Abstract
Acquired perineovulvar defects are usually the result of excision of vulvar intraepithelial neoplasia (VIN) or invasive squamous cell carcinoma. Because both VIN and vulvar carcinoma have a tendency toward local recurrence, future reconstructive options should be reckoned with during treatment of the primary and all subsequent (pre-) malignant perineovulvar lesions. Hence, a proposal of sequence of reconstructive options for these defects is called for. In cases where local skin flaps suffice, these are preferably designed in such a fashion as not to sever the branches of the internal pudendal vascular system. In cases where either a pudendal thigh flap or an infragluteal flap may be used to close the perineovulvar defect, the pudendal thigh flap is to be preferred to preserve the infragluteal flap for future use. Only when these flaps no longer are available or sufficient to cover the defect should the gluteal thigh flap be applied. The use of myocutaneous flaps is rarely indicated to close isolated superficial perineovulvar defects.
Keywords: Vulvar carcinoma, vulvar intraepithelial neoplasia, vulvar reconstruction, perineum reconstruction, internal pudendal artery
ACQUIRED PERINEOVULVAR DEFECTS
Acquired perineovulvar defects are usually the result of excision of (pre-)malignant lesions and rarely caused by infection, trauma, or the excision of burn scars or lichen sclerosus.1,2,3 The (pre-)malignant lesions occur in 5 to 10 per 100,000 women in developed countries and include vulvar intraepithelial neoplasia (VIN) and frank invasive carcinoma, predominately squamous cell carcinoma.4,5,6,7 VIN is commonly diagnosed in the fifth decade of life and occurs multifocal in half of the patients whereas vulvar carcinoma predominately affects the 55- to 80-years age group.6,8,9
Surgery is still considered the cornerstone of treatment of these (pre-)malignancies,10,11 but there is a definite trend toward reducing the extent of the perineovulvar excision.5 Modified radical vulvectomy or wide local excision may be successful in terms of cure and do better to address the concerns regarding the psychological and behavioral sequelae secondary to disfigurement, loss of body image, and perceived loss of sexual function related to more extensive, radical vulvectomy.6,11,12,13,14 Consequently, optimal esthetic and functional perineovulvar reconstruction is now considered an integral part of treatment of these (pre-)malignancies as it may markedly improve the quality of life, self-esteem, and sexual rehabilitation of the patient with perineovulvar damage.3,6,13
Because of its relative low incidence and its significant physical, sexual, and psychological morbidity, treatment of perineovulvar intraepithelial or invasive neoplasia is best handled in multidisciplinary oncology teams.1,2,6,7,8,13,15,16 Centralization of these teams allows for the concentration of the otherwise limited experience in management of these diseases and immediate perineovulval reconstruction illustrates the merits of such an interdisciplinary approach between gynecology and plastic surgery in these patients.13
OPTIONS OF PERINEOVULVAR RECONSTRUCTION
Recently, it has been recognized that the choice of technique for perineovulvar reconstruction is largely dictated by the extent of the resection and the involvement of the surrounding anatomical structures rather than by the stage of the disease.5,17,18 Based on this observation and the principles of the reconstructive ladder, algorithms for such reconstruction have been proposed.6,11,17,18 A detailed description of each of these reconstructive procedures is beyond the scope of this review; therefore, references to such descriptions are provided instead.
The broad range of techniques employed for perineovulvar reconstruction implies that no one technique can address the requirements of such reconstruction in all patients and that the technique is to be selected in accordance with the condition of each individual patient and the skill and preference of her reconstructive surgeon. Although the achievable reconstructive results are esthetically quite crude compared with the normal state without disease, perineovulvar reconstruction should not only aim to close the defect. To date, it has been acknowledged that the ideal reconstructive procedure should (1) bring a comparable thickness of well-vascularized subcutaneous tissue and skin, (2) allow for adaptation of the flap surface to the size of the recipient defect, (3) reestablish the function and sensibility of the perineovulvar area, (4) allow for the recreation of a natural perineovulvar appearance, (5) allow for a single-stage closure of the defect, and (6) minimize donor site morbidity.4,16,18,19
Reproducing the region's basic functional anatomy and the natural convexity of the major labia implies that healing by secondary intention should not be allowed in any but the smallest defects because it results in significant morbidity.2,6,11,20 In women who previously underwent local radiotherapy, wounds that are left to heal secondarily can remain open for up to 6–12 months.1 This is an unacceptable burden to the patient and the implicit delay to return to normal function can no longer be justified. Primary closure often leads to the same outcome as up to 85% of primarily closed vulvectomy wounds may become dehiscent.5,15 Primary closure should be applied only in cases where the maximum width of the excision defect perpendicular to the skin release tension lines measures up to 2 cm maximal. Such closure may easiest be accomplished in elderly women in whom the minor or major labia often offer abundant tissue surplus.
When primary closure does not suffice, split thickness or full thickness skin grafts may be used to cover vulvar, but not perineal defects. However, even when used in the vulvar region skin grafts are bothersome to fix and damage from previous or future radiotherapy may further limit the use of these grafts.3,7 Moreover, once grown in, grafts tend to retract and distort the local anatomy and such distortion often results in functional, aesthetic, and psychological sequelae.11,21
Transposition of skin flaps may prevent short-term wound dehiscence resulting from undue tension and a long-term “open” distortion of the introitus, urethra, or anus.6,7,18 The abundance of perineovulvar blood supply offers the possibility of various designs for multiple local skin flaps.17 Traditionally, rhomboid flaps have been applied and this flap alone may be designed in many different ways to address the individual need of the patient after an excision that is restricted to a maximum of 20 cm2.3,12 For larger defects, other local transposition flaps have been suggested.2,11,13,18
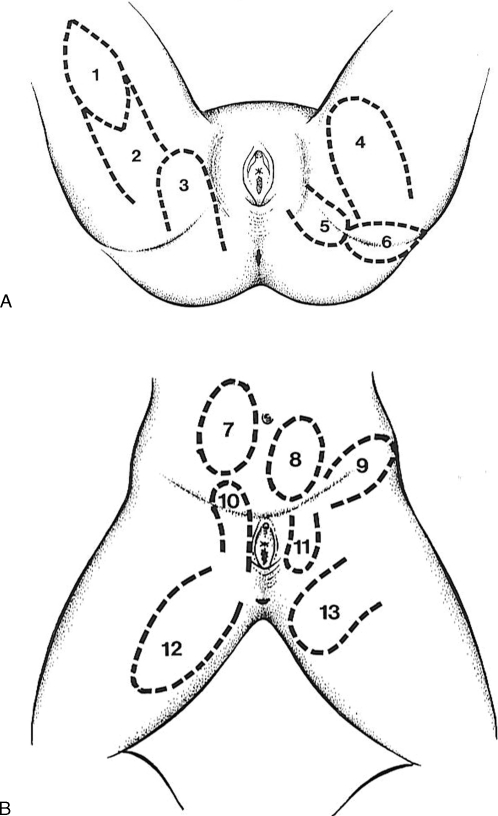
The multiple vascular supply of the perineal, pubic, inguinal, gluteal, infragluteal, and upper thigh regions allows for many regional fasciocutaneous flaps to be raised (Fig. 1).2,9,16,20,21,22,23,24,25,26 Most of these flaps may be applied either as V-Y flaps,2,9,20,25 pedicled transposition flaps,2,16,26 subcutaneously pedicled flaps,22,23,25 or true island flaps.20 Because the vascular systems of the inguinal and perineovulvar region anastomose with each other, these flaps may be raised as extended flaps or as reversed flaps depending on a retrograde blood flow. In all cases, care has to be taken that the resulting donor or recipient scars do not perpendicularly cross the inguinal, vulvocrural, or infragluteal fold as contractures of such scars would compromise the mobility of the upper legs and often leave the perineovulvar structures and orifices exposed.1,9
Figure 1.
(A, B) Examples of fasciocutaneous flaps that may be used for perineo-vulvar reconstruction. 1, gluteal thigh island flap; 2, gluteal thigh pedicled flap; 3, gluteal flap; 4, transverse medial thigh flap; 5, infragluteal flap; 6, inferior gluteal flap; 7, superficial epigastric flap; 8, external pudendal flap; 9, inguinal flap; 10, pudendal thigh flap; 11, reversed pudendal thigh flap; 12, longitudinal medial thigh flap; 13, superomedial thigh flap.
Of the region's vascular pedicles, the terminal branches of the internal pudendal vascular system allow for the “workhorse” fasciocutaneous flaps for perineovulvar reconstruction: the pudendal thigh or vulvoperineal flap anteriorly,16,21,22,26,27 and the infragluteal flap posteriorly.2,4,9,19,20,21,25 Pudendal thigh flaps may be used as a V-Y advancement flap for lateral vulvar defects, as a transposition or island flap reaching the perineum and perianal region (Fig. 2), or as a reversed flap for pubic defects. The infragluteal flap may be used as a V-Y advancement flap to close perianal, perineal, or lateral vulvar defects, or it may be further dissected circumferentially to be rotated anteriorly to unilaterally replace the major labium and close lateral and anterior vulvar defects (Fig. 3).4 Moreover, a long infragluteal transposition may be rotated 180 degrees to be draped over the midline and simultaneously fill a perineal, a median posterior commissural, a contralateral introital, and a contralateral labial defect, with the most distal tip of the flap reaching the urethral meatus. In this, we disagree with Salgarello et al18 that bilateral flaps are to be preferred in cases of bilateral semivulvar reconstruction because a unilateral flap placed across the midline will experience distal necrosis.
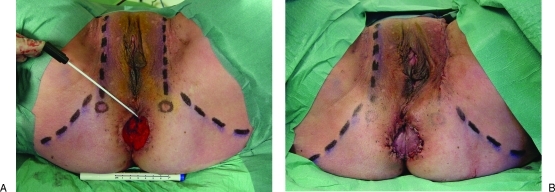
Figure 2.
Following excision of vulvar intraepithelial neoplasia in a 48-year-old woman (A), a pedicled pudendal thigh island flap easily reached the perianal defect (B). Note that the swabstick points to the anus and both circles bilaterally indicate the exit point of the internal pudendal vessels. Both vertical broken lines indicate the posterior labial vascular system that anastomoses with the external pudendal vascular system anteriorly; both horizontal broken lines bilaterally indicate the infragluteal folds.
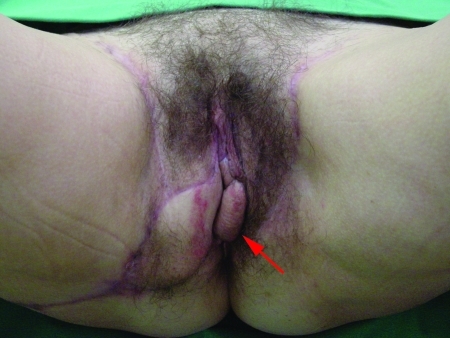
Figure 3.
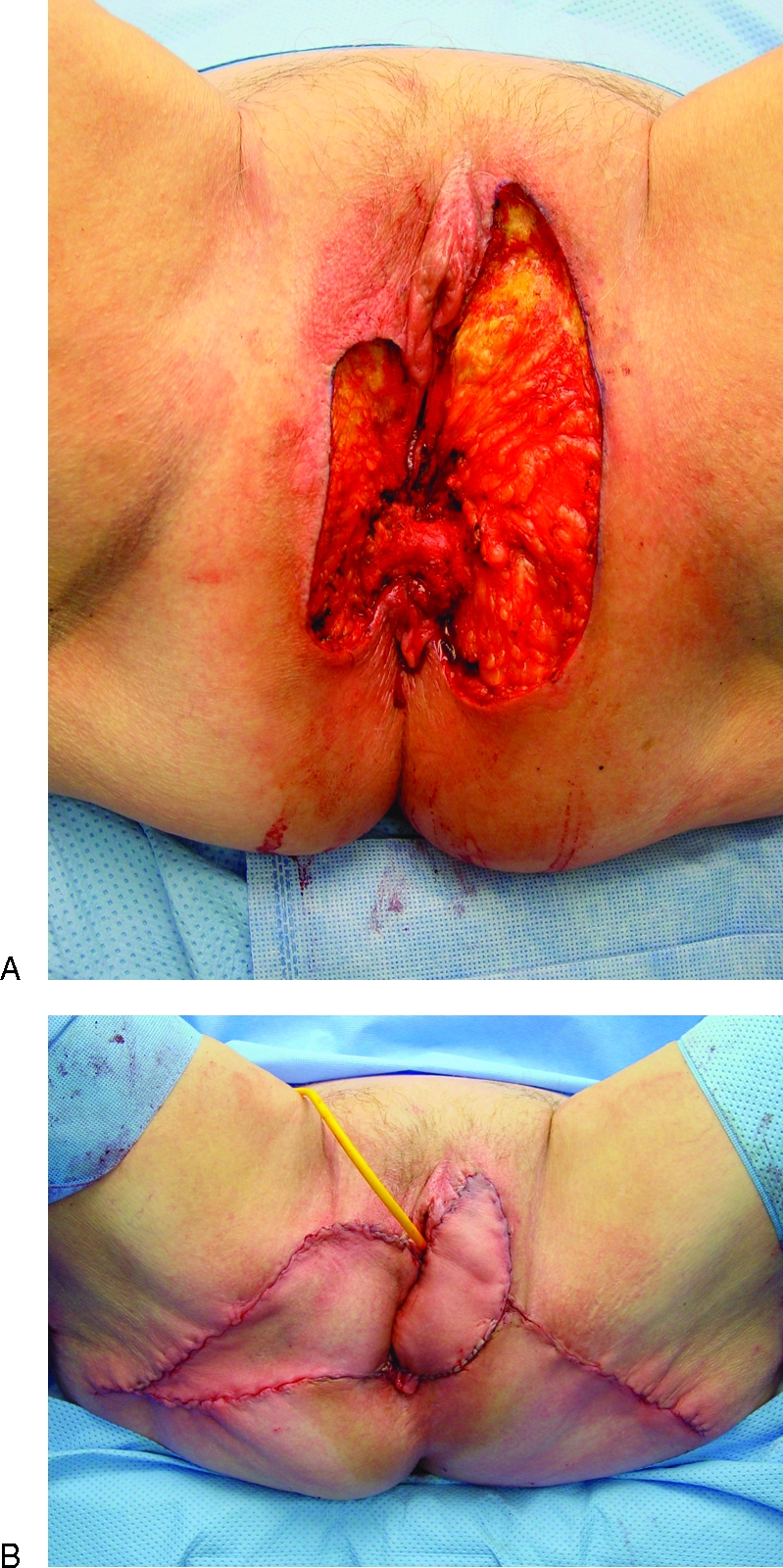
Following resection of recurrent vulvar squamous cell carcinoma in a 74-year-old woman (A), the right infragluteal flap was advanced as a V-Y flap while the left infragluteal flap was dissected circumferentially to be used as an island flap (B). Note that the infragluteal flap may easily reach anterior vulvar defects.
The reliability of these flaps based on the internal pudendal system has been repeatedly confirmed.19,27,28 The internal pudendal vessels consistently exit at a point just medial of the ischial tuberosity and reliably give rise to a medial perineal branch and lateral infragluteal branch before continuation as the dorsal labial vascular system. When raised primarily or secondarily, the infragluteal island flap may be thinned up to the level of Scarpa's fascia.4,19,25 Instead of the workhorse flaps based on the internal pudendal vascular system, lower abdominal flaps based on the external pudendal vessels, inguinal flaps based on the circumflex inguinal vessels, or gluteal thigh flap based on the descending branches of the inferior gluteal vessels may be applied.12,23,24,29
Consequently, there is little need for musculocutaneous flaps in cases where superficial defects are restricted to the perineovulvar region. Of these, the gracilis myocutaneous flap is of limited use for perineovulvar reconstruction as it has a short arc of rotation with a pivot point that is located some 10 cm caudal from the recipient defect and a poor reputation of the vascularization of its skin paddle.5,6,11,15,16,22,30 Most, or probably all, of the indications of a pedicled gracilis myocutaneous or muscular flap may be addressed by use of the infragluteal flap that is closer and more reliable. The transversely, vertically, or obliquely designed skin paddle of the rectus abdominis myocutaneous flap is well suited to close perineovulvar defects that are part of more extended tissue losses such as in patients who underwent pelvic exenteration or in whom wound repair includes a reconstruction of the vagina.6,7,11,18
Given the number of local and regional flaps available, microsurgical free flap transfers are seldom required for perineovulvar reconstruction.3 If any are, the radial forearm flap, the latissimus dorsi flap, and the subscapular flaps have been suggested for this.1,31
RECURRENT ACQUIRED PERINEOVULVAR DEFECTS
Both vulvar intraepithelial neoplasia and vulvar squamous cell carcinoma often recur. Up to 50% of all women need more than one intervention for VIN within the first 14 years after initial diagnosis, either for disease persistence or recurrence.8 Moreover, progression of VIN to invasive carcinoma occurs in 3% of patients after treatment, and is more likely to develop in VIN associated with infection with transcriptionally active human papillomavirus than in noninfectious VIN.8,32,33
Nonlocal recurrences of vulvar carcinoma are usually diagnosed within 2 years after primary surgery, whereas only local recurrences were observed after these 2 years.14 Although the majority of patients (70%) survive,5 the 5-year local recurrence rate of vulvar carcinoma is 21–33%14,34,35 depending on the technique of vulvectomy and lymphadenectomy.15 Nearly 10% of all patients present with a vulvar recurrence after the first 5 years.35 Many of the vulvar recurrences are amenable to repeat excision with a control rate comparable to that of primary disease (60%),10,30,35 and for patients in whom the first recurrence site is the vulva, the second recurrence (or reoccurrence) may invariably also be expected on the vulva.35
Because of possible, or even probable surgical excision of residual or recurrent disease, future reconstructive options should be reckoned with during treatment of the primary and all subsequent (pre-)malignant perineovulvar lesions. Hence, a sequence of reconstructive options for these defects is indicated.
A PROPOSAL OF SEQUENCE OF RECONSTRUCTIVE OPTIONS FOR PERINEOVULVAR DEFECTS
In cases where local skin flaps may address perineovulvar reconstructive needs, these are preferably designed in such a fashion as not to sever the posterior labial branches of the internal pudendal system to the pudendal thigh flap or its branches to the infragluteal flap. Ideally, the local flaps are designed as part of one of these two flaps, that, then are initially used in part only. This way, in the future, the local flap may be raised as part of a larger flap needed to close the new, larger defect. Alternatively, a pudendal thigh or infragluteal flap that may appear “too large” for the primary defect is raised and transposed in only a limited fashion to allow for possible reuse and more extensive transplantation in the future.
In cases where either an external pudendal, an inguinal, or a reversed pudendal thigh flap may be used to close an anterior vulvar or pubic defect, the inguinal flap is preferred. Use of an external pudendal flap or the reversed pudendal thigh flap in these cases would imply severing the posterior labial branches of the internal pudendal vascular system and,27 consequently, loss of the anterograde pudendal thigh flap for future use.
Likewise the pudendal thigh flap is to be preferred in cases where either a pudendal thigh flap or an infragluteal flap may be used to close the perineovulvar defect. The perforating vessels of the internal pudendal system at the base of the flap and its immediate branches to the infragluteal fold need not be seen, dissected, or circumcised when raising the pudendal thigh flap as a fasciocutaneous flap with a skin pedicle, as a subcutaneously pedicled flap, or as a true island flap. This allows for transplantation of the pudendal thigh flap without severing the infragluteal branches. This way, the infragluteal flap is still available for future use (Fig. 4). Dissection of the infragluteal flap as a V-Y flap or a transposition flap, on the other hand, principally involves severing the posterior labial branches, and consequently offering the possibility of future use of a pudendal thigh flap.
Figure 4.
Following resection of vaginal carcinoma in a 58-year-old woman, bilateral pudendal thigh flaps were used to reconstruct the distal half of the vagina and the left side of the introitus (arrow). Six months later, an infragluteal island flap could still be used to close a subsequent right-sided vulvar defect. Note the inguinal scars that bilaterally indicate the donor sites of the pudendal thigh flap.
Primary use of the pudendal thigh flap may only be contraindicated for technical reasons in women in whom inguinal lymph node dissection is similarly performed.21 Still, even this contraindication is relative as no hard oncological contraindication exists. In cases of lymph node involvement, the flap will undergo adjuvant radiotherapy whether it is transposed to the perineovulvar region or not. Unlike others,4,18,22 therefore, we feel that the possibility of lymphatic spread to the groin should not preclude the use of the pudendal thigh flap in postoncologic vulvar reconstruction.
Once a pudendal thigh or infragluteal V-Y flap has been used, it may secondarily be reused by circumferential dissection to allow for its transpositioning as an island flap. Again, this implies that it may be wise to design and advance each V-Y flap of larger dimensions than strictly needed primarily; the extra skin may be needed secondarily as part of the transposition flap used to close a larger future defect.
Only when these internal pudendal flaps are no longer available or no longer sufficient to cover the defect should the gluteal thigh flap be applied.12,29 Again, this pedicled flap may be raised as a fasciocutaneous flap with a skin pedicle, as a subcutaneously pedicled flap, or as a true island flap. These flaps may all be designed much larger than either of the internal pudendal flaps and should be reserved for the reconstruction of major perineovulvar defects. Reliable use of the gluteal thigh flap is still possible in cases where the resulting scar of a previous infragluteal transposition flap extends over the full length of the infragluteal fold (Fig. 5). The descending branches of the inferior gluteal vessels and its accompanying nerve enter the subcutaneous tissue of the gluteal thigh flap distal to the gluteus maximus muscle and distal to the donor scar of the infragluteal flap.
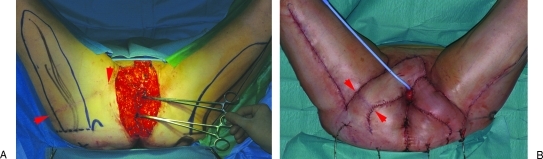
Figure 5.
Following resection of a third recurrence of vulvar carcinoma in a 62-year-old woman (A), bilateral gluteal thigh flaps were transpositioned to close the defect (B). Note that the donor site scar of the previously applied infragluteal flap runs the full width of the skin pedicle of the right-sided gluteal thigh flap (arrows).
In case of a perineovulvar defect without the need of intrapelvic bulk or vaginal reconstruction, the use of a rectus abdominis myocutaneous flap may be limited to a salvage procedure after the gluteal thigh flaps have been used.16,20 Because of its bulk, the aesthetic and functional outcome of such use of the rectus abdominis flap is inferior, and abdominal wall weakness and lower back problems are well-known sequela of the loss of this muscle.7,16
CONCLUSIONS
Although perineovulvar (pre-)malignancies have a low incidence, their surgical treatment often implies anatomically, functionally, and aesthetically complex defects. Therefore, this treatment is best achieved in full cooperation between the oncologic gynecologist and a plastic surgeon with vast experience in perineovulvar reconstructions. Given the high recurrence rates of both VIN and vulvar carcinoma, future reconstructive options should be reckoned with during the reconstruction of treatment of (pre-)malignant perineovulvar lesions. We propose a sequence of options that enhances such reconstruction of possible, or even probable future perineovulvar defects.
References
- McCraw J B, Papp C, Ye Z, Huang V, McMellin A. Reconstruction of the perineum after tumor surgery. Surg Oncol Clin N Am. 1997;6(1):177–189. [PubMed] [Google Scholar]
- Niranjan N S. Perforator flaps for perineal reconstructions. Semin Plast Surg. 2006;20:133–144. [Google Scholar]
- Friedman J D. Reconstruction of the perineum. In: Thorne C H, editor. Grabb and Smith's Plastic Surgery. Philadelphia: Lippincott Williams Wilkins; 2007. pp. 708–716. [Google Scholar]
- Ragoowansi R, Yii N, Niranjan N. Immediate vulvar and vaginal reconstruction using the gluteal-fold flap: long-term results. Br J Plast Surg. 2004;57(5):406–410. doi: 10.1016/j.bjps.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Weikel W, Hofmann M, Steiner E, Knapstein P G, Koelbl H. Reconstructive surgery following resection of primary vulvar cancers. Gynecol Oncol. 2005;99(1):92–100. doi: 10.1016/j.ygyno.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Höckel M, Dornhöfer N. Vulvovaginal reconstruction for neoplastic disease. Lancet Oncol. 2008;9(6):559–568. doi: 10.1016/S1470-2045(08)70147-5. [DOI] [PubMed] [Google Scholar]
- Staiano J J, Wong L, Butler J, Searle A E, Barton D P, Harris P A. Flap reconstruction following gynaecological tumour resection for advanced and recurrent disease—a 12 year experience. J Plast Reconstr Aesthet Surg. 2009;62(3):346–351. doi: 10.1016/j.bjps.2007.12.050. [DOI] [PubMed] [Google Scholar]
- Jones R W, Rowan D M, Stewart A W. Vulvar intraepithelial neoplasia: aspects of the natural history and outcome in 405 women. Obstet Gynecol. 2005;106(6):1319–1326. doi: 10.1097/01.AOG.0000187301.76283.7f. [DOI] [PubMed] [Google Scholar]
- Lee P K, Choi M S, Ahn S T, Oh D Y, Rhie J W, Han K T. Gluteal fold V-Y advancement flap for vulvar and vaginal reconstruction: a new flap. Plast Reconstr Surg. 2006;118(2):401–406. doi: 10.1097/01.prs.0000227683.47836.28. [DOI] [PubMed] [Google Scholar]
- Maggino T, Landoni F, Sartori E, et al. Patterns of recurrence in patients with squamous cell carcinoma of the vulva. A multicenter CTF Study. Cancer. 2000;89(1):116–122. doi: 10.1002/1097-0142(20000701)89:1<116::aid-cncr16>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Fowler J M. Incorporating pelvic/vaginal reconstruction into radical pelvic surgery. Gynecol Oncol. 2009;115(1):154–163. doi: 10.1016/j.ygyno.2009.05.040. [DOI] [PubMed] [Google Scholar]
- Loree T R, Hempling R E, Eltabbakh G H, Recio F O, Piver M S. The inferior gluteal flap in the difficult vulvar and perineal reconstruction. Gynecol Oncol. 1997;66(3):429–434. doi: 10.1006/gyno.1997.4790. [DOI] [PubMed] [Google Scholar]
- Narayansingh G V, Cumming G P, Parkin D P, McConell D T, Honey E, Kolhe P S. Flap repair: an effective strategy for minimising sexual morbidity associated with the surgical management of vulval intra epithelial neoplasia. J R Coll Surg Edinb. 2000;45(2):81–84. [PubMed] [Google Scholar]
- De Hullu J A, Hollema H, Lolkema S, et al. Vulvar carcinoma. The price of less radical surgery. Cancer. 2002;95(11):2331–2338. doi: 10.1002/cncr.10969. [DOI] [PubMed] [Google Scholar]
- Carramaschi F, Ramos M L, Nisida A C, Ferreira M C, Pinotti J A. V-Y flap for perineal reconstruction following modified approach to vulvectomy in vulvar cancer. Int J Gynaecol Obstet. 1999;65(2):157–163. doi: 10.1016/s0020-7292(99)00016-8. [DOI] [PubMed] [Google Scholar]
- Monstrey S, Blondeel P, Landuyt K Van, Verpaele A, Tonnard P, Matton G. The versatility of the pudendal thigh fasciocutaneous flap used as an island flap. Plast Reconstr Surg. 2001;107(3):719–725. doi: 10.1097/00006534-200103000-00011. [DOI] [PubMed] [Google Scholar]
- Friedman J, Dinh T, Potochny J. Reconstruction of the perineum. Semin Surg Oncol. 2000;19(3):282–293. doi: 10.1002/1098-2388(200010/11)19:3<282::aid-ssu10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Salgarello M, Farallo E, Barone-Adesi L, et al. Flap algorithm in vulvar reconstruction after radical, extensive vulvectomy. Ann Plast Surg. 2005;54(2):184–190. doi: 10.1097/01.sap.0000141381.77762.07. [DOI] [PubMed] [Google Scholar]
- Hashimoto I, Nakanishi H, Nagae H, Harada H, Sedo H. The gluteal-fold flap for vulvar and buttock reconstruction: anatomic study and adjustment of flap volume. Plast Reconstr Surg. 2001;108(7):1998–2005. doi: 10.1097/00006534-200112000-00025. [DOI] [PubMed] [Google Scholar]
- Moschella F, Cordova A. Innervated island flaps in morphofunctional vulvar reconstruction. Plast Reconstr Surg. 2000;105(5):1649–1657. doi: 10.1097/00006534-200004050-00008. [DOI] [PubMed] [Google Scholar]
- Yii N W, Niranjan N S. Lotus petal flaps in vulvo-vaginal reconstruction. Br J Plast Surg. 1996;49(8):547–554. doi: 10.1016/s0007-1226(96)90132-0. [DOI] [PubMed] [Google Scholar]
- Wee J T, Joseph V T. A new technique of vaginal reconstruction using neurovascular pudendal-thigh flaps: a preliminary report. Plast Reconstr Surg. 1989;83(4):701–709. doi: 10.1097/00006534-198904000-00018. [DOI] [PubMed] [Google Scholar]
- Mayer A R, Rodriguez R L. Vulvar reconstruction using a pedicle flap based on the superficial external pudendal artery. Obstet Gynecol. 1991;78(5 Pt 2):964–968. [PubMed] [Google Scholar]
- Spear S L, Pellegrino C J, Attinger C E, Potkul R K. Vulvar reconstruction using a mons pubis flap. Ann Plast Surg. 1994;32(6):602–605. doi: 10.1097/00000637-199406000-00007. [DOI] [PubMed] [Google Scholar]
- Knol A C, Hage J J. The infragluteal skin flap: a new option for reconstruction in the perineogenital area. Plast Reconstr Surg. 1997;99(7):1954–1959. doi: 10.1097/00006534-199706000-00022. [DOI] [PubMed] [Google Scholar]
- Giraldo F, González C. The versatility of the pudendal thigh fasciocutaneous flap used as an island flap. Plast Reconstr Surg. 2001;108(7):2172–2174. doi: 10.1097/00006534-200112000-00083. [DOI] [PubMed] [Google Scholar]
- Giraldo F, Mora M J, Solano A, Abehsera M, Ferrón M, Smith J M. Anatomic study of the superficial perineal neurovascular pedicle: implications in vulvoperineal flap design. Plast Reconstr Surg. 1997;99(1):100–108. doi: 10.1097/00006534-199701000-00016. [DOI] [PubMed] [Google Scholar]
- Salmon M. The external genitalia and the perineum. In: Taylor G I, Tempest M N, editors. Arteries of the Skin. London: Churchill Livingstone; 1988. pp. 109–128. [Google Scholar]
- Hurwitz D J, Swartz W M, Mathes S J. The gluteal thigh flap: a reliable, sensate flap for the closure of buttock and perineal wounds. Plast Reconstr Surg. 1981;68(4):521–532. [PubMed] [Google Scholar]
- Weikel W, Schmidt M, Steiner E, Knapstein P G, Koelbl H. Surgical therapy of recurrent vulvar cancer. Am J Obstet Gynecol. 2006;195(5):1293–1302. doi: 10.1016/j.ajog.2006.03.049. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Maxwell R. Vulval reconstruction by free tissue transfer. Case report. Br J Obstet Gynaecol. 1991;98(1):98–100. doi: 10.1111/j.1471-0528.1991.tb10318.x. [DOI] [PubMed] [Google Scholar]
- Seters M van, Beurden M van, de Craen A J. Is the assumed natural history of vulvar intraepithelial neoplasia III based on enough evidence? A systematic review of 3322 published patients. Gynecol Oncol. 2005;97(2):645–651. doi: 10.1016/j.ygyno.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Eva L J, Ganesan R, Chan K K, Honest H, Malik S, Luesley D M. Vulval squamous cell carcinoma occurring on a background of differentiated vulval intraepithelial neoplasia is more likely to recur: a review of 154 cases. J Reprod Med. 2008;53(6):397–401. [PubMed] [Google Scholar]
- Rouzier R, Haddad B, Plantier F, Dubois P, Pelisse M, Paniel B J. Local relapse in patients treated for squamous cell vulvar carcinoma: incidence and prognostic value. Obstet Gynecol. 2002;100(6):1159–1167. doi: 10.1016/s0029-7844(02)02501-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez Bosquet J, Magrina J F, Gaffey T A, et al. Long-term survival and disease recurrence in patients with primary squamous cell carcinoma of the vulva. Gynecol Oncol. 2005;97(3):828–833. doi: 10.1016/j.ygyno.2005.03.006. [DOI] [PubMed] [Google Scholar]