservative management with the cohort subdivided into tumors confined to IAC and those extending into the CPA. Tumors confined to the internal acoustic canal at diagnosis show a significantly higher probability of continued successful conservative management over time (log rank test: chi-squared 8.68, p = 0.0032).
Figure 2.
Kaplan-Meier survival curve adapted to illustrate probability of ongoing successful conservative management with the cohort divided into tumors confined to the internal auditory canal (IAC) at diagnosis (Group 1) and these extending from internal auditory canal to cerebellopontine angle (CPA) (Group 2). Log rank test: chi-squared 8.68, p = 0.0032.
The growth rate of 28 of the 34 tumors located solely in the internal acoustic meatus was measureable. These intracanalicular tumors had a mean growth rate of 0.16 mm/y and median of 0 mm/y. This compares with a mean growth rate of 1.52 mm/y and median of 0.31 mm/y for 17 measureable tumors found, at diagnosis, to be extending from internal acoustic meatus into CPA. This is a statistically significant difference in growth rate between the two groups (p = 0.0045, Mann-Whitney U test). Overall mean growth rate for all measureable tumors was 0.66 mm/y.
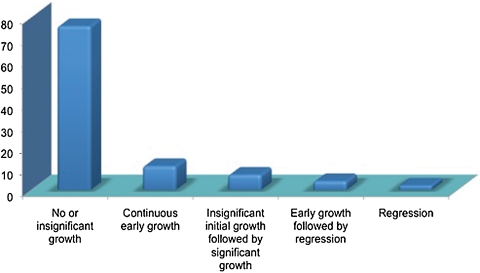
In addition to objective measurement of tumor growth rate (possible in 45 of 59 tumors), a descriptive categorization of growth was possible for all but three cases. Fig. 3 illustrates this characterization.
Figure 3.
Subjective categorization of tumor growth patterns by % (n = 56).
Age at diagnosis did not correlate with growth rate (r = −0.036, p = 0.811, CI -0.323 to 0.257). There was no difference in growth rates with respect to patient sex (p = 0.262), laterality of tumor (p = 0.238), facial nerve function at diagnosis, or degree of hearing impairment at diagnosis (Mann-Whitney U tests).
DISCUSSION
Contemporary Understanding of VS Growth Patterns and Natural History
Many patients will live undisturbed by their VS while others will progress to life-threatening neurological complications as a consequence of significant tumor growth. As conservative management was first made feasible, numerous attempts have been made to gain a better understanding of the natural history of VS. A recent Heath Technology Assessment systematic review15 identified 171 articles (published from 1990 to 2008) that assessed, in various ways, VS natural history. Two meta-analyses and two systematic reviews have concurrently attempted to consolidate some of the key findings.
Tumor Growth and Failure Rates of Conservative Management
Published literature to date16,17,18,19 has predominantly found slow VS tumor growth in expectantly managed patients (ranging from 1.2 to 1.9 mm/y). However, among these cases there are examples of exceptionally rapid growth (up to 30 mm/y). The current study is in general agreement with these meta-analyses and systematic reviews, finding an average growth rate of 1.5 mm/y and a failure rate for conservative management of 19%.
The failure of conservative management occurs, by definition, when a treatment intervention occurs. Failure, in the most part, is prompted by significant observed growth of a tumor. Intervention occurred in nine patients due to significant tumor growth. What constitutes “significant” growth is debatable. In this series, decision-making occurred on a case-by-case basis without explicit or decisive criteria. However, a growth rate exceeding 5 mm/y and/or extracanalicular size exceeding (or evidently trending toward) 20 mm were key determinants in halting conservative management. In addition to growth, other potential reasons may prompt intervention. In this study, one patient requested intervention in the context of a radiographically indolent tumor while another was treated due to worsening symptoms while the tumor showed no signs of growth. Meta-analysis and systematic review suggests an overall failure rate of between 18 and 26%. This study is consistent with this finding with a failure rate of 19%, despite the inclusion of two cases that might justifiably have continued with a conservative approach.
Time Frame of VS Growth
The time frame during which tumor growth occurs is equally, if not more, important than average growth rates. Specifically, if it were noted that no observed growth during a specified time period could predict future tumor behavior, patient follow-up and management would be better informed. Many previous studies20,21,22,23,24,25 have suggested that tumor behavior during the first year postdiagnosis is particularly important in determining future growth. However, the duration of patient follow-up is clearly critical when making conclusions of this kind. It is notable that mean duration of follow-up in the majority of previously published studies is inadequate (36 to 38 months). It is highly likely, therefore, that several late “failures” in these patient groups have not yet had time to manifest. This study has a mean follow-up duration of 68 months (range, 11 to 156 months). The finding of two conservative management failures beyond 5 years of surveillance is in opposition to studies to some previous findings. However, certain other studies and case reports corroborate this finding of late failures. Quaranta26 found that 65% of VS grew within 4 years but that 14% showed growth even after 60 months of stability. It would be interesting to investigate the histology of those tumors that fail early and those that fail late.
Given the inconsistent way in which VS is diagnosed (ranging from screening MRI, to incidental finding on MRI, to MRI provoked by a suggestive constellation of symptoms and signs) it is unsurprising that growth observed within what is, for many, an arbitrary window of tumor existence is not reliably predictive. Stratification of tumors according to circumstances of diagnosis and duration of symptoms might enable a more informed assessment of “cohort” behavior. Currently, however, the key clinical conclusion must be that surveillance ought to continue beyond 5 years even in the context of an apparently indolent tumor. The frequency of imaging has not been considered explicitly here, but will be dictated by prior tumor behavior, tumor size, and resource considerations.
Predictors of VS Tumor Growth
Reliable predictors of growth at time of diagnosis would prove helpful for patient counseling and planning management. In this cohort tumors located, at diagnosis, solely within the IAC grew significantly more slowly than those found to be extending into the CPA. This is in concordance with several other reports. Hajioff et al27 noted significantly slower growth in IAC tumors compared with tumors extending from IAC to CPA. Solares and Panizza28 found also that intracanalicular tumors are less likely to grow than grade II tumors. The finding of a significant difference in growth rate is also reflected via Kaplan-Meier plot (Fig. 2) illustrating that intracanalicular tumors show a significantly higher probability of successful continuing conservative management with time.
In this study, no association with growth rate was found when assessing age, sex, laterality of tumor, and selected clinical findings (VII and VIII nerve function) at the time of diagnosis. Previous literature is inconsistent but failure to identify useful predictors of growth predominates. Bedersen et al,29 Flint et al,30 Rosenburg,31 and Walsh et al32 found no correlation between growth and initial symptoms, initial size, duration of symptoms, laterality, gender, and age.
Limitations of this Study
A key factor in assessing VS growth is the technique used to measure tumor size. Often, studies of VS growth have opted to measure simply the maximum diameter of the tumor in any plane. Tumors are three-dimensional structures and this therefore constitutes a significant oversimplification. In addition, VS are unique in terms of their spatial characteristics due to their origin within a constrained cylindrical bony structure (IAC) and extension into the less constrained CPA. Many other methods of assessing growth have been reported resulting in confusion and undermining cross-study comparisons. It has been proposed that volumetric measurements of tumor size may provide more accurate growth assessment.33 Measurement of tumor volume with specific area tracing software is more accurate than the use of two-dimensional measurements. Although some studies support this hypothesis for nonintracanalicular tumors,34 the required software is not yet widely available nor is the technique standardized.
Inter- and intraobserver variation is another source of inaccuracy. One group has assessed the reliability of VS size measurement using various methods (including that proposed by the Committee on Hearing and Equilibrium guidelines) and found a margin of error of 2 mm.35 This finding undermines, in particular, the calculations made regarding growth of intracanalicular tumors in which the observed changes in tumor size are typically very small (calculated mean growth of these tumors in this study was just 0.16 mm/y).
Regarding duration of patient follow-up, although the mean follow-up in this series was appreciably longer than that of several previous studies, it is still fundamentally inadequate. VS has moved from being an urgent/emergent surgical disease toward, in many cases, a chronic disease. As such, there must be similar frame shift with respect to follow-up. Long-term follow-up of the order of 20 + years is required to make firm conclusions. In reality, the best method of understanding VS tumor growth and behavior is a prospective study using serial MRI with a consistent and standardized method for reporting size and defining growth, and with significantly longer durations of follow-up. The initiation of centralized national VS tumor registries would begin to satisfy these criteria.
CONCLUSION
Over three-quarters of patients in this study showed no evidence of significant tumor growth during wait-rescan follow-up. Most tumors managed in this way do not grow and the “failure rate” of conservative management was found to be 19% during a mean follow-up period of 68 months. Conservative management with surveillance MRI is therefore an acceptable method of managing a subset of VS patients. The size of tumor at time of diagnosis is important. Tumors found to be extending into the CPA at diagnosis grow significantly faster and have a higher probability of “failing” conservative management than those confined to the internal auditory meatus. No other reliable predictors of tumor growth are identified. Importantly, the absence of observed growth during the first 5 years of surveillance does not guarantee a continued indolent growth pattern. Surveillance must therefore continue beyond this time to safely monitor for potential late tumor growth.
ACKNOWLEDGMENT
This study was conducted as part of, and with the support of, the Edinburgh Surgical Sciences Qualification (www.essq.rcsed.ac.uk/).
References
- Stangerup S E, Tos M, Caye-Thomasen P, Tos T, Klokker M, Thomsen J. Increasing annual incidence of vestibular schwannoma and age at diagnosis. J Laryngol Otol. 2004;118(8):622–627. doi: 10.1258/0022215041917989. [DOI] [PubMed] [Google Scholar]
- Hardell L, Hansson Mild K, Sandström M, Carlberg M, Hallquist A, Påhlson A. Vestibular schwannoma, tinnitus and cellular telephones. Neuroepidemiology. 2003;22(2):124–129. doi: 10.1159/000068745. [DOI] [PubMed] [Google Scholar]
- Howitz M F, Johansen C, Tos M, Charabi S, Olsen J H. Incidence of vestibular schwannoma in Denmark, 1977-1995. Am J Otol. 2000;21(5):690–694. [PubMed] [Google Scholar]
- Tos M, Charabi S, Thomsen J. Incidence of vestibular schwannomas. Laryngoscope. 1999;109(5):736–740. doi: 10.1097/00005537-199905000-00011. [DOI] [PubMed] [Google Scholar]
- Stangerup S-E, Tos M, Caye-Thomasen P, Tos T, Klokker M, Thomsen J. Increasing annual incidence of vestibular schwannoma and age at diagnosis. J Laryngol Otol. 2004;118(8):622–627. doi: 10.1258/0022215041917989. [DOI] [PubMed] [Google Scholar]
- Tos M, Charabi S, Thomsen J. Clinical experience with vestibular schwannomas: epidemiology, symptomatology, diagnosis, and surgical results. Eur Arch Otorhinolaryngol. 1998;255(1):1–6. doi: 10.1007/s004050050012. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos T P, Johnson I, O'Donoghue G M. Quality of life after acoustic neuroma surgery. Laryngoscope. 1998;108(9):1382–1385. doi: 10.1097/00005537-199809000-00024. [DOI] [PubMed] [Google Scholar]
- Bennett M, Haynes D S. Surgical approaches and complications in the removal of vestibular schwannomas. 2007. Neurosurg Clin N Am. 2008;19(2):331–343. vii. doi: 10.1016/j.nec.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Roland P S, Eston D. Stereotactic radiosurgery of acoustic tumors. Otolaryngol Clin North Am. 2002;35(2):343–355. doi: 10.1016/s0030-6665(02)00002-6. [DOI] [PubMed] [Google Scholar]
- Pollock B E, Link M J, Foote R L. Failure rate of contemporary low-dose radiosurgical technique for vestibular schwannoma. J Neurosurg. 2009;111(4):840–844. doi: 10.3171/2009.3.JNS08949. [DOI] [PubMed] [Google Scholar]
- Battaglia A, Mastrodimos B, Cueva R. Comparison of growth patterns of acoustic neuromas with and without radiosurgery. Otol Neurotol. 2006;27(5):705–712. doi: 10.1097/01.mao.0000226302.59198.87. [DOI] [PubMed] [Google Scholar]
- Roche P H, Régis J, Devèze A, Delsanti C, Thomassin J M, Pellet W. [Surgical removal of unilateral vestibular schwannomas after failed Gamma Knife radiosurgery] Neurochirurgie. 2004;50(2-3 Pt 2):383–393. [PubMed] [Google Scholar]
- Committee on Hearing and Equilibrium. Guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma). American Academy of Otolaryngology-Head and Neck Surgery Foundation, INC. Otolaryngol Head Neck Surg. 1995;113(3):179–180. doi: 10.1016/S0194-5998(95)70101-X. [DOI] [PubMed] [Google Scholar]
- Kanzaki J, Tos M, Sanna M, Moffat D A, Monsell E M, Berliner K I. New and modified reporting systems from the consensus meeting on systems for reporting results in vestibular schwannoma. Otol Neurotol. 2003;24(4):642–648. discussion 648–649. doi: 10.1097/00129492-200307000-00019. [DOI] [PubMed] [Google Scholar]
- Fortnum H, O'Neill C, Taylor R, et al. The role of magnetic resonance imaging in the identification of suspected acoustic neuroma: a systematic review of clinical and cost effectiveness and natural history. Health Technol Assess. 2009;13(18):iii–iv. ix–xi, 1–154. doi: 10.3310/hta13180. [DOI] [PubMed] [Google Scholar]
- Yamakami I, Uchino Y, Kobayashi E, Yamaura A. Conservative management, gamma-knife radiosurgery, and microsurgery for acoustic neurinomas: a systematic review of outcome and risk of three therapeutic options. Neurol Res. 2003;25(7):682–690. doi: 10.1179/016164103101202075. [DOI] [PubMed] [Google Scholar]
- Yoshimoto Y. Systematic review of the natural history of vestibular schwannoma. J Neurosurg. 2005;103(1):59–63. doi: 10.3171/jns.2005.103.1.0059. [DOI] [PubMed] [Google Scholar]
- Smouha E E, Yoo M, Mohr K, Davis R P. Conservative management of acoustic neuroma: a meta-analysis and proposed treatment algorithm. Laryngoscope. 2005;115(3):450–454. doi: 10.1097/00005537-200503000-00011. [DOI] [PubMed] [Google Scholar]
- Selesnick S H, Johnson G. Radiologic surveillance of acoustic neuromas. Am J Otol. 1998;19(6):846–849. [PubMed] [Google Scholar]
- Flint D, Fagan P, Panarese A. Conservative management of sporadic unilateral acoustic neuromas. J Laryngol Otol. 2005;119(6):424–428. doi: 10.1258/0022215054273089. [DOI] [PubMed] [Google Scholar]
- Moller P, Myrseth E, Pedersen P-H, Lund-Johannessen M, Krakenes J, Moen G. Small vestibular schwannoma: results with observation, surgery and gamma-knife. Proceedings of the Fourth International Conference on Vestibular Schwannoma and Other CPA Lesions. Cambridge, UK: July 13–17, 2003. [Google Scholar]
- O'Reilly B, Murray C D, Hadley D M. The conservative management of acoustic neuroma: a review of forty-four patients with magnetic resonance imaging. Clin Otolaryngol Allied Sci. 2000;25(2):93–97. doi: 10.1046/j.1365-2273.2000.00331.x. [DOI] [PubMed] [Google Scholar]
- Bederson J B, von Ammon K, Wichmann W W, Yasargil M G. Conservative treatment of patients with acoustic tumors. Neurosurgery. 1991;28(5):646–650. discussion 650–651. doi: 10.1097/00006123-199105000-00002. [DOI] [PubMed] [Google Scholar]
- Glasscock M E, III, Pappas D G, Jr, Manolidis S, Von Doersten P G, Jackson C G, Storper I S. Management of acoustic neuroma in the elderly population. Am J Otol. 1997;18(2):236–241. discussion 241–242. [PubMed] [Google Scholar]
- Stangerup S-E, Caye-Thomasen P, Tos M, Thomsen J. The natural history of vestibular schwannoma. Otol Neurotol. 2006;27(4):547–552. doi: 10.1097/01.mao.0000217356.73463.e7. [DOI] [PubMed] [Google Scholar]
- Quaranta N, Baguley D M, Axon P R, Moffat D A. Conservative management of vestibular schwannomas. Proceedings of the Fourth International Conference on Vestibular Schwannoma and Other CPA Lesions. Cambridge, UK: July 13–17, 2003; pp. 256–257. [Google Scholar]
- Hajioff D, Raut V V, Walsh R M, et al. Conservative management of vestibular schwannomas: third review of a 10-year prospective study. Clin Otolaryngol. 2008;33(3):255–259. doi: 10.1111/j.1749-4486.2008.01705.x. [DOI] [PubMed] [Google Scholar]
- Solares C A, Panizza B. Vestibular schwannoma: an understanding of growth should influence management decisions. Otol Neurotol. 2008;29(6):829–834. doi: 10.1097/MAO.0b013e318180a4c4. [DOI] [PubMed] [Google Scholar]
- Bederson J B, von Ammon K, Wichmann W W, Yasargil M G. Conservative treatment of patients with acoustic tumors. Neurosurgery. 1991;28(5):646–650. discussion 650–651. doi: 10.1097/00006123-199105000-00002. [DOI] [PubMed] [Google Scholar]
- Flint D, Fagan P, Panarese A. Conservative management of sporadic unilateral acoustic neuromas. J Laryngol Otol. 2005;119(6):424–428. doi: 10.1258/0022215054273089. [DOI] [PubMed] [Google Scholar]
- Rosenberg S I. Natural history of acoustic neuromas. Laryngoscope. 2000;110(4):497–508. doi: 10.1097/00005537-200004000-00002. [DOI] [PubMed] [Google Scholar]
- Walsh R M, Bath A P, Bance M L, Keller A, Rutka J A. Consequences to hearing during the conservative management of vestibular schwannomas. Laryngoscope. 2000;110(2 Pt 1):250–255. doi: 10.1097/00005537-200002010-00012. [DOI] [PubMed] [Google Scholar]
- Vokurka E A, Herwadkar A, Thacker N A, Ramsden R T, Jackson A. Using Bayesian tissue classification to improve the accuracy of vestibular schwannoma volume and growth measurement. AJNR Am J Neuroradiol. 2002;23(3):459–467. [PMC free article] [PubMed] [Google Scholar]
- de Langenberg R van, de Bondt B J, Nelemans P J, Baumert B G, Stokroos R J. Follow-up assessment of vestibular schwannomas: volume quantification versus two-dimensional measurements. Neuroradiology. 2009;51(8):517–524. doi: 10.1007/s00234-009-0529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall A H, Owen VMF, Nikolopoulos T P, O'Donoghue G M. Acoustic schwannomas: awareness of radiologic error will reduce unnecessary treatment. Otol Neurotol. 2005;26(3):512–515. doi: 10.1097/01.mao.0000169782.69341.6d. [DOI] [PubMed] [Google Scholar]