Abstract
The analysis of the treatment results in patients with cerebellopontine angle (CPA) tumors, manifested as trigeminal neuralgia (TN). During the 10-year period from 1998 to 2008, 14 patients with verified CPA tumors that had the typical manifestations of TN were operated on at our hospital (5.8% from all patients with TN who underwent surgery). In nine cases the epidermoid was identified; three patients had meningioma, one patient had acoustic neurinoma, and one patient had lipoma. The follow-up of all patients lasted at least 12 months. The intraoperative assessment identified the three variants of relationship between the tumors and neurovascular structures: (1) tumor grows around the trigeminal nerve; (2) the tumor causes compression and displacement of the trigeminal nerve; and (3) tumor presses the arterial vessel to the trigeminal nerve by moving the vessel or nerve. For six patients, with removal of tumor a microvascular decompression of the trigeminal nerve was performed. Complete pain relief was achieved in 12 patients (86%). TN is an expectative symptom of CPA tumors. The most frequent cause of secondary TN of CPA tumors is epidermoid. All patients with manifestations of TN should undergo the magnetic resonance imaging for early diagnosis of CPA tumor.
Keywords: Trigeminal neuralgia, cerebellopontine angle tumor, microvascular decompression, epidermoid
According to the International Classification of Headache Disorders, 2 ed. (2004), trigeminal neuralgia (TN) in its etiology is divided into classic TN (caused by vascular compression of trigeminal nerve root) and symptomatic one (caused by other factors as tumors, vascular disorders, demyelination in multiple sclerosis).1 We can assume from different sources that from 1 to 9.9 % cases of TN are caused by cerebellopontine angle tumors (CPA).2,3,4,5
This study was done to identify and evaluate neurovascular and tumor interrelations in 14 patients with symptomatic (secondary) TN caused by CPA tumors. In this article 10 years of clinical experience in management of patients with TN secondary to CPA tumors is summarized.
We analyzed 14 patients with typical manifestations of TN due to CPA tumors. These patients made up 5.8% from all TN patients operated in our department (242 cases). Between 1998 and 2008, 242 consecutive patients underwent surgery for classical TN in Neurosurgical Department #1, Saint-Petersburg City Hospital #2. All the operations were performed by the senior author (YSh). The patients' mean age was 51.2 years (age range, 28 to 78 years) and there were predominantly females (11 females and 3 males).
By comparing patients with primary TN and secondary TN, we note that symptoms duration was significantly longer and patients' age on clinical manifestation was significantly lower in group with CPA tumors (t-test for two independent samples, p < 0.05). In spite of female predominance among patients with tumors the distribution by sex in groups was statistically insignificant (cross-tabulation analysis with Fisher exact test, p = 0.0928) (Table 1).
Table 1.
Comparison of Age, Symptoms, Duration, and Male–Female Distribution in Two Groups
| Data | Secondary TN in CPA Tumors (Average) | Classic Primary TN (Average) |
|---|---|---|
| Age on admission (y) | 51.2 | 61.4 |
| Age at onset (y) | 39.8 | 54.1 |
| Symptom duration (y) | 9.5 | 7.2 |
| Female (%) | 79 | 57 |
| Male (%) | 21 | 43 |
TN, trigeminal neuralgia; CPA, cerebellopontine angle.
The histopathology revealed epidermoids in nine patients, meningioma in three patients, vestibular schwannoma in one case, and lipoma in one case. The mean follow-up duration was 4.5 years (range, 1 to 10 years and not less than 12 months).
CLINICAL MANIFESTATION
Table 2 shows the clinical features and the most essential information of all 14 patients. It is noteworthy that all these patients had typical manifestations of neuralgia, which implies the existence of short paroxysms of sharp pain on one side of the face in the zone of innervation of the branches of the trigeminal nerve. The presence of trigger points on the face or mouth, as well as positive effects from taking carbamazepine is typical for these patients.
Table 2.
Clinical Characteristics of 14 Patients with Secondary TN
| Age/Sex | TN Duration (y) | Side | Spreading of Pain | Neurological Symptoms (Except TN) | Tumor Type | Removal of Tumor | Outcome | Complications | Follow-Up (y) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45/F | 6 | Le | V2 | Hypesthesia a/d (V n.) | M | Total | Ex | 10 | |
| 2 | 56/M | 11 | R | V3 | E | Total | Ex | Liquorrhea | 8 | |
| 3 | 48/F | 9 | R | V2–3 | L | Subtotal | Good | Hypesthesia (V n.) | 7 | |
| 4 | 54/F | 8 | R | V3 | E | Total | Ex | Dizziness | 6 | |
| 5 | 39/F | 4 | Le | V1–2-3 | Hearing loss, hypesthesia a/d (V n.) | N | Total | Ex | 6 | |
| 6 | 62/F | 19 | R | V2–3 | E | Total | Ex | 5 | ||
| 7 | 44/M | 6 | Le | V3 | HFS | E | Total | Ex | Hearing loss | 5 |
| 8 | 28/F | 2 | Le | V2–3 | HFS | E | Total | Ex | 4 | |
| 9 | 59/F | 9 | R | V3 | Hypesthesia (V n.) | M | Total | Ex | 4 | |
| 10 | 34/M | 5 | Le | V1–2-3 | Wryneck | E | Subtotal | Good | 3 | |
| 11 | 53/F | 8 | Le | V1–2-3 | Hypesthesia a/d (V n.) | E | Total | Ex | 2 | |
| 12 | 66/F | 12 | R | V3 | E | Total | Ex | 2 | ||
| 13 | 78/F | 23 | Le | V1–2 | Dizziness | M | Total | Ex | Dizziness | 1 |
| 14 | 51/F | 11 | R | V2–3 | Dizziness | E | Subtotal | Ex | 1 |
E, epidermoid; Ex, excellent; HFS, hemifacial spasm; L, lipoma; Le, left; a\d, after alcohol destruction; M, meningioma; N, neurinoma; R, right; TN, trigeminal neuralgia.
In two patients TN manifestation was combined with the hemifacial spasm, which was also typical and characterized by paroxysmal involuntary contractions in facial muscles on one side of the face. Spastic torticollis was observed in one patient. Four patients had hypesthesia of ipsilateral half of the face; however, three of them had hypoesthesia after destructive procedures (trigeminal nerve branches destruction with alcohol) during outpatient treatment. At the preliminary stage neuroimaging techniques were not used to any patient in this group. Hypoesthesia was CPA tumor-related only in one patient. Two patients noted fairly intense episodes of vertigo caused by irritation of the vestibulocochlear nerve.
When analyzing the clinical manifestations of secondary TN in patients with tumors we drew our attention to the almost complete absence of spontaneous remission of pain that was specific for the patients with classical TN.
DIAGNOSTICS
Brain magnetic resonance imaging (MRI) brain was performed in all which showed ipsilateral CPA lesion with mass effect. Brain computed tomography (CT) without contrast enhancement was performed in six cases before MRI.
CLINICAL ASSESSMENT AND FOLLOW-UP
An assessment of the clinical results was performed for all patients, via personal or telephone interview. The main criterion for clinical assessment was degree of facial pain relief. Results were evaluated at the discharge of the patients and consecutive follow-up.
The patients were classified into the following four grades, on the basis of the degree of facial pain relief: “excellent” if patient was pain-free; “good” if the pain relief was more than 75%; “poor” if the pain was less than 25% resolved; and “failure” for all remaining results.
RESULTS
Neuroimaging Findings
MRI revealed characteristic abnormal signal in lateral pontine cistern in nine patients: hypointense T1 signal at the pathological lesion and hyperintense T2 signal relative to the brain tissue and light hyperintense T2 signal relative to cerebrospinal fluid density (Fig. 1).
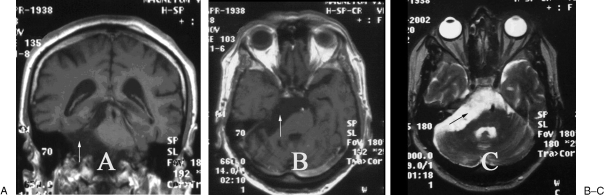
Figure 1.
Right CPA epidermoid tumor. T1-weighted (A, B) and T2-weighted (C) magnetic resonance imaging (MRI) showed a large heterogeneous tumor with no enhancement. (A, coronal image; B and C, axial images with mass lesion [arrows].)
In all patients these findings corresponded to epidermoid tumor. One patient demonstrated marked caudal tumor growth (clinically he had torticollis) and one patient had bilateral tumor spread with both trigeminal nerves involved (Fig. 2) but with unilateral clinical manifestation.
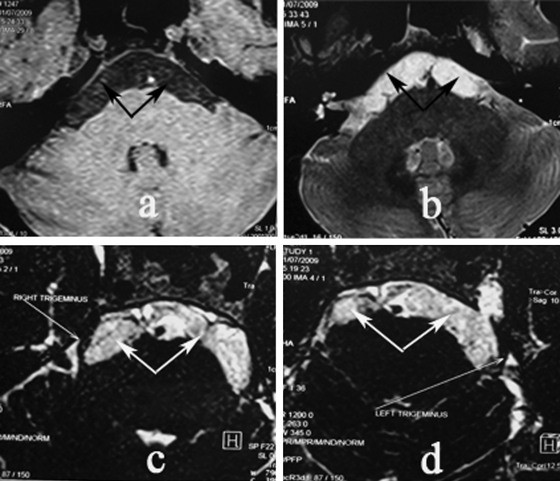
Figure 2.
Epidermoid with bilateral growth. Enhanced T1-weighted (a) and T2-weighted (b, c, d) magnetic resonance imaging (MRI) showed tumor with spreading to both sides (tumor pointed by arrow). Trigeminal nerves involved in tumor bilaterally.
Vestibular schwannoma, diameter of 10 mm, was detected in one patient. MRI scanning showed hypodense lesion at the trigeminal nerve root entry zone, there was lipoma of prepontine cistern (Fig. 3).
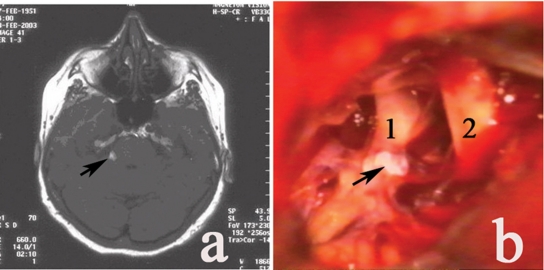
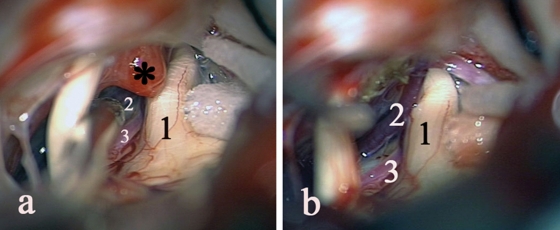
Figure 3.
Lipoma (a) unenhanced axial T1-weighted MRI (tumor pointed by arrow); (b) intraoperative picture. 1, V n.; 2, VII–VIII nn; tumor pointed by arrow.
Of the remaining three patients, two of them had posterior petrous meningiomas, diameter of 15 mm, which were identified on MRI (Fig. 4). In one case routine MRI showed no abnormality in CPA. The clinical manifestations corresponded to the criteria of classical TN. However, during the surgery meningioma of the posterior surface of a pyramid, diameter of 5 mm, compressing ipsilateral trigeminal nerve was found (Fig. 5).
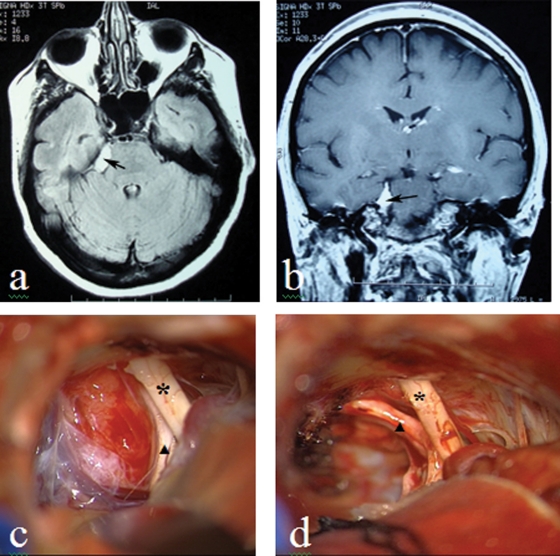
Figure 4.
Meningioma (a) axial T1-weighted MRI; (b) coronal T1-weighted MRI; (c, d) intraoperative picture before and after tumor removal. (V nerve pointed by triangle, VII–VIII nerves bundle pointed by asterisk).
Figure 5.
Meningioma (pointed by asterisk): (a) before tumor removal (tumor presses the superior cerebellar artery and vein to the trigeminal nerve); (b) after tumor removal. 1, V n.; 2, vein; 3, superior cerebellar artery.
Surgery
All patients were operated using a standard retrosigmoid approach with supine position on the contralateral side. Three-point rigid fixation of the head and microneurosurgical technique with operating microscope were used.3
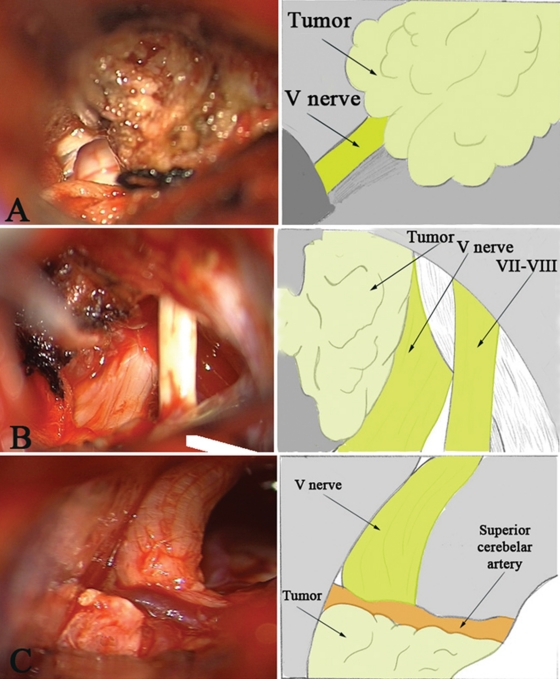
We have identified three variants of relationship of the tumor and neurovascular structures in accordance with the intraoperative findings (Fig. 6):
Figure 6.
Three variants of relationship of the tumor and neurovascular structures. (A) Trigeminal nerve becomes overgrown with tumor tissue; (B) the tumor causes compression and displacement of the trigeminal nerve; (C) the tumor pushes the arteries to the trigeminal nerve due to displacement of a vessel or nerve.
Trigeminal nerve becomes overgrown with tumor tissue (Fig. 6A).
The tumor causes compression and displacement of the trigeminal nerve (Fig. 6B).
The tumor pushes the arteries to the trigeminal nerve due to displacement of a vessel or nerve (Fig. 6C).
Variant A met with lipoma and epidermoid tumor in five cases, variant B met with epidermoid in four cases, and variant C met with meningiomas, neurinoma, and intracranial epidermoid in five cases (Table 3).
Table 3.
Variants of Compression and the Tumor Type
| Variant of Relationship | Lipoma | Epidermoid | Meningioma | Neurinoma |
|---|---|---|---|---|
| Variant A | 1 | 5 | − | − |
| Variant B | − | 4 | − | − |
| Variant C | − | 1 | 2 | 1 |
The tumor mass was removed totally in 11 cases and subtotally in 3 cases when total removal of the tumor was not possible. In six patients tumor removal was combined with trigeminal nerve microvascular decompression (MVD) (in two cases in combination with facial nerve MVD), as neurovascular conflict or proximity of the vessel to the trigeminal nerve were identified.
In the case of lipoma with solid elastic structure of the tissue, which was tightly fixed to the pons and had many perforating vessels, total removal was associated with high risk of serious neurological complications. In two cases epidermoids were removed subtotally, one of which was extended to the contralateral side (Fig. 2), and its total removal from unilateral approach presented significant technical difficulties. However, in view of one-sided clinical symptoms, the intervention from the opposite side was decided not to perform. In the second case the tumor extended caudally into the region of craniovertebral junction, so the capsule of the tumor in the area of the caudal nerve was not removed.
TN was completely relieved in 12 patients (86%) after surgery (excellent result). Two patients (14%) achieved good result. In patient with lipoma, facial pain decreased by 75% with facial hypesthesia arising. Patient with caudal epidermoid, extension and concomitant spastic torticollis was pain-free but torticollis symptoms recurred 3 months after surgery (no signs of tumor recurrence on the MRI), therefore, this result was assessed as good, even in the complete absence of pain in his face. Life-threatening and disabling complications were not observed in our study. One patient was reoperated in the early postoperative period in connection with the development of nasal cerebrospinal fluid leakage.
Transient vertigo and nystagmus were seen in two patients and that were mostly diminished several months later in the follow-up period. One patient had hearing impairment which relieved in a month after surgery. Postoperative facial hypoesthesia in zones of trigeminal branches V2 and V3 innervation was observed in one patient, but it was diminished after 6 months.
DISCUSSION
CPA tumors are not frequent cause of secondary TN. According to different authors, from 1 to 9.9% of cases of TN occurred due to CPA tumors.2,3,4,5,6
There were 242 patients with typical manifestations of TN operated in our department for 10 years. In 14 cases, CPA tumors were identified that accounted of 5.8%. Patients with CPA tumors who had unilateral facial pain but did not corresponded the criteria for TN (permanent pain of varying intensity combined with severe neurological disorders) were excluded from this study. In virtually all cases, these patients had large and huge CPA tumors (in most cases of meningiomas and neurinomas).
The most frequent cause of TN are intracranial epidermoids.4,7,8 The incidence of TN in patients with CPA epidermoids accounted of 76.9%, while according to Kobata et al (2002) accounted of 90.6%.4 Meningiomas and neurinomas are more rare causes of TN, but according to several authors facial pain may be present in more than 35% of patients with meningiomas of CPA and up to 10% of patients with neurinoma of this location.5,9,10,11 Mention of the lipoma as the cause of TN was not found on review of the literature.
In our group the most frequent cause of TN was epidermoids, which consisted 65% of the cases (9 out of 14), second place is occupied by the occurrence of meningioma—21% (3 of 14), and third place is occupied by neurinoma and lipoma—7% (1 case). An interesting question is the mechanism of TN development in patients with tumors.
Vascular compression of the trigeminal nerve root, leading to demyelination in the nerve root entry zone, currently recognized the cause of the classical TN.1,3
In five cases (36%) we observed tumor pressed against the arterial vessel (in three cases of superior cerebellar artery and in two cases of anterior inferior cerebellar artery) to the root of trigeminal nerve, causing the mechanism of the disease similar to the mechanism of classical TN (Figs. 5 and 6C). This mechanism was observed in all meningiomas, neurinomas, and in one case of epidermoid tumor.
The tumors resulted in the changes of the normal anatomical relationship between the neurovascular structures, causing the development of neurovascular compression. In such a case tumor did not impact nerve (only in one patient meningioma impacted the nerve sufficiently, manifested as hypoesthesia).
In four patients with epidermoids, neurovascular compression was not revealed, but tumors caused compression and displacement of the trigeminal nerve (Fig. 6B), and in five patients epidermoids completely overgrown nerve (Fig. 6A). Several authors believe that the chemical effects of cholesterol on the nerve can cause the development of TN in such cases, but this assumption has no direct evidence.7,11
Epidermoid tumors are congenital, slow-growing entities that fill the arachnoid space, causing its expansion, and with a gradual increase in the volume begin to have a mass effect. (They begin to have a mass effect with a gradual increase in volume.) Most authors believe that the main mechanism of TN development in this case is the direct pressure of the tumor on the nerve with its deformation, or compression of the nerve capsule of the tumor at its fouling.7,8,11
Analysis of the cases (especially the case with lipoma, overgrown nerve, but with no significant mass effect) suggests another important TN mechanism: a compression of the tumor on the ventral surface of the pons, which can cause irritation of the trigeminal nerve nuclei to the implementation in TN. Such a mechanism of TN described in vascular compression of the ventral surface of the pons by the large vessels (in basilar artery dolichoectasia).
When analyzing the results of surgical treatment in our group the degree of pain relief was taken into account as the main criterion (however, in our series of observations, along with the trait, symptoms of other cranial nerve dysfunction were also seen; see Table 2).
After tumor removal the relationship of cranial nerves with the offending vessels should be carefully assessed for optimal functional results and complete pain relief. Tumor resection should be supplemented with MVD in disturbed neurovascular relationships. In some cases exploration and decompression of the ventral side of the pons are required.
Our results demonstrate a good efficiency of CPA tumors microsurgical treatment and the low rate of complications, especially in the early diagnosis of the disease. We would like to note the usefulness of neuroimaging methods (especially MRI) in all patients with clinical manifestations of TN, and facial pain as a whole (as stated in AAN-EFNS guidelines on TN management).12 In our study, three patients (21%) who underwent destructive procedure, if they have CPA, only confirms this position.
CONCLUSION
TN is an expectative and typical symptom of CPA tumors, including one of the first symptoms. The most common cause of secondary TN in CPA tumors is epidermal intracranial tumors.
Brain MRI is indicated for all patients with clinical manifestations of TN for early diagnosis of tumors and effective microsurgical treatment. Careful examination of the trigeminal nerve and the ventral surface of the pons are required during tumor removal. It is essential that tumor removal has to be supplied with the MVD in neurovascular relationships disruption.
References
- Headache Classification Committee of the International Headache Society Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia. 2004;24(1):1–156. [PubMed] [Google Scholar]
- Cruccu G, Leandri M, Feliciani M, Manfredi M. Idiopathic and symptomatic trigeminal pain. J Neurol Neurosurg Psychiatry. 1990;53(12):1034–1042. doi: 10.1136/jnnp.53.12.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannetta P J. Microvascular decompression of trigeminal nerve for tic doloureux. In: Youmans J R, editor. Neurological Surgery. London: WB Saunders; 1997. pp. 3728–3756. [Google Scholar]
- Kobata H, Kondo A, Iwasaki K. Cerebellopontine angle epidermoids presenting with cranial nerve hyperactive dysfunction: pathogenesis and long-term surgical results in 30 patients. Neurosurgery. 2002;50(2):276–285. discussion 285–286. doi: 10.1097/00006123-200202000-00008. [DOI] [PubMed] [Google Scholar]
- Jamjoom A B, Jamjoom Z A, al-Fehaily M, el-Watidy S, al-Moallem M. Nain-Ur-Rahman. Trigeminal neuralgia related to cerebellopontine angle tumors. Neurosurg Rev. 1996;19(4):237–241. doi: 10.1007/BF00314838. [DOI] [PubMed] [Google Scholar]
- Puca A, Meglio M. Typical trigeminal neuralgia associated with posterior cranial fossa tumors. Ital J Neurol Sci. 1993;14(7):549–552. doi: 10.1007/BF02339213. [DOI] [PubMed] [Google Scholar]
- Puca A, Meglio M, Tamburrini G, Vari R. Trigeminal involvement in intracranial tumours. Anatomical and clinical observations on 73 patients. Acta Neurochir (Wien) 1993;125(1-4):47–51. doi: 10.1007/BF01401827. [DOI] [PubMed] [Google Scholar]
- Ogleznev KYa, Grigoryan YuA, Slavin K V. Parapontine epidermoid tumours presenting as trigeminal neuralgias: anatomical findings and operative results. Acta Neurochir (Wien) 1991;110(3-4):116–119. doi: 10.1007/BF01400677. [DOI] [PubMed] [Google Scholar]
- Matsuka Y, Fort E T, Merrill R L. Trigeminal neuralgia due to an acoustic neuroma in the cerebellopontine angle. J Orofac Pain. 2000;14(2):147–151. [PubMed] [Google Scholar]
- Samii M, Matthies C. Acoustic neurinomas associated with vascular compression syndromes. Acta Neurochir (Wien) 1995;134(3-4):148–154. doi: 10.1007/BF01417682. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Kondo A, Otsuka S, Hasegawa K, Ohbayashi T. Painful tic convulsif caused by a brain tumor: case report and review of the literature. Neurosurgery. 1992;30(6):916–919. doi: 10.1227/00006123-199206000-00017. [DOI] [PubMed] [Google Scholar]
- Cruccu G, Gronseth G, Alksne J, et al. American Academy of Neurology Society. European Federation of Neurological Society AAN-EFNS guidelines on trigeminal neuralgia management. Eur J Neurol. 2008;15(10):1013–1028. doi: 10.1111/j.1468-1331.2008.02185.x. [DOI] [PubMed] [Google Scholar]