Abstract
Extensive defects of the ear require satisfactory cosmetic reconstruction to enable the patient to achieve full social integration. Although surgical procedures are the gold standard for reconstruction of the ear, in some cases they cannot be performed because of extended scars, threatening tumor, or congenital tissue abnormalities. Prosthetic reconstruction of the auricle is an established and reliable alternative technique to autologous surgical reconstructions. Since studies performed by Brånemark, osseointegrated implants have been widely used to provide a reliable and stable anchorage for a prosthesis (prosthesis anchored to bone). To allow good osseointegration of the titanium screw implants, two stages are necessary. After careful preparation for the surgical procedure (local and general examination, computed tomography scan, skin preparation), screws are implanted into bone, which are then covered by a skin flap. During the second stage, the skin is incised, and penetrating fixtures are attached to the screw implants, which allow fixation of the prosthesis. This procedure is reliable and reproducible, with good to excellent results and stability over time.
Keywords: Prosthesis, ear, osseointegration, plastic surgery
Historically, prostheses were used in many places, since 1500 B.C. in India, 300 B.C. in China, and in European civilizations.1 The technique has been significantly improved since Ambroise Paré and Petronius in the 16th century. The main issue with the use of these prostheses was their attachment to the body. Many methods were used: from laces and wires to glasses or pieces of tape.
Acquired loss of the auricle is a source of psychological distress; patients focus on it and it impairs their social life.1 Prosthetic reconstruction of the auricle (PRA) has two main goals: reconstruction of an aesthetically pleasing auricle, and return to a normal life without the anxiety of having a prosthesis not perfectly fit.
Studies by Brånemark on bone healing and vascularization, as well as osseointegration of foreign metal in the body, made possible the use of titanium screw implants to hold prosthesis perfectly. First used for oral implants, their use has been rapidly extended to extraoral indications. In the field of prosthetics, it has been a major milestones. By using three or more stable bone anchored fixtures, we have precisely placed, easily handled prostheses.
Extraoral implants are currently widely used by surgeons to reconstruct difficult cases of massive tissue loss on the head and neck. The auricle, in particular, is a complex structure that is difficult to reconstruct and requires a satisfactory result to allow the patient a normal social life. When general or local conditions are not adequate to permit surgical reconstruction, an auricular prosthesis anchored to bone implants is a technique that gives excellent results. The technique is reliable and reproducible, even in irradiated tissues. We report our experience with the indications, technique, and results of auricular prostheses anchored to extraoral implants.
INDICATIONS
The three major indications for prosthetic auricular reconstruction are trauma, oncologic defects, and congenital abnormalities.
Trauma
A complex wound of the auriculotemporal area with massive soft tissue loss is a good indication for PRA, especially when the retroauricular skin is affected by trauma. Total otopiesis performed with costal cartilage is possible only in cases where skin and subcutaneous tissue are intact.2,3,4,5,6,7 Therefore, a large wound affecting both the auricle as well as the temporal region precludes the possibility of an autologous ear reconstruction. Deep burns of the auriculotemporal area affect local tissues in the same way, with fibrotic scars preventing a satisfactory autologous reconstruction.8
Oncologic Defects
Aggressive tumors of the auricle often require a large excision followed by radiotherapy, and follow-up of these lesions is critical. Radiotherapy also affects local tissue vascularity and therefore limits the possibility of good autologous reconstruction. Prosthetic reconstruction allows local monitoring for recurrence and may be a temporary step before an autologous reconstruction.8,9,10
Congenital Abnormalities
Microtia or partial auricular congenital abnormalities should be treated by total otopiesis whenever possible.2,5 In cases of previous surgical failure, or lack of healthy skin in the temporal region, a prosthesis anchored to bone (PAB) is a good choice for an aesthetic reconstruction. In these cases, however, temporal bone may not be thick enough to allow osseointegration of implants, highlighting the importance of bone imaging preoperatively.
Patient Selection
A patient may require a PAB rather than an autologous reconstruction because of medical contraindications to the multiple surgical procedures needed, such as cachexia.11
Contraindications
Absolute contraindications of a PAB are exceptional, and belong to logic; local conditions (osteitis, total absence of hygiene) or general conditions (terminally ill patient, leukemia, lymphoma, advanced cirrhosis, psychological conditions). The need to avoid general anesthesia is not a contraindication, as the procedures can be performed under local anesthesia. Radiotherapy is not a contraindication of a PAB, as long an extremely careful implantation is done, and if possible using hyperbaric oxygen therapy (HBO). Cases of thin temporal bone (<2.5 mm) contraindicate the use of 3-mm-long extraoral implants, because of the risk of fracture and bleeding of the lateral sinus vein. Juxtaosseus plaque like Farmand's EPITEC system allows the use of a percutaneous anchoring implant for the prosthesis where screws are placed far from the thin bone.12,13
TECHNIQUE
Preoperative Examination and Surgical Planning
CLINICAL EXAMINATION
Clinical examination should focus on loss etiology and evolution of the auricle. Careful evaluation of the skin and subcutaneous tissues of the auriculotemporal area must be made to look for tumor evolution, scars, skin quality, fibrosis or any dermatologic pathology, as well as the presence of hair, or any other conditions that contraindicate the procedure.
Local examination must highlight the health of the skin and the subcutaneous tissues. Irradiated skin is not a contraindication, but requires precautions when raising the skin flap and for healing. Skin and tissue thickness overlying the mastoid is an important parameter; thick surrounding soft tissue may create fixture movement by a leverage effect.
If hair is present at the site of the fixtures, it must be permanently removed before surgery. Hair follicles or hair growing deep to the prosthesis may become a source of infection. Many removal techniques are possible. Our first therapeutic approach is laser destruction of hair follicles. Alternatively, hair roots may be removed during the thinning of the subcutaneous tissue during the second stage.14
BONE IMAGING: DETERMINING FIXTURE POSITIONS
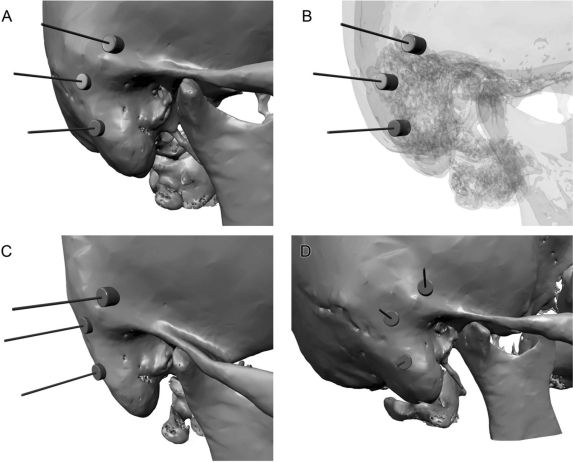
A computed tomography (CT) scan is always needed for presurgical examination of the auricular area.15,16 Whenever bone has been altered by previous therapies or congenital abnormalities, or when bone has been partially resected either with tumor or surgery of the facial nerve, a CT scan with three-dimensional (3D) reconstruction reveals adequate sites for bone implants (Figs. 1A, C, D). Implants are usually placed into the mastoid bone; part of the screws can be in a mastoid air cell without requiring relocation of the implant (Fig. 1B). Osseointegrated implants require a well-vascularized bone, thick enough to solidify around them and accept the load of the prosthesis.16 In our cases of auricular reconstruction by a PAB, we used 3- and 4-mm-long screws. In healthy bone, an adequate site for fixtures has been proposed by Tjellström on an arc of a circle located 18 mm from the external ear canal,17 which is then confirmed by morphometric studies.18
Figure 1.
Three-dimensional reconstruction of a computed tomography scan showing locations for fixtures. (A) Anterolateral view of the mastoid. (B) Same view with translucent bone showing fixtures in contact with mastoid cells. (C,D) Different views.
Use of 3D bone models, built by stereolithography using the CT scan as a template, may allow surgeons to address difficult cases.15
A lateral cephalometric radiogram is also performed as a basis for further comparisons.
RADIOTHERAPY
Radiotherapy may have an impact on the outcome of this surgery, but radiotherapy alone is not a contraindication for extraoral implants. Bone irradiation is required in many cases of tumors of the auriculotemporal region. Irradiated bone is at increased risk of infection, loosening of the fixture, and osteoradionecrosis.
The dramatic effects of irradiation on all kinds of tissues are well known.19,20,21,22 Wide variations in dose, type, fractionation scheme of irradiation; surgical technique; and time interval between radiotherapy and implant placement explain the various ranges in osseointegration and survival rate of fixtures and make comparative studies difficult.23 If available, hyperbaric oxygen (HBO) was found to increase bone healing, whenever the bone had been irradiated more than 50 Gy. In a well-defined protocol of 20 sessions of HBO preoperatively and 10 sessions after, at 2.5 Atm and 90 minutes each, one session each day, Granström lowered the failure rate of fixtures to the same level as nonirradiated bone.24,25,26
With extra precautions taken during implantation and without HBO we did not experience any fixture loosening or replacement of bone around the implant by fibrotic scar which could not efficiently hold the prosthesis. The success rate is excellent, as found by other authors.27 As tobacco induces small arterial vasoconstriction, its effect lowers oxygen in tissues, especially in irradiated tissues. It could also alter the efficacy of HBO. Therefore, smoking cessation is an important measure, and some authors even consider smoking a contraindication to a PAB.28
In soft tissues, periabutment skin infections are more frequent and harder to treat.29 Irradiated tissues are also less resistant to wound pressure ulcers. Care should be taken that prostheses are not in tight contact with the tissues to prevent these ulcers, which can lead to osteradionecrosis.29
The optimal delay between radiotherapy and implantation of fixtures depends on multiple factors. The minimal delay seems to be 6 months, when acute irradiation lesions have healed. From an oncologic point of view, depending upon the aggressiveness of the tumor and excision margins, delay should be 1 to 3 years after the completion of radiotherapy.29 In conclusion, an overall delay of 6 to 18 months after irradiation seems to be reasonable.
Surgery
FIRST STAGE: TITANIUM BONE IMPLANTS
Based upon clinical and imaging findings preoperatively, a skin flap is designed to place implants into the bone, not directly under the scar. Subcutaneous tissues are infiltrated with epinephrine diluted in normal saline for a final concentration of 1μg/mL (1mg of epinephrine per liter of normal saline). Skin is incised down to periosteum, which is also incised. The flap is then raised in the subperiosteal plane. When adequate sites are reached, holes are created in the bone to insert the implants.
Protocols may differ between manufacturers; however, all of the bone preparation is done with continuous irrigation of cold normal saline to prevent overheating, which can lead to bone necrosis. The first step is made with a 3-mm diamond burr at 2000 rev/min. The drilling is performed with a twist drill equipped with a drill stop (to limit the depth of the hole) at 2000 rev/min. Then, in cases of hard bone, tapping is done at low speed, 12 rev/min.30,31,32 Implants are positioned at the same low speed into the holes. Cover screws are placed to prevent tissue ingrowth inside the implant. The flap is repositioned and sutured in two layers. Medical treatment following this first stage consists of antibiotic prophylaxis. Our protocol includes amoxicillin 2 g per day and calcitherapy 250 mg per day for 2 weeks postoperatively.24,29 Three implants are needed to ensure a perfect stabilization of the prosthesis. In some difficult cases, only two implants may be used.
SECOND STAGE: PERCUTANEOUS FIXTURE
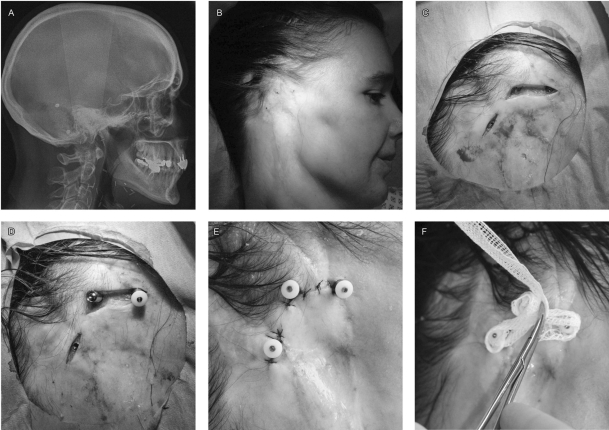
The second stage is performed 6 to 8 months later to allow the bone to heal and osseointegrate the implants. Beyond 6 to 9 months, failure of osseointegration is rare.22 A lateral cephalogram is performed to localize the positions of the implants (Fig. 2A). Palpation of tissues permits finding of the screws (Fig. 2B). The skin is incised at the implant locations, the cover screws are removed and the fixtures are positioned (Figs. 2C, D). If needed, the skin is thinned to attenuate the overall thickness of the cutaneous and subcutaneous tissues.14 Some authors propose skin grafting directly onto the periosteum as the best configuration because it cannot move around the fixtures.14 The skin is closed using sutures to limit exposure of subcutaneous tissues (Fig. 3E). Iodoform gauze dressings are applied to prevent the formation of granulation tissue until complete healing (Fig. 2F).
Figure 2.
Skin incision and fixture positioning. A 39-year-old woman who underwent resection of a cystic adenoid carcinoma of the parotid followed by irradiation of 50 Gy. (A) Lateral cephalogram showing four fixtures, three upon the mastoids for prosthesis, one posterior for bone anchored hearing aid. (B) Preoperative localization of implants. (C) Skin incisions showing implants with their cover screw. (D) Placement of transcutaneous fixtures, the posterior implant has the cover screw removed, the middle one has the transcutaneous fixture and the anterior one has the protective cap on top of the fixture. (E) Sutured skin closure to obtain good contact between fixtures and the skin, limiting subcutaneous tissue exposure. (F) Dressing with iodoform gauze, to prevent granulation tissue (even on irradiated tissues)
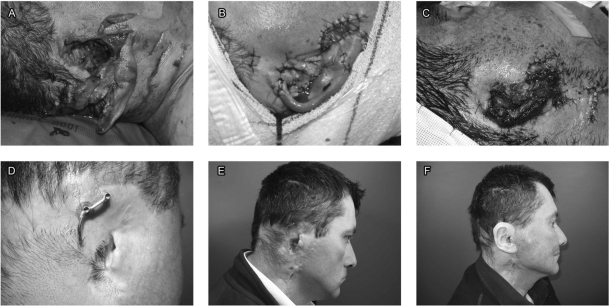
Figure 3.
Traumatic loss of the auricle. (A) Lateral aspect of the wound. (B) The next day, the auricle presented an absence of vascularization. (C) Complete necrosis of the auricle. (D) Bar positioned on the fixtures, the posterior one shows granulation tissue around the fixture. (E) Lateral view of the bar attached to fixtures. (F) Prosthesis, prior to application of color.
THIRD STAGE: PROSTHESIS
The prosthesis is constructed of silicone, by a specialized technician. The details are beyond the scope of the present article. In summary, an impression is made of the contralateral auricle, then based on this imprint a wax model of the lost auricle is designed. Several adjustments are usually required to create the final silicone prosthesis. After two months, the fixtures can be loaded with the final retention system, and can accept the prosthesis. It has to be rebuilt every 2 years, to adjust color and correct attrition.
Complications
INFECTION
Perifixture skin infection is a serious and complicated issue, arising in 15 to 20% of patients.23 The incidence increases with skin mobility around the fixture, as well as poor hygiene with deposits around the fixtures (Fig. 4A). Microflora found around the percutaneous implants is usually Staphylococcus aureus, Streptococcus species, and gram-negative bacteria.23,33,34,35,36,37 Yeast such as Candida parapsilosis is frequently found as normal flora. Topical antibiotics used to treat perifixture skin infection lead to a selection of yeast versus bacteria, but their pathologic role has not been proven.23 Usually general and topical treatments lead to a complete resolution of the infection, without loss of the fixture. Our initial treatment is oral Augmentin and topically fucidic acid. The prosthesis must not be worn until complete resolution of the infection.
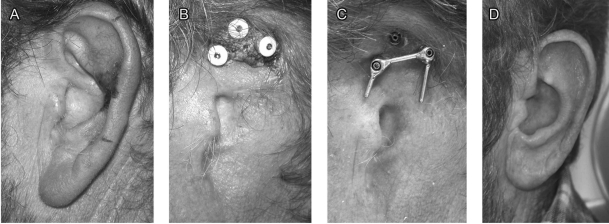
Figure 4.
Squamous cell carcinoma of the auricle. (A) Lateral aspect of the auricle, extensive carcinoma on the helix. (B) Deposits around the fixtures. (C) Bar attached to two fixtures. (D) Anterolateral aspect of the prosthesis.
GRANULATION TISSUE
As a result of colonization of the perifixture skin by bacteria, local inflammatory granulation tissue may arise (Fig. 4B). To prevent this complication, skin around the fixtures should be carefully sutured during the second stage. If granulation tissue appears, and especially if it is infected, bleeding may occur.
To treat this complication, topical treatment using silver nitrate is our first choice. If infected, or if perifixture exudates are not completely clean,23 antibiotics must be used. In our hands, the thinner the subcutaneous tissue, the lower the complication rate. The best dressing is iodoform gauze, as it tends to dry the wound as opposed to Vaseline-based dressings. We did not use corticotherapy as it may conceal a deep infection in a site close to the meninges. Treatment necessitates not wearing the prosthesis until the complete healing of the perifixture tissues. Prevention is based on perfect local hygiene of the fixtures and removing deposits around them, in particular.
RESULTS
Case 1: Traumatic Loss of the Auricle
The patient is a 40-year-old man who was attacked by a panther. At his arrival in our center, there was a large wound with lacerated tissues, transection of the facial nerve and nearly complete avulsion of the auricle (Fig. 3A). We initially tried to replant the auricle. Over the next few days, there was complete necrosis of the auricle, which required debridement (Figs. 3B, C). As massive soft tissue defects make autologous reconstruction using cartilage difficult, we decided to use a PAB. We used two fixtures; granulation tissue developed around the posterior one (Fig. 3D). Figure 3E demonstrates the bar joining the fixtures. Figure 3F shows the silicone prosthesis, prior to the application of color.
Case 2: Cancer-Related Amputation of the Auricle
A 50-year-old patient, who underwent a renal transplant in 1996, presented with a squamous cell carcinoma of the auricle in 2007 (Fig. 4A). Because of multiple skin carcinomas operated over the years, we chose to reconstruct his auricle by a PAB. Lack of hygiene and large deposits around the fixtures forced us to use only two fixtures (Figs. 4B, C). The patient has an overall good result, thanks to the presence of the tragus and preauricular wrinkles, which hide the anterior border of the prosthesis (Fig. 4D).
Case 3: Congenital Absence of the Auricle
A 17-year-old woman was referred to our center for treatment of hemifacial microsomia. We used a PAB to build her ear, positioned slightly above the remnants of the auricle (Fig. 5).
Figure 5.
Congenital case of hemifacial microsomia. Auricular prosthesis placed above the remnants of the malformed ear.
CONCLUSION
A PAB of the auricle is limited by the low number of patients who have compatible pathology and who are not candidates for other surgical reconstructions. It provides good to excellent results, with low morbidity, and is therefore a good alternative to other methods. A PAB is easily implemented, provides significant benefits to patients, and is well accepted.
Such characteristics make the PAB a good solution for a quick, painless, satisfactory reconstruction of the auricle that enables patients to return rapidly to normal life.
ACKNOWLEDGMENT
We thank Cécile Caufourier for her help in the preparation of the manuscript.
References
- Benoist M. Réhabilitation et prothèse maxillo-faciales. Paris: Prélat; 1978. p. 454. [Google Scholar]
- Firmin F. State-of-the-art autogenous ear reconstruction in cases of microtia. Adv Otorhinolaryngol. 2010;68:25–52. doi: 10.1159/000314561. [DOI] [PubMed] [Google Scholar]
- Firmin F. [Secondary reconstruction of the external ear destroyed by burns. Indications and techniques. Apropos of 30 clinical cases] Ann Chir Plast Esthet. 1995;40(3):252–263. [PubMed] [Google Scholar]
- Firmin F. [Value of tissue expansion in total reconstruction of the external ear] Ann Chir Plast Esthet. 1996;41(5):495–502. [PubMed] [Google Scholar]
- Firmin F. Ear reconstruction in cases of typical microtia. Personal experience based on 352 microtic ear corrections. Scand J Plast Reconstr Surg Hand Surg. 1998;32(1):35–47. doi: 10.1080/02844319850158930. [DOI] [PubMed] [Google Scholar]
- Firmin F, Sanger C, O'Toole G. Ear reconstruction following severe complications of otoplasty. J Plast Reconstr Aesthet Surg. 2008;61(Suppl 1):S13–S20. doi: 10.1016/j.bjps.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Jost G, Firmin M F. [Restoration of the relief of the helix root in reconstructions of the external ear] Ann Otolaryngol Chir Cervicofac. 1969;86(10):675–678. [PubMed] [Google Scholar]
- Labbé D, Bénateau H, Compère J F, Sabin P. [Extra-oral implants: indications and contraindications] Rev Stomatol Chir Maxillofac. 2001;102(5):239–242. [PubMed] [Google Scholar]
- Sabin P, Bonin B. [Indications for extra-oral implants: strategic methods apropos of a clinical case] Rev Stomatol Chir Maxillofac. 2001;102(5):249–252. [PubMed] [Google Scholar]
- Wilkes G H, Wolfaardt J F. Osseointegrated alloplastic versus autogenous ear reconstruction: criteria for treatment selection. Plast Reconstr Surg. 1994;93(5):967–979. doi: 10.1097/00006534-199404001-00011. [DOI] [PubMed] [Google Scholar]
- Thorne C H, Brecht L E, Bradley J P, Levine J P, Hammerschlag P, Longaker M T. Auricular reconstruction: indications for autogenous and prosthetic techniques. Plast Reconstr Surg. 2001;107(5):1241–1252. doi: 10.1097/00006534-200104150-00024. [DOI] [PubMed] [Google Scholar]
- Sabin P, Cadre B, Ferrand J Y, Pacini R, Labbé D. [Bone-anchored implants: comparison of the techniques and values of endo- and juxta-bone implants] Rev Laryngol Otol Rhinol (Bord) 1997;118(2):103–107. [PubMed] [Google Scholar]
- Farmand M. [A new implant system for the fixation of facial prostheses] Dtsch Z Mund Kiefer Gesichtschir. 1991;15(6):421–427. [PubMed] [Google Scholar]
- Federspil P. Kugler, 1992, pp 47–71 Delb W: Treatment of congenital malformations of the external and middle ear. In Ars B (ed): Congenital External and Middle Ear Malformations. Amsterdam, New York: [Google Scholar]
- Halsnad S M, Srinivasan D, Jeynes P, Sharp I. Re: P. Gentile, F. Nicoli, R. Caruso, G. Gravante, V. Cervelli. Alternative strategy to reconstruct the nose after excision: extra-oral implant anchored to bone [Br. J. Oral Maxillofac. Surg. 47 (2009) 50-51] Br J Oral Maxillofac Surg. 2009;47(6):491–492. doi: 10.1016/j.bjoms.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Badie-Modiri B, Kaplanski P. [Extra-oral implants: principal areas of implantation] Rev Stomatol Chir Maxillofac. 2001;102(5):229–233. [PubMed] [Google Scholar]
- Tjellström A. Osseointegrated implants for replacement of absent or defective ears. Clin Plast Surg. 1990;17(2):355–366. [PubMed] [Google Scholar]
- Jensen O T, Brownd C, Blacker J. Nasofacial prostheses supported by osseointegrated implants. Int J Oral Maxillofac Implants. 1992;7(2):203–211. [PubMed] [Google Scholar]
- Jacobsson M G, Jönsson A K, Albrektsson T O, Turesson I E. Short- and long-term effects of irradiation on bone regeneration. Plast Reconstr Surg. 1985;76(6):841–850. doi: 10.1097/00006534-198512000-00006. [DOI] [PubMed] [Google Scholar]
- Jacobsson M, Tjellström A, Thomsen P, Albrektsson T, Turesson I. Integration of titanium implants in irradiated bone. Histologic and clinical study. Ann Otol Rhinol Laryngol. 1988;97(4 Pt 1):337–340. doi: 10.1177/000348948809700402. [DOI] [PubMed] [Google Scholar]
- Kosmidou L, Toljanic J A, Moran W J, Panje W R. The use of percutaneous implants for the prosthetic rehabilitation of orbital defects in irradiated cancer patients: a report of clinical outcomes and complications. Int J Oral Maxillofac Implants. 1998;13(1):121–126. [PubMed] [Google Scholar]
- Jacobsson M, Tjellstrom A, Fine L, Andersson H. A retrospective study of osseointegrated skin-penetrating titanium fixtures used for retaining facial prostheses. Int J Oral Maxillofac Implants. 1992;7(4):523–528. [PubMed] [Google Scholar]
- Abu-Serriah M M, Bagg J, McGowan D A, Moos K F, MacKenzie D. The microflora associated with extra-oral endosseous craniofacial implants: a cross-sectional study. Int J Oral Maxillofac Surg. 2000;29(5):344–350. [PubMed] [Google Scholar]
- Granström G, Jacobsson M, Tjellström A. Titanium implants in irradiated tissue: benefits from hyperbaric oxygen. Int J Oral Maxillofac Implants. 1992;7(1):15–25. [PubMed] [Google Scholar]
- Granström G, Tjellström A, Brånemark P I. Osseointegrated implants in irradiated bone: a case-controlled study using adjunctive hyperbaric oxygen therapy. J Oral Maxillofac Surg. 1999;57(5):493–499. doi: 10.1016/s0278-2391(99)90059-9. [DOI] [PubMed] [Google Scholar]
- Johnsson A A, Sawaii T, Jacobsson M, Granström G, Turesson I. A histomorphometric study of bone reactions to titanium implants in irradiated bone and the effect of hyperbaric oxygen treatment. Int J Oral Maxillofac Implants. 1999;14(5):699–706. [PubMed] [Google Scholar]
- Andersson G, Andreasson L, Bjelkengren G. Oral implant rehabilitation in irradiated patients without adjunctive hyperbaric oxygen. Int J Oral Maxillofac Implants. 1998;13(5):647–654. [PubMed] [Google Scholar]
- Bodard A G, Gourmet R, Lucas R, Bonnet E, Breton P. [Dental implants in irradiated areas: a series of 33 patients] Rev Stomatol Chir Maxillofac. 2006;107(3):137–142. discussion 143–144. doi: 10.1016/s0035-1768(06)77007-3. [DOI] [PubMed] [Google Scholar]
- Bénateau H, Crasson F, Labbé D, Riscala S, Alix T. [Extra-oral implants and irradiation: current trends] Rev Stomatol Chir Maxillofac. 2001;102(5):266–269. [PubMed] [Google Scholar]
- Brix M, Badie-Modiri B, Delcampe P. [Extra-oral implants: surgical procedures] Rev Stomatol Chir Maxillofac. 2001;102(5):243–247. [PubMed] [Google Scholar]
- Gentile P, Nicoli F, Caruso R, Gravante G, Cervelli V. Alternative strategy to reconstruct the nose after excision: extra-oral implant anchored to bone. Br J Oral Maxillofac Surg. 2009;47(1):50–51. doi: 10.1016/j.bjoms.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Sabin P, Labbé D, Ferrand J Y, Daburon P, Compere J F. [Maxillofacial prosthesis fixed on endosseous implants. Apropos of 15 cases] Ann Chir Plast Esthet. 1995;40(4):363–370. [PubMed] [Google Scholar]
- Gitto C A, Plata W G, Schaaf N G. Evaluation of the peri-implant epithelial tissue of percutaneous implant abutments supporting maxillofacial prostheses. Int J Oral Maxillofac Implants. 1994;9(2):197–206. [PubMed] [Google Scholar]
- Holgers K M, Bjursten L M, Thomsen P, Ericson L E, Tjellström A. Experience with percutaneous titanium implants in the head and neck: a clinical and histological study. J Invest Surg. 1989;2(1):7–16. doi: 10.3109/08941938909016500. [DOI] [PubMed] [Google Scholar]
- Holgers K M, Ljungh A. Cell surface characteristics of microbiological isolates from human percutaneous titanium implants in the head and neck. Biomaterials. 1999;20(14):1319–1326. doi: 10.1016/s0142-9612(99)00033-2. [DOI] [PubMed] [Google Scholar]
- Holgers K M, Roupe G, Tjellström A, Bjursten L M. Clinical, immunological and bacteriological evaluation of adverse reactions to skin-penetrating titanium implants in the head and neck region. Contact Dermat. 1992;27(1):1–7. doi: 10.1111/j.1600-0536.1992.tb05189.x. [DOI] [PubMed] [Google Scholar]
- Toljanic J A, Morello J A, Moran W J, Panje W R, May E F. Microflora associated with percutaneous craniofacial implants used for the retention of facial prostheses: a pilot study. Int J Oral Maxillofac Implants. 1995;10(5):578–582. [PubMed] [Google Scholar]