Abstract
Patients undergoing balloon retrograde transvenous obliteration (BRTO) are mostly decompensated cirrhotic with either bleeding gastric varices (GV) or hepatic encephalopathy. It is crucial that clinicians are up-to-date with the assessments needed prior to BRTO to anticipate and prevent complications, and to deliver critical quality care. These patients will require preprocedural assessments and management, including endoscopic, clinical, laboratory, and imaging evaluation. Endoscopic evaluation is mandatory prior to BRTO, and it is highly recommended that it be performed at the same institution where BRTO will be performed. It is essential that clinicians are aware of the potential benefits and complications that may result from BRTO. These complications should be anticipated and prevented when possible. For GV bleeders, there should be consideration of a transvenous intrahepatic portosystemic shunt (TIPS) during or before BRTO in patients with refractory ascites or pleural effusion, as well as endoscopic banding or a TIPS in patients with high-risk esophageal varices. Patients undergoing BRTO are usually complicated and require a team approach. In this article, the authors address these assessment and preparatory management and planning procedures prior to the BRTO procedure as well as expected outcomes and potential complications.
Keywords: Gastric varices, portal hypertension, liver cirrhosis, BRTO, TIPS, splenorenal shunt
Patients who undergo balloon retrograde transvenous obliteration (BRTO) for bleeding gastric varices (GV) will require preprocedural assessments and management including endoscopic, clinical, laboratory, and imaging evaluations (see Figs. 1 and 2). It is crucial that clinicians are appraised of these needed assessments pre-BRTO to prevent complications and deliver critical quality care. The majority of patients undergoing BRTO are cirrhotics and require a team approach with involvement of gastroenterologists/hepatologists, endoscopists, and interventional radiologists. Open dialogue and collaboration is essential in the management of pre-BRTO patients. In this article, we will address these assessment and preparatory management and planning issues prior to the BRTO procedure and what expected outcomes and potential complications to anticipate. To our knowledge none of the management strategies discussed carry U.S. Food and Drug Administration (FDA) approval. Clearly, this is an area in need of carefully planned research to optimize management of GV bleeds.
Figure 1.
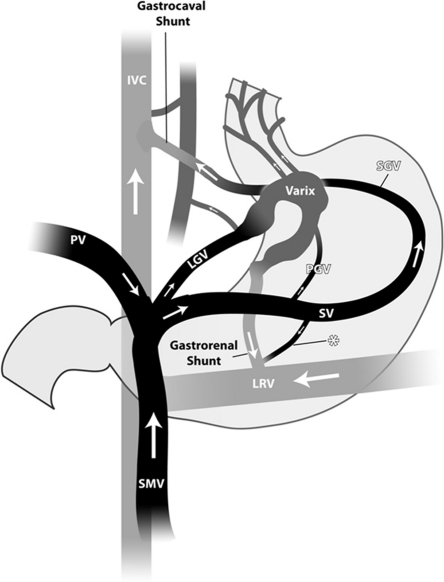
Anatomy of the portal circulation: Gastric varices due to splenic vein thrombosis tend to arise from the short gastric veins running from the hilum of the spleen to the greater curvature aspect of the stomach rather than through splenorenal (asterisk) or gastrorenal shunts common with portal hypertensive fundal varices. IVC, inferior vena cava; PV, portal vein; LGV,left gastric vein; SMV, superior mesenteric vein; SV, splenic vein; SGV, short gastric vein; LRV, left renal vein. (Courtesy of Dr. Saher Sabri.)
Figure 2.
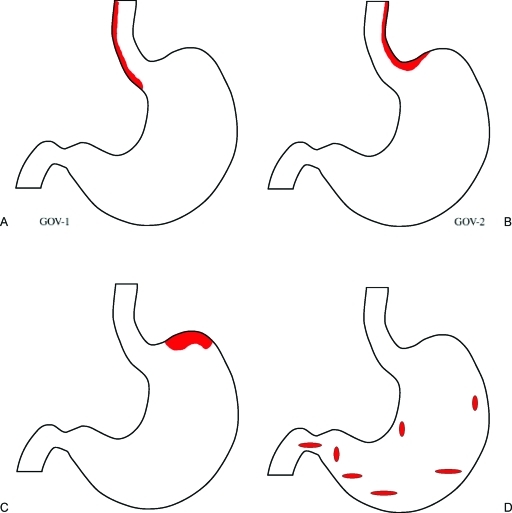
Schematic diagram of Sarin's Endoscopic Classification of Gastric Varices: Fundal varices are included in two of the groups: (A) Type 1 gastroesophageal varices (GOV 1) are typically continuation of esophageal varices into the lesser curvature varices. (B) Type 2 gastroesophageal varices (GOV 2) when the esophageal and fundal varices are present in continuity over the cardia, (C) Type 1 isolated gastric varices (IGV 1), that are usually fundal gastric varices. (D) Type 2 isolated gastric varices (IGV 2) are gastric varices at ectopic sites in the stomach outside the cardiofundal region or the first part of the duodenum. An alternative classification based on vascular anatomy (determined by dominant feeder veins) has been proposed and is especially useful in consideration of balloon retrograde transvenous obliteration.
CLINICAL ASSESSMENTS
Patients who are considered for BRTO are usually cirrhotics with bleeding varices and/or hepatic encephalopathy (HE).1,2,3,4,5 Thus, assessment of these patients' liver function, including coagulation parameters, bilirubin, nutrition, in addition to blood counts, and renal function is crucial to assess synthetic reserve. In addition, assessment for presence of ascites, pleural effusion (hepatic hydrothorax), anasarca, and hypotension is of paramount importance as these variables influence outcomes in our experience. In patients with GV bleed, we emphasize the need for upper endoscopy in the same institution performing BRTO, to confirm the diagnosis and ensure careful team consideration of management options. In patients with GV bleed and uncontrolled ascites and/or hepatic hydrothorax, or high-risk esophageal varices, consideration of TIPS with BRTO is advisable to simultaneously effect portal decompression.
Endoscopic Management of Bleeding Gastric Varices
Initial management of GV bleeding involves diagnostic and therapeutic considerations. Basic measures common to GI bleeding in general (such as establishment of intravenous access and airway control if the patient is unstable) are beyond the focus of this article, but it should be mentioned that overaggressive volume resuscitation can exacerbate portal hypertension and should be avoided as discussed below. Cirrhosis may be suspected by prior evaluation or through historical, physical, and laboratory findings although this is not always apparent at the outset. In patients with known or suspected portal hypertension, medical therapy also usually includes antiportal hypertension medications and antibiotic prophylaxis.
Once the patient is stabilized, upper endoscopic examination is usually undertaken as a routine early measure to evaluate upper GI bleeding. Detailed endoscopic management has been discussed in “Medical and Endoscopic Management of Gastric Varices” in this issue of Seminars of Interventional Radiology.
Volume, Plasma, and ProCoagulants
Animal and human studies established that volume expansion increases portal vein pressure.6,7,8 Variceal bleeding is predominantly portal pressure driven, thus it is apparent that minimizing portal pressure is an important goal in managing these patients and similarly avoidance of overaggressive volume resuscitation is warranted. Blood pressures lower than normal are therefore acceptable and relatively lower target hematocrit with red blood cell transfusions are key goals. This relationship has led to the target hematocrit level of 21% from the Baveno IV Conference on Variceal and Portal Hypertensive Bleeding,9 whereas optimization of platelet function suggests a target of 25% for hematocrit (due to flow rheology and effective platelet margination).10 Renal support may be necessary for volume control. In liver transplantation, maintaining low volumes and associated low portal pressures played a significant role in controlling bleeding during transplantation.11 Detailed fluid and transfusion management has been discussed in an earlier article in this issue, Al-Osaimi AMS, Caldwell SH. Medical and endoscopic management of gastric varices. Semin Intervent Radiol 2011; 28:273–282.
Anticipated Benefits, Outcomes, and Complications
Studies have demonstrated improvement of the hepatic functional reserve, increased portal blood flow, reduction of gastric variceal hemorrhage and improvement in impaired glucose tolerance testing after BRTO.12,13,14,15,16,17,18,19,20 Recent studies have shown improvement or stability of hepatic function via Child-Pugh (CP) score and MELD (Model of End-Stage Liver Disease).13,21 On the other hand, most of these patients are cirrhotics, thus detailed attention to the patient' liver and renal function pre- and post-BRTO is essential. Patients with fluid overload (including ascites, lower extremity edema, and/or hepatic hydrothorax[HH]), and esophageal varices will need close monitoring, as they may worsen post-BRTO in up to 40% of patients due to enhanced portal flow to the liver following BRTO.13,22
In the immediate and early post-BRTO period, complications have been noted including fever, chest or epigastric pain, hemoglobinuria, transient hypertension, nausea or vomiting, and pleural effusion (see Table 1 for comprehensive list).13,22,23 Thus, a routine check of blood oxygenation and a chest roentgenogram is recommended in all patients following BRTO.22 Lactate dehydrogenase, aspartate aminotransferase, and bilirubin may increase in the first couple of days, but usually return to baseline; it is thought to be due to intravascular hemolysis.22,24 Renal function might be affected, which could be related to multiple factors including diuretic use (for ascites or fluid overload), acute tubular necrosis (most likely due to hemodynamic instability postvariceal bleed or ethanolamine oleate or other sclerosant agents), contrast-induced nephropathy or hepatorenal syndrome.22,24 In 5–10% of patients undergoing BRTO, TIPS is performed either simultaneously or subsequently before or after BRTO; thus TIPS-specific complications should be anticipated, and prevented and corrected as clinically indicated.
Table 1.
| Adverse Events/Complications | % |
|---|---|
| GI Complications | |
| Worsening esophageal varices | 40 |
| Nausea and/or vomiting | 21 |
| Rebleeding GV | 10 |
| Gastric ulcers | 9 |
| Worsening ascites | 7 |
| Hepatic encephalopathy | 2 |
| Hemorrhagic PHG | 2 |
| Non-GI Complications | |
| Epigastric, chest and/or back pain | 56–76.5 |
| Hemoglobinuria | 49 |
| Transient systemic hypertension | 35 |
| Transient fever | 33 |
| New or worsening pleural effusion | 12 |
| Pulmonary infarction | 2 |
BRTO, balloon retrograde transvenous obliteration; GV, gastric varices; PGH, portal hypertensive gastropathy.
PREPROCEDURAL IMAGING
Obtaining good quality imaging is required to decide if the patient is a candidate for the BRTO procedure and is critical for planning the procedure. The main anatomic features (Fig. 1) to be considered prior to performing the BRTO procedure include the patency of the main portal vein, the presence of a gastrorenal or gastrocaval shunt, and the presence or absence of gastric varices.
Patency of the Main Portal Vein
The main portal vein provides outflow pathway for the mesenteric and splenic venous circulation (Fig. 1). With elevation of portal venous pressure, usually secondary to chronic liver disease, collateral veins are formed and provide portosystemic pathways. Two of the common pathways include a gastorenal shunt, which is a communication between gastric veins, such as the left, posterior, or short gastric veins and the left renal vein. The other is a gastrocaval shunt, which is a communication between the aforementioned gastric veins and the inferior vena cava through phrenic vein pathways.25,26,27 Commonly these collateral pathways course within the wall of the stomach and result in gastric varices (Fig. 2). These pathways provide essential outflow pathway for the mesenteric and splenic venous circulation secondary to portal hypertension. However, these pathways may be formed secondary to thrombosis of the main portal vein and provide the main and occasionally the only outflow for the mesenteric and splenic venous circulation. In such instances, when patients present with bleeding or high-risk gastric varices and complete thrombosis of the main portal vein, it would be contraindicated to perform the BRTO procedure as occluding the portosystemic shunts may result in mesenteric venous hypertension and mesenteric ischemia. Partial thromboses of the main portal vein or a small diminutive but patent main portal vein, however, are not contraindications for the procedure. Actually, occluding the competing portosystemic shunts may result in improved flow through the main portal vein.1,23,26,27,28,29 Chronic occlusion of the main portal vein with cavernous transformation provides a challenging condition where there remains the risk that the collateral veins around the main portal vein forming the cavernous transformation may not be sufficient to provide outflow for the portal venous system after occluding the portosystemic shunts. However, if the cavernous transformation is robust, it may be acceptable to proceed with the BRTO procedure after weighing the risks and benefits of the procedure.
Presence of a Gastrorenal or Gastrocaval Shunt
With the BRTO procedure, an occlusion balloon is advanced into a portosystemic shunt through the systemic venous outflow pathway for the purpose of occluding the shunt. This allows stagnation of the sclerosing agent within the shunt and gastric varices resulting in obliteration of the varix. Preprocedural imaging is critical in documenting the presence of this portosystemic pathway (Fig. 3). The most common shunt to be occluded during a BRTO procedure is a gastrorenal shunt, which provides venous outflow in 90% of gastric varices cases with the remaining 10% draining through a gastrocaval shunt. Preprocedural imaging is important in measuring the size of the shunt. The diameter of the gastrorenal shunt is measured at the base of the shunt at the communication point with the left renal vein, which where the occlusion balloon will be placed. The balloon diameter is chosen to match the diameter of the shunt. In roughly 20% of patients with gastric varices and a gastrorenal shunt, the anastomosis is too small to be safely accessed via the vena cava and alternative methods of managing the varices should be considered.
Figure 3.
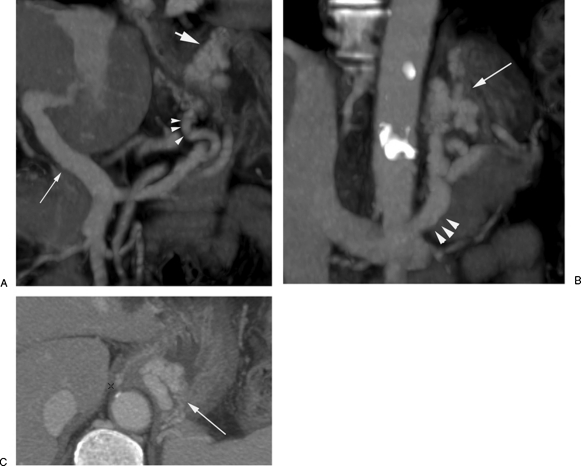
Preprocedural imaging with contrast-enhanced computed tomography (CECT).(A) Oblique coronal CECT shows patency of the main portal vein (small arrow). The posterior gastric vein (arrowhead) is the main feeding “afferent” vein for the gastric varices (large arrow). (B) Oblique coronal CECT shows gastric fundal varices (arrow) and gastrorenal shunt (arrowheads). (C) Axial CECT shows gastric fundal varices (arrow).
Presence or Absence of Gastric Varices
BRTO procedure can be performed to occlude portosystemic shunts to treat bleeding and high-risk gastric varices as well as treating hepatic encephalopathy. In the case of encephalopathy, gastrorenal or gastrocaval shunts may be present, but endoscopically evident gastric varices may be absent.
TRANSABDOMINAL DOPPLER ULTRASOUND
Doppler ultrasound usually provides useful information on patency of the main portal vein and the direction of flow in the intrahepatic portal vein branches. However, it is limited in evaluating the presence and size of gastric varices and gastrorenal or gastrocaval shunts. For that reason, it is necessary to obtain good quality contrast-enhanced computed tomography (CECT) or magnetic resonance imaging (CEMRI).
CONTRAST-ENHANCED COMPUTED TOMOGRAPHY
CECT provides optimal visualization of the main portal vein and documents patency, and the presence of thrombus or cavernous transformation. The gastrorenal shunt can usually be visualized well with CECT (Fig. 3). The gastrocaval shunts are usually smaller in diameter and may be harder to visualize. The gastric varices can be visualized as enhancing vascular structures in the fundus or cardia of the stomach (Fig. 3). It is important not to use oral contrast agents these may obscure the visualization of the varices. The afferent gastric veins contributing to the gastric varices can be identified on CECT, especially if it is a type 1 gastric varix (defined by vascular anatomy) with afferent flow from one dominant afferent vein, most commonly the posterior or left gastric veins (Fig. 3). However, it may be difficult to know how many afferent veins contribute to the varices.
CONTRAST-ENHANCED MAGNETIC RESONANCE IMAGING
CEMRI similar to CECT provides optimal visualization of the portal veins and its branches as well as the gastrorenal and gastrocaval shunts, the gastric varices, and the afferent gastric veins. The dynamic postcontrast sequences provide the needed information to plan the BRTO procedure. T2 weighted sequences can provide adequate visualization of the components of the gastric varices, inflow and outflow veins, and may compliment the contrast-enhanced sequences (Fig. 4).
Figure 4.
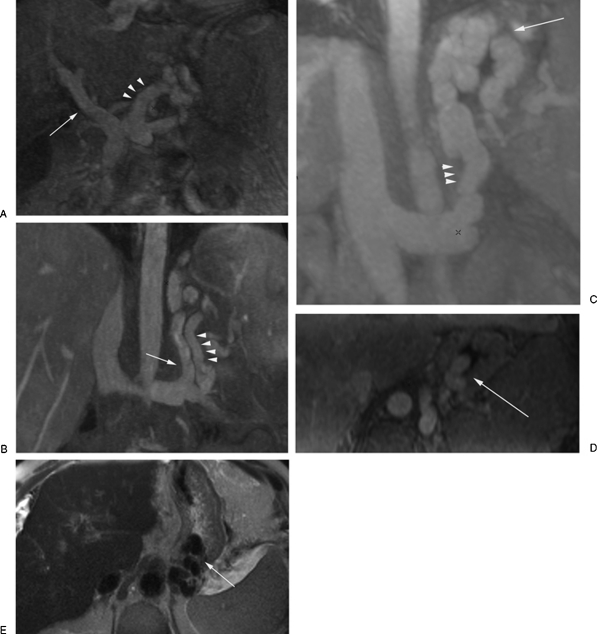
Preprocedural magnetic resonance imaging (MRI). (A) Contrast-enhanced MRI (CEMRI) in the coronal plane showing patency of the portal vein and the left gastric vein (arrowheads) as the main feeding “afferent” vein. (B) CEMRI in the coronal plane showing the posterior gastric vein (arrowheads) as the main feeding “afferent” vein. The gastrorenal shunt is also noted (arrow). CEMRI in the coronal (C) and axial (D) planes showing the gastrorenal shunt (arrowheads) and the opacified gastric fundal varices (arrow). (E) Axial T2-weighted MR image showing the gastric fundal varices as flow voids (arrow).
SUMMARY
It is crucial that clinicians are up-to-date with the assessments needed prior to BRTO to anticipate and prevent complications, and to deliver critical quality care. These patients will require preprocedural assessments and management, including endoscopic, clinical, laboratory, and imaging to consider and optimize management options. It is essential that clinicians are aware of the potential benefits and complications that may result from BRTO. These complications should be anticipated and prevented when possible. The treatment of patients undergoing BRTO is usually complicated and requires a team approach.
DISCLOSURE
Dr. SH Caldwell disclosed financial support for research from CL Behring; the other authors had no disclosure relating to this manuscript.
ACKNOWLEDGMENT
We thank Reem Asseri for the schematics of the gastric varices.
References
- Kanagawa H, Mima S, Kouyama H, Gotoh K, Uchida T, Okuda K. Treatment of gastric fundal varices by balloon-occluded retrograde transvenous obliteration. J Gastroenterol Hepatol. 1996;11(1):51–58. doi: 10.1111/j.1440-1746.1996.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Koito K, Namieno T, Nagakawa T, Morita K. Balloon-occluded retrograde transvenous obliteration for gastric varices with gastrorenal or gastrocaval collaterals. AJR Am J Roentgenol. 1996;167(5):1317–1320. doi: 10.2214/ajr.167.5.8911204. [DOI] [PubMed] [Google Scholar]
- Kitamoto M, Imamura M, Kamada K, et al. Balloon-occluded retrograde transvenous obliteration of gastric fundal varices with hemorrhage. AJR Am J Roentgenol. 2002;178(5):1167–1174. doi: 10.2214/ajr.178.5.1781167. [DOI] [PubMed] [Google Scholar]
- Sakurabayashi S, Sezai S, Yamamoto Y, Hirano M, Oka H. Embolization of portal-systemic shunts in cirrhotic patients with chronic recurrent hepatic encephalopathy. Cardiovasc Intervent Radiol. 1997;20(2):120–124. doi: 10.1007/s002709900118. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Hirota S, Sugimura K. Long-term results of balloon-occluded retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy. J Vasc Interv Radiol. 2001;12(3):327–336. doi: 10.1016/s1051-0443(07)61912-5. [DOI] [PubMed] [Google Scholar]
- Kravetz D, Bosch J, Arderiu M, Pilar Pizcueta M, Rodés J. Hemodynamic effects of blood volume restitution following a hemorrhage in rats with portal hypertension due to cirrhosis of the liver: influence of the extent of portal-systemic shunting. Hepatology. 1989;9(6):808–814. doi: 10.1002/hep.1840090603. [DOI] [PubMed] [Google Scholar]
- Castañeda B, Morales J, Lionetti R, et al. Effects of blood volume restitution following a portal hypertensive-related bleeding in anesthetized cirrhotic rats. Hepatology. 2001;33(4):821–825. doi: 10.1053/jhep.2001.23437. [DOI] [PubMed] [Google Scholar]
- Villanueva C, Ortiz J, Miñana J, et al. Somatostatin treatment and risk stratification by continuous portal pressure monitoring during acute variceal bleeding. Gastroenterology. 2001;121(1):110–117. doi: 10.1053/gast.2001.25536. [DOI] [PubMed] [Google Scholar]
- de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43(1):167–176. doi: 10.1016/j.jhep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Gabriel D A, Li X, Monroe DMIII, III, Roberts H R. Recombinant human factor VIIa (rFVIIa) can activate factor FIX on activated platelets. J Thromb Haemost. 2004;2(10):1816–1822. doi: 10.1111/j.1538-7836.2004.01015.x. [DOI] [PubMed] [Google Scholar]
- Porte R J, Molenaar I Q, Begliomini B, et al. EMSALT Study Group Aprotinin and transfusion requirements in orthotopic liver transplantation: a multicentre randomised double-blind study. Lancet. 2000;355(9212):1303–1309. doi: 10.1016/s0140-6736(00)02111-5. [DOI] [PubMed] [Google Scholar]
- Tanabe N, Ishii M, Sato Y, et al. Effects of collateral vessel occlusion on oral glucose tolerance test in liver cirrhosis. Dig Dis Sci. 2000;45(3):581–586. doi: 10.1023/a:1005461611262. [DOI] [PubMed] [Google Scholar]
- Kumamoto M, Toyonaga A, Inoue H, et al. Long-term results of balloon-occluded retrograde transvenous obliteration for gastric fundal varices: hepatic deterioration links to portosystemic shunt syndrome. J Gastroenterol Hepatol. 2010;25(6):1129–1135. doi: 10.1111/j.1440-1746.2010.06262.x. [DOI] [PubMed] [Google Scholar]
- Takuma Y, Nouso K, Makino Y, Saito S, Shiratori Y. Prophylactic balloon-occluded retrograde transvenous obliteration for gastric varices in compensated cirrhosis. Clin Gastroenterol Hepatol. 2005;3(12):1245–1252. doi: 10.1016/s1542-3565(05)00744-5. [DOI] [PubMed] [Google Scholar]
- Nakano R, Iwao T, Oho K, Toyonaga A, Tanikawa K. Splanchnic hemodynamic pattern and liver function in patients with cirrhosis and esophageal or gastric varices. Am J Gastroenterol. 1997;92(11):2085–2089. [PubMed] [Google Scholar]
- Choi Y H, Yoon C J, Park J H, Chung J W, Kwon J W, Choi G M. Balloon-occluded retrograde transvenous obliteration for gastric variceal bleeding: its feasibility compared with transjugular intrahepatic portosystemic shunt. Korean J Radiol. 2003;4(2):109–116. doi: 10.3348/kjr.2003.4.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Uematsu T, Nishigaki Y, Sugihara J, Tomita E, Moriwaki H. Therapeutic effect of balloon-occluded retrograde transvenous obliteration on portal-systemic encephalopathy in patients with liver cirrhosis. Intern Med. 2001;40(8):688–691. doi: 10.2169/internalmedicine.40.688. [DOI] [PubMed] [Google Scholar]
- Akahane T, Iwasaki T, Kobayashi N, et al. Changes in liver function parameters after occlusion of gastrorenal shunts with balloon-occluded retrograde transvenous obliteration. Am J Gastroenterol. 1997;92(6):1026–1030. [PubMed] [Google Scholar]
- Miyamoto Y, Oho K, Kumamoto M, Toyonaga A, Sata M. Balloon-occluded retrograde transvenous obliteration improves liver function in patients with cirrhosis and portal hypertension. J Gastroenterol Hepatol. 2003;18(8):934–942. doi: 10.1046/j.1440-1746.2003.03087.x. [DOI] [PubMed] [Google Scholar]
- Cardoso J E, Gautreau C, Jeyaraj P R, et al. Augmentation of portal blood flow improves function of human cirrhotic liver. Hepatology. 1994;19(2):375–380. [PubMed] [Google Scholar]
- Saad W, Darwish W, Anderson , et al. The effect of balloon-occluded retrograde transvenous obliteration (BRTO) on the model of end-stage liver disease (MELD) score. J Vasc Interv Radiol. 2011;22:S34–S35. [Google Scholar]
- Shimoda R, Horiuchi K, Hagiwara S, et al. Short-term complications of retrograde transvenous obliteration of gastric varices in patients with portal hypertension: effects of obliteration of major portosystemic shunts. Abdom Imaging. 2005;30(3):306–313. doi: 10.1007/s00261-004-0270-8. [DOI] [PubMed] [Google Scholar]
- Hiraga N, Aikata H, Takaki S, et al. The long-term outcome of patients with bleeding gastric varices after balloon-occluded retrograde transvenous obliteration. J Gastroenterol. 2007;42(8):663–672. doi: 10.1007/s00535-007-2077-1. [DOI] [PubMed] [Google Scholar]
- Wada H, Hashizume M, Yamaga H, Kitano S, Sugimachi K. Hemodynamic and morphological changes in the dog kidney after injection of 5% ethanolamine oleate into the superior vena cava. Eur Surg Res. 1990;22(2):63–70. doi: 10.1159/000129084. [DOI] [PubMed] [Google Scholar]
- Chikamori F, Shibuya S, Takase Y, Ozaki A, Fukao K. Transjugular retrograde obliteration for gastric varices. Abdom Imaging. 1996;21(4):299–303. doi: 10.1007/s002619900068. [DOI] [PubMed] [Google Scholar]
- Kiyosue H, Mori H, Matsumoto S, Yamada Y, Hori Y, Okino Y. Transcatheter obliteration of gastric varices. Part 1. Anatomic classification. Radiographics. 2003;23(4):911–920. doi: 10.1148/rg.234025044. [DOI] [PubMed] [Google Scholar]
- Kiyosue H, Mori H, Matsumoto S, Yamada Y, Hori Y, Okino Y. Transcatheter obliteration of gastric varices: Part 2. Strategy and techniques based on hemodynamic features. Radiographics. 2003;23(4):921–937. discussion 937. doi: 10.1148/rg.234025135. [DOI] [PubMed] [Google Scholar]
- Chikamori F, Kuniyoshi N, Shibuya S, Takase Y. Eight years of experience with transjugular retrograde obliteration for gastric varices with gastrorenal shunts. Surgery. 2001;129(4):414–420. doi: 10.1067/msy.2001.112000. [DOI] [PubMed] [Google Scholar]
- Cho S K, Shin S W, Lee I H, et al. Balloon-occluded retrograde transvenous obliteration of gastric varices: outcomes and complications in 49 patients. AJR Am J Roentgenol. 2007;189(6):W365–W372. doi: 10.2214/AJR.07.2266. [DOI] [PubMed] [Google Scholar]