Abstract
Balloon-occluded retrograde transvenous obliteration (BRTO) is an endovascular technique used as a therapeutic adjunct or alternative to transjugular intrahepatic shunts (TIPS) in the management of gastric varices. Occlusion balloons are strategically placed to modulate flow within the gastrorenal or gastrocaval shunt to allow stagnation of the sclerosant material within the gastric varix. The approach and complexity of the procedure depends on the anatomic classification of inflow and outflow veins of the varix. Ethanolamine oleate has been described as the main sclerosant used in this procedure. Recently, foam sclerosants have gained popularity as alternative embolization agents, which provide the advantage of better variceal wall contact and potentially less dose of sclerosant.
Keywords: Gastric varices, portal hypertension, liver cirrhosis, BRTO, portosystemic shunt, embolization
Balloon-occluded retrograde transvenous obliteration (BRTO) is an endovascular technique that was refined in Japan1,2,3,4,5,6,7,8,9,10,11,12 as a therapeutic adjunct or alternative to TIPS in the management of gastric varices. It is also an effective therapy for sclerosis of de novo portosystemic shunts complicated by hepatic encephalopathy. A BRTO procedure involves occlusion of outflow veins of the portosystemic shunt, such as a gastrorenal shunt, using an occlusion balloon followed by the injection of a sclerosing agent directly into the varix endovascularly.13
Critical to the procedure is the stagnation of the sclerosant within the varix or shunt without reflux into either the portal or systemic vasculature, which might result in serious complications. To avoid these events, occlusion balloons are strategically placed to modulate the flow within the varix and/or shunt. Additionally, microcatheters and embolization coils are adjunctive tools employed to administer the sclerosant in high concentration within the varix and prevent reflux to nontarget sites.
TECHNIQUE
Basic Procedural Steps
Access of the right femoral or internal jugular vein using standard angiographic technique and placement of a 6–12 French (Fr) sheath. Most of the reported cases in the literature have been described using the right femoral vein approach. Certain institutions have adopted the jugular vein approach exclusively.14 The authors suggest reviewing the preprocedure computed tomography (CT) or magnetic resonance imaging (MRI) scans and decide the approach that provides the best angle for selecting the shunt.
Catheterization of the gastrorenal shunt via the left renal vein is typically accomplished using a catheter with a mounted occlusion balloon. Reversed shaped balloon catheters are available in Asia and provide easier and stable access into the shunt. However, such catheters are not readily available in the United States. The approach suggested by the authors is to place an appropriate size access sheath (6–12 Fr) within the inferior vena cava (IVC) or the distal renal vein. Next, the left renal vein is selected using a 5 Fr diagnostic catheter (Cobra; Angiodynamics, Queensbury, NY) (Fig. 1A). The diagnostic catheter is then exchanged for an angled tip catheter (Slip-cath; Cook Inc, Bloomington, IN), which is used to select the shunt. Alternatively, a 5 Fr Simmons II catheter (Angiodynamics, Queensbury, NY) is used to select the left renal vein and is pulled back until the tip is positioned into the shunt. Next, a 0.035-inch stiff wire (Rosen, Angioynamics, or TAD II, Mallinckrodt Inc., St. Louis, MO) is advanced as deep as possible into the shunt followed by an occlusion balloon catheter with diameters ranging from 8.5 to 32 mm (Fig. 1B). The diameter of the balloon is chosen to occlude the communicating gastrorenal shunt vein of interest. Several occlusion balloons are available in the United States and include but are not limited to the Python occlusion balloon (Applied Medical, Rancho Santa Margarita, CA), the Equalizer (Boston Scientific, Natick, MA), standard occlusion balloon catheters (Boston Scientific), flow-directed occlusion balloon catheters (Cook Inc, Bloomington, IN) and the Coda (Cook Inc.).
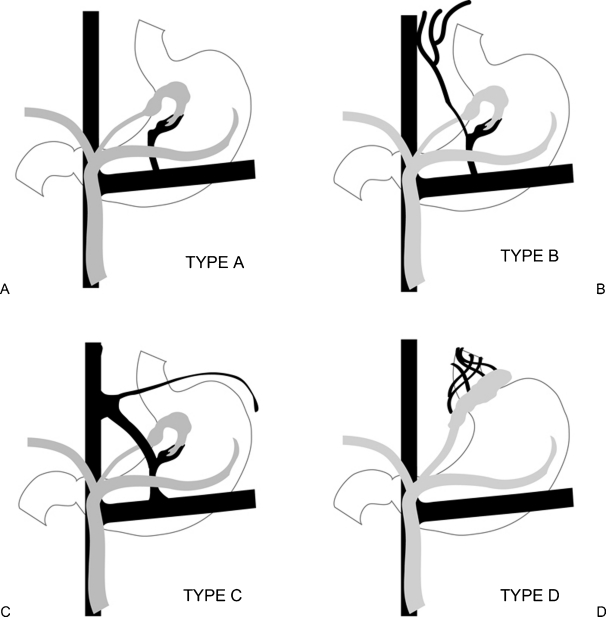
Balloon occlusion venography is then performed to define the type of varix/varices and the anatomy of the venous drainage (Fig. 1C). The cases are classified according to the venous drainage pattern into type A, B, or C (Fig. 2).15,16 Type D drainage patterns do not have a definable shunt and cannot be treated with the BRTO procedure.
Infusion of sclerosant follows. The goal is filling the full extent of the varix with the embolization endpoint being minimal filling of the afferent vein/portal vasculature. The injection of a sclerosing agent can be performed with or without use of a microcatheter for more selective injection. We suggest advancing a microcatheter (Rapid transit, Codman & Shurtleff, Inc, Raynham, MA) through the occlusion balloon as deep as possible into the varix. This provides the benefit of selective delivery of the sclerosant into the varix and minimizing the volume used as well as minimizing the risk of balloon rupture when the sclerosant comes in close proximity to the balloon. The sclerosant is infused through the microcatheter with the goal of filling the full extent of the varix. The embolization endpoint is minimal filling of the afferent vein/portal vasculature (Fig. 1D). Leaking collateral veins such as the inferior phrenic or paravertebral veins are commonly present and prevent full opacification of gastric varices. These veins are occluded using coils or Gelfoam pledgets through a microcatheter. This would modulate flow in the veins in an effort to concentrate the sclerosant at the varix and minimize nontarget distribution in the portal or systemic vasculature.
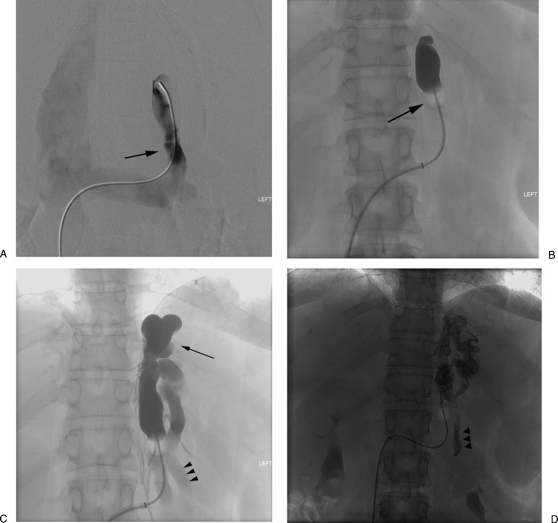
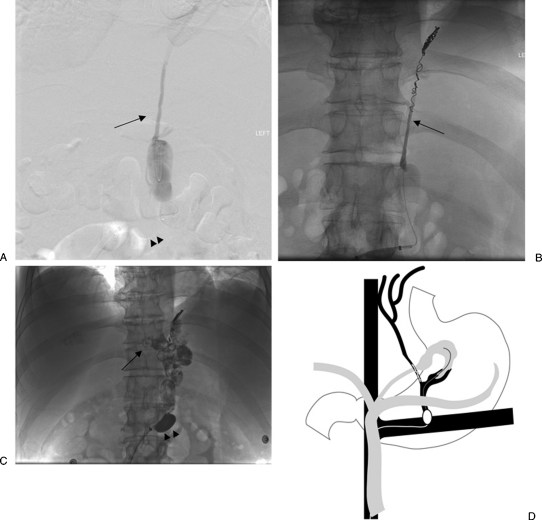
Figure 1.
Balloon-occluded retrograde transvenous obliteration procedural steps. (A) Catheterization of the gastrorenal shunt (arrow). (B) Advancement of an occlusion balloon (arrow) into the shunt and performing retrograde venogram. (C) Retrograde venogram shows opacification of the gastric varices (arrow) as well as the afferent posterior gastric vein (arrow heads). (D) Administration of the sclerosant mixture with the endpoint being filling of the afferent posterior gastric vein (arrowheads).
Figure 2.
Classification based on venous drainage. (A) Type A has a shunt with a single draining vein. (B) Type B has a shunt with multiple collateral draining veins. (C) Type C has both a gastrocaval and gastrorenal shunt. (D) Type D has multiple small draining veins and no shunt.
Treatment Modification According to Draining Venous Pattern
TYPE A
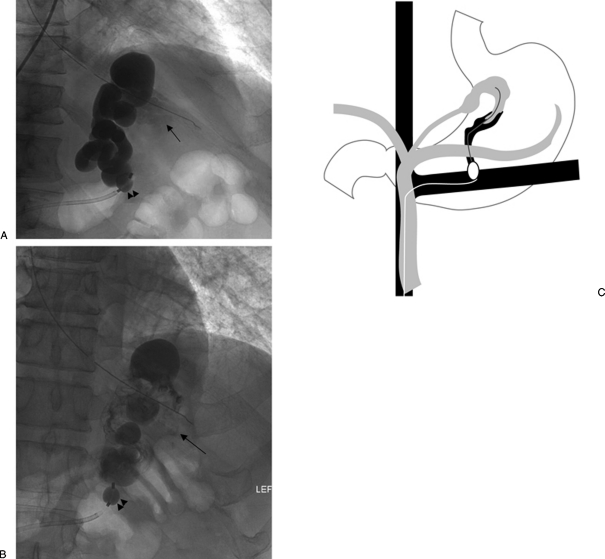
Varices are contiguous with a single draining shunt, commonly a gastrorenal shunt and less commonly a gastrocaval shunt, which drains through an enlarged inferior phrenic vein directly into the IVC. Due to the absence of leaking collateral veins, or additional shunts, this type is the most straightforward type to treat. The entire varix can be visualized during balloon-occluded retrograde venography. The microcatheter is then advanced as deep as possible into the varix and the sclerosant is administered until the embolization endpoint of minimal filling of the afferent vein/portal vasculature is achieved (Fig. 3).
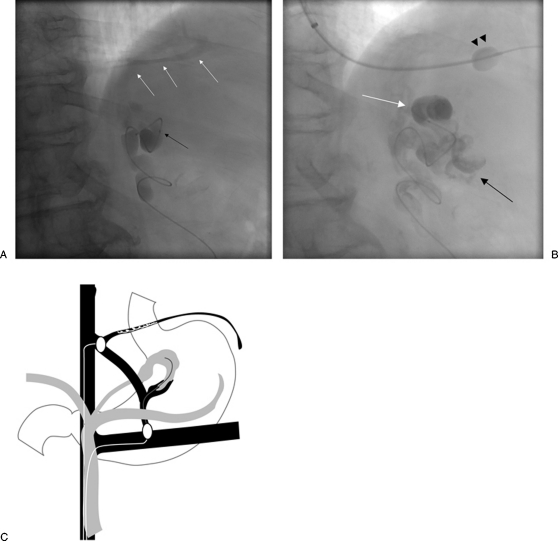
Figure 3.
Type A venous outflow. (A) With the occlusion balloon (arrowheads) positioned at the base of the gastrorenal shunt, balloon-occluded retrograde venography shows filling of the gastric varices as well as the afferent vein (arrow). (B) With the occlusion balloon (arrowheads) inflated, the sclerosant was administered with minimal filling of the afferent vein (arrow). (C) Overview of type A venous outflow treatment.
TYPE B
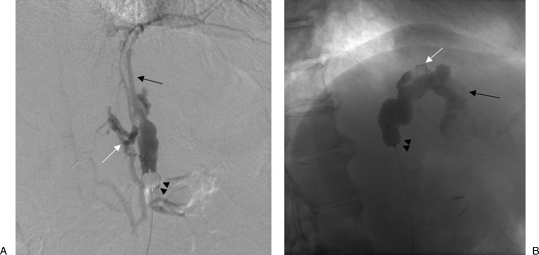
Varices are contiguous with a single shunt, usually a gastrorenal shunt, and one or multiple collateral veins. These veins drain though a plexus of vessels back to the right atrium or IVC, but do not form a discrete shunt. These draining veins include pericardiophrenic, ascending lumbar, intercostal, perivertebral veins, and rarely the azygous vein. On retrograde venography, full opacification of the varices is not achieved secondary to preferential flow of contrast into the leaking collateral veins. For types B1 and some type B2 varices where the collateral veins are too small or numerous to catheterize, there have been several proposed treatment strategies. The occlusion balloon can be advanced beyond the leaking collaterals and if repeat venography shows the varix and no leaking collaterals, the sclerosant can be administered from that location (Fig. 4). However, this is usually hard to achieve due to the tortuosity of the shunt and the balloons are likely to not advance beyond the base of the shunt. Fukuda et al17 reported on a technique to downgrade the varices by advancing the occlusion balloon over the microcatheter as deep as possible into the varix and were able to be successful in (87%) of cases. Another treatment strategy is to perform flow-directed embolization of the collaterals using the sclerosant or Gelfoam pledgets, which can be injected from the shunt in a stepwise fashion till a repeat venography opacifies the varices. Absolute alcohol has also been used for this application. Lastly, the microcatheter can simply be advanced beyond the leaking collaterals into the varix and if venography through the microcatheter opacifies the varix with no leaking collaterals, the sclerosant can be administered from that location. For B3 varices with larger size leaking collateral veins, these collaterals can be selectively catheterized using a microcatheter and embolized using metallic coils (Nester or Tornado coils; Cook Inc, Bloomington, IN). Next, a repeat venography opacifies the varix and the sclerosant can be administered after the microcatheter is advanced as deep as possible into the varix (Fig. 5).
Figure 4.
Type B venous outflow. (A) With the occlusion balloon (arrowheads) positioned at the base of the gastrorenal shunt, balloon-occluded retrograde venography shows filling of multiple “leaking” collateral veins including inferior phrenic (black arrow) and perivertebral veins (white arrow).(B) After the occlusion balloon (arrowheads) was advanced beyond the “leaking” collateral veins, venography through a microcatheter (white arrow) now shows opacification of the gastric varices (black arrow).
Figure 5.
Type B venous outflow. (A) With the occlusion balloon (arrowheads) positioned at the base of the gastrorenal shunt, balloon-occluded retrograde venography shows filling of the inferior phrenic vein (black arrow). (B) Using a microcatheter (arrow), the vein was catheterized and embolized using metallic coils. (C) With the occlusion balloon (arrowheads) inflated, the sclerosant was administered with minimal filling of the afferent vein (arrow). (D) Overview of type B venous outflow using metallic coils.
TYPE C
Varices are contiguous with both a gastrorenal and gastrocaval shunt. If the shunt is small in size (C1) and can be catheterized through the gastrorenal shunt using a microcatheter, this shunt can be coil embolized and treated similar to type B3 varices. For type C2 where the second gastrocaval shunt is large enough in size to be catheterized with an occlusion balloon catheter, then a second occlusion balloon is usually advanced into this gastrocaval shunt using the internal jugular approach. A repeat venography will then opacify the varix and administration of the sclerosant can be performed (Fig. 6).
Figure 6.
Type C venous outflow. (A) With the occlusion balloon positioned at the base of the gastrorenal shunt (not shown), balloon-occluded retrograde venography through a microcatheter (black arrow) shows filling of a gastrocaval shunt (white arrows). (B) A second occlusion balloon was advanced to occlude the gastrocaval shunt (arrowheads) .The sclerosant was administered through a microcatheter (white arrow) with opacification of the varices and minimal filling of the afferent vein (black arrow). (C) Overview of type C venous outflow treatment with a second occlusion balloon.
Treatment Modification Based on Afferent Vein Anatomy
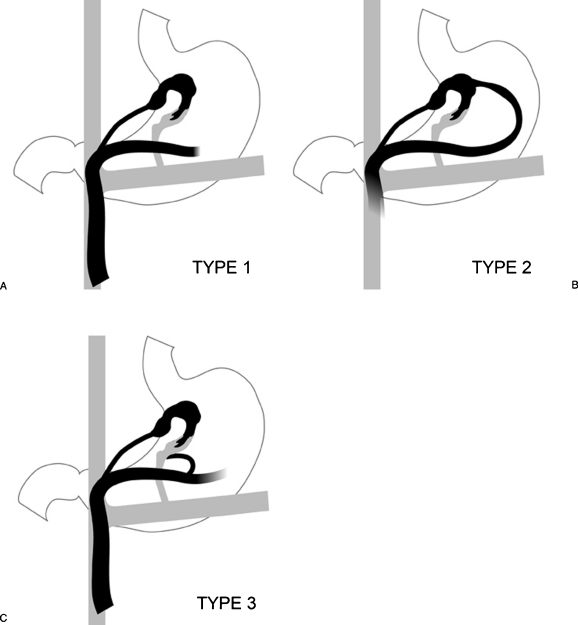
The gastric varices are also divided based on their afferent vein anatomy into three types (Fig. 7).
Figure 7.
Classification based on venous inflow. (A) Type 1 gastric varices are supplied by a single afferent gastric vein. This is the most common and simplest type to treat. (B) In type 2, the gastric varices are supplied by multiple afferent gastric veins; and (C) type 3, by single or multiple gastric veins with coexistent gastric veins that are directly contiguous with the shunt, but do not contribute to the varices.
TYPE 1
Gastric varices are usually supplied by a single afferent gastric vein, most commonly the left or posterior gastric vein. Because there is only one inflow (afferent) vein and provided that the outflow veins are appropriately occluded, the sclerosant administered through occlusion balloons usually refluxes into the gastric variceal complex and stagnates due to the high pressure from the portal system, which allows slow minimal reflux into the afferent vein. Any additional forceful injection of sclerosant can actually overcome the high portal pressure and cause further reflux into the portal system. That is why it is crucial to identify the endpoint of embolization, which is minimal filling of the afferent vein.
TYPE 2
Gastric varices are usually supplied by multiple afferent gastric veins. In this type, there are two separate afferent veins (left and posterior gastric veins). Provided the draining veins are appropriately occluded, the sclerosant, similar to type 1, will stagnate in the gastric varices and minimally reflux into one or both afferent veins. Unfortunately, commonly the pressure in one of the afferent veins may be lower than in the other vein and reflux will be noted in the lower pressure vein, which will be an indication that the endpoint of embolization has been achieved and the procedure is usually completed at that point. This usually results in partial obliteration of the varices because the other, higher pressure, afferent vein remains patent and continues to feed at least a portion of the gastric varices, which routinely requires a second BRTO procedure to occlude the remaining varices and reflux the sclerosant into this afferent vein. The difficulty in this type is realizing that there are two separate afferent veins, which are hard to identify on preprocedural imaging and unless both have similar pressures and reflux is documented in both afferent veins, partial obliteration is the usual result. In the rare instance that preprocedural imaging clearly shows two afferent veins, it may still be difficult to reflux into both afferent veins if these have different pressures. Further administration of sclerosant may result in reflux into the portal system. If that is the case, it is prudent to stage the procedure and plan on a repeat BRTO after several weeks to allow for the low-pressure afferent vein to thrombose and to embolize the higher pressure afferent vein in the second session.
TYPE 3
In type 3 gastric varices, a separate afferent vein drains directly into the shunt and does so without communication to the gastric varices. This demonstrates a challenge because sclerosant administered will usually flow into this afferent vein rather than the gastric varices route and cause reflux into the portal system. To avoid such a complication, it would be essential to advance a microcatheter as deep as possible into the gastric varices and perform a planning venogram to document the ability of achieving stagnation within the varices and reflux into the afferent vein(s) feeding the gastric varices. If that cannot be achieved, then the separate afferent vein that drains directly into the shunt needs to be embolized by percutaneous transhepatic or transjugular route to eliminate it from the circuit.
Embolization Agents/Sclerosants
Agents such as ethanolamine oleate iopamidol (EO, Oldamin; Grelan Pharmaceutical, Tokyo, Japan), sodium tetradecol sulfate (Sotradecol, AngioDynamics, Inc. Queensbury, NY), and polidocanol (Polidocasklerol, ZERIA Pharmaceutical, Tokyo, Japan) as foam and n-butyl-2-cyanoacrylate (NBCA) are examples that have been frequently used in therapy. Ethanolamine oleate is the original agent used for this procedure and is the agent of choice in Asia.1,2,3,4,5,6,7,8,9,10,11,12 5% EO is the most common concentration used and consists of a mixture of 10% EOI and the same dose of a contrast medium.3,5,6 EO causes hemolysis in the blood vessels. As a result, free hemoglobin is released, which may cause renal tubular disturbances and acute renal failure. To prevent renal insufficiency, 4,000 U of haptoglobin (Green Cross, Osaka, Japan) is administered intravenously during the BRTO to bind with the circulating free hemoglobin to minimize its nephrotoxic effects. Other reported complications associated with the use of EO include cardiogenic shock, pulmonary edema, and disseminated intravascular coagulation.3,5,6 Therefore, some authors recommend limiting the use of EO to a volume of 40 mL per procedure.5 One other limiting factor for using EO in the United States is the lack of experience with this agent in other vascular beds. The most important limitation to use of this agent in the United States is its lack of availability in the desirable concentrations and the fact that haptoglobin, a preventive measure against EO–induced hemolysis routinely injected intravenously during the procedure, is not approved by the United States Food and Drug Administration (FDA).
Sodium tetradecyl sulfate (STS) has been widely used for sclerotherapy of superficial lower extremity varicosities in both the liquid and foam forms.18,19 It has also been utilized as a foam sclerosant in treating male varicoceles, pelvic congestion syndromes, and venous malformations.20,21,22,23 The STS foam has the potential advantage of minimizing the dose of the sclerosant, especially in large varicose veins. In one study, the mean total volume of sclerosing mixture used was 34.1 mL (range: 10–65 mL) and the mean volume of 3% STS was 10 mL (1–20 mL).24 These volumes of the sclerosant and its mixtures compares favorably to the reported volumes of EO in some of the larger reported series (means of 23–26 mL).3,10 The use of a lower dose of sclerosant has the potential advantage of lowering the systemic effects such as hemolysis and renal failure without affecting the technical success or obliteration rate.
The STS foam also provides the potential advantage of better contact with the variceal wall when compared with liquid agents.18 In treating lower extremity varicose veins, foam sclerotherapy was found to be far more effective than liquid sclerotherapy by creating air bubbles that not only displace the blood volume, but also provide a larger surface area of the sclerosant to contact the venous endothelium.19
Moreover, when performing balloon-occluded retrograde transvenous obliteration, foam sclerosants tend to ascend immediately into the nondependent target gastric varices, which are located more ventral than the gastrorenal shunt, as opposed to heavy liquid sclerosants, which remain in a dependent location (i.e., dorsal in a supine patient).25
In vivo studies have demonstrated the pathologic damage of the foam to be rapid with complete damage of the endothelium within the first 2 minutes of contact with the foam, followed by intimal edema, progressive intimal separation from the tunica media and formation of microthrombi in the successive 30 minutes.26 Sotradecol is available in two different concentrations (1% and 3%). Three percent Sotradecol is favored to provide the highest dose possible in the markedly dilated varices even though one study suggested that there is no added benefit from utilizing 3% polidocanol foam compared with 1% polidocanol foam.19 The authors recommend using 3% Sotradecol mixed with gas (air or CO2) to provide foam consistency. Lipiodol is added to aid in visualization of the sclerosant mixture. The STS is mixed with lipiodol and gas (air or CO2) in a ratio of 2 mL STS: 1mL lipiodol: 3 mL air/CO2.24 The authors' observation has shown that room air remains in the foam solution for much longer than CO2 and we have switched exclusively to using room air. However, there remains the concern that room air can potentially embolize either to the lungs or the systemic circulation via a patent foramen ovale as it comes out of solution.27,28 Other proposed sclerosant mixtures include foam ethanolamine oleate, which is made by mixing 10 mL of 10% ethanolamine oleate (Oldamin; Aska Pharmaceutical, Tokyo, Japan) with 10 mL of iodinated contrast medium and 20 mL of air, and foam polidocanol, which is made by mixing 2 mL of 3% polidocanol (Polidocasklerol; ZERIA Pharmaceutical, Tokyo, Japan) and 8 mL of air.25
The occlusion balloons were inflated and remained in place for a minimum of 4 hours and a maximum of 24 hours. The balloons can be left in place inflated overnight and deflated under fluoroscopy the next day. Leaving the balloon in place for such a long time carries the potential risk of increased access site bleeding, higher infection rates, and patient inconvenience. In addition, there are logistical and cost concerns from having the patients stay overnight in a monitored bed. The authors recommend leaving the balloons inflated for 4 hours and have not noticed any change in obliteration rate or complication rate compared when the balloon was inflated for 24 hours.24 On the other hand, rupture of the occlusion balloon during or after administration of the sclerosing mixture can lead to serious complications such as pulmonary embolism or systemic toxicity due to the sclerosant, and may also result in a technical failure. Park et al experienced occlusion balloon rupture in 6 of 69 (8%) of their cases, which resulted in one suspected case of pulmonary embolism and two cases of in-hospital mortality.29 In the authors' experience, two cases of balloon rupture (9%) were encountered and occurred early in the experience in performing this procedure when the sclerosing mixture was not exclusively injected through the microcatheter.24 We postulate that the direct contact of the balloon with the sclerosing mixture led to the balloon rupture. In the subsequent cases, the microcatheter was advanced as deep as possible into the varix and the sclerosing mixture was injected through the microcatheter to minimize the risk of the mixture coming in to close proximity to the balloon, to provide the benefit of selective delivery of the sclerosing mixture into the varix and potentially minimizing the volume of sclerosant used.
The authors recommend utilizing cone beam CT (Dyna CT, Siemens Medical, Malvern, PA) to document filling of the entire varix with sclerosant. Koizumi et al have demonstrated that cone beam CT provides sufficient visualization of foam sclerosant distribution and confirms filling of the target vessel which is usually the afferent portal vein branch, even despite its lower contrast resolution than conventional CT and allowed the reduction of the amount of injected sclerosants with theoretically improved safety profile, and achieved a 100% technical success rate in a single procedure25 (Fig. 8).
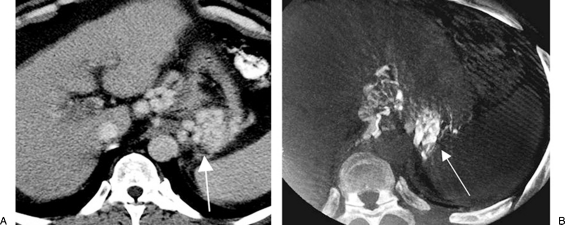
Figure 8.
Cone beam computed tomography (CT) utilization in a balloon-occluded retrograde transvenous obliteration procedure. (A) Contrast-enhanced CT scan shows gastric fundal varices (arrow). (B) Cone beam CT after administration of the sclerosant showed opacification of the same gastric fundal varices (arrow).
Failure to Obliterate the Varix Can Be Attributed to Several Factors
The gastrorenal shunt may not be fully occluded with the balloon catheter due to the large shunt size and rapid flow through the shunt. Some authors suggest partial splenic embolization to decrease the flow in the shunt and reattempting the BRTO procedure in 2 weeks.
Lack of a defined gastrorenal or gastrocaval shunt or a very tortuous shunt, which may prevent catheterization and balloon occlusion
Extensive leaking collateral veins that prevent full opacification of the varix. Selective catheterization of these collaterals may not be possible. Slow nonselective injection of Gelfoam pledgets may help decrease flow into these collaterals and further opacify the varix. Advancement of a micocatheter deep into the varix may bypass these leaking collaterals. However, this can be difficult to perform without the varix being opacified.
Inadequate volume of sclerosant administered. As stated earlier, the endpoint should be filling of the entire varix and visualization of the afferent vein.
References
- Sarin S K, Lahoti D, Saxena S P, Murthy N S, Makwana U K. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16(6):1343–1349. doi: 10.1002/hep.1840160607. [DOI] [PubMed] [Google Scholar]
- Kim T, Shijo H, Kokawa H, et al. Risk factors for hemorrhage from gastric fundal varices. Hepatology. 1997;25(2):307–312. doi: 10.1053/jhep.1997.v25.pm0009021939. [DOI] [PubMed] [Google Scholar]
- Ryan B M, Stockbrugger R W, Ryan J M. A pathophysiologic, gastroenterologic, and radiologic approach to the management of gastric varices. Gastroenterology. 2004;126(4):1175–1189. doi: 10.1053/j.gastro.2004.01.058. [DOI] [PubMed] [Google Scholar]
- Rössle M, Haag K, Ochs A, et al. The transjugular intrahepatic portosystemic stent-shunt procedure for variceal bleeding. N Engl J Med. 1994;330(3):165–171. doi: 10.1056/NEJM199401203300303. [DOI] [PubMed] [Google Scholar]
- Rössle M, Siegerstetter V, Olschewski M, Ochs A, Berger E, Haag K. How much reduction in portal pressure is necessary to prevent variceal rebleeding? A longitudinal study in 225 patients with transjugular intrahepatic portosystemic shunts. Am J Gastroenterol. 2001;96(12):3379–3383. doi: 10.1111/j.1572-0241.2001.05340.x. [DOI] [PubMed] [Google Scholar]
- Tripathi D, Therapondos G, Jackson E, Redhead D N, Hayes P C. The role of the transjugular intrahepatic portosystemic stent shunt (TIPSS) in the management of bleeding gastric varices: clinical and haemodynamic correlations. Gut. 2002;51(2):270–274. doi: 10.1136/gut.51.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Z W, Teng G J, Jeffery R F, Gemery J M, Janne d'Othee B, Bettmann M A. Long-term results and quality of life in patients treated with transjugular intrahepatic portosystemic shunts. AJR Am J Roentgenol. 2002;179(6):1597–1603. doi: 10.2214/ajr.179.6.1791597. [DOI] [PubMed] [Google Scholar]
- Boyer T D, Haskal Z J, American Association for the Study of Liver Diseases The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology. 2005;41(2):386–400. doi: 10.1002/hep.20559. [DOI] [PubMed] [Google Scholar]
- Jalan R, Hayes P C, British Society of Gastroenterology UK guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. 2000;46(Suppl 3-4):III1–III15. doi: 10.1136/gut.46.suppl_3.iii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi-Jaoudeh N, Matsumoto A H, Angle J F, et al. TIPS rebleeding rates for different subtypes of varices in the era of covered stents. (Abstract) J Vasc Interv Radiol. 2008;19(Suppl 1):S77–S78. [Google Scholar]
- Kanagawa H, Mima S, Kouyama H, Gotoh K, Uchida T, Okuda K. Treatment of gastric fundal varices by balloon-occluded retrograde transvenous obliteration. J Gastroenterol Hepatol. 1996;11(1):51–58. doi: 10.1111/j.1440-1746.1996.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Chikamori F, Shibuya S, Takase Y, Ozaki A, Fukao K. Transjugular retrograde obliteration for gastric varices. Abdom Imaging. 1996;21(4):299–303. doi: 10.1007/s002619900068. [DOI] [PubMed] [Google Scholar]
- Hiraga N, Aikata H, Takaki S, et al. The long-term outcome of patients with bleeding gastric varices after balloon-occluded retrograde transvenous obliteration. J Gastroenterol. 2007;42(8):663–672. doi: 10.1007/s00535-007-2077-1. [DOI] [PubMed] [Google Scholar]
- Chikamori F, Kuniyoshi N, Shibuya S, Takase Y. Eight years of experience with transjugular retrograde obliteration for gastric varices with gastrorenal shunts. Surgery. 2001;129(4):414–420. doi: 10.1067/msy.2001.112000. [DOI] [PubMed] [Google Scholar]
- Kiyosue H, Mori H, Matsumoto S, Yamada Y, Hori Y, Okino Y. Transcatheter obliteration of gastric varices. Part 1. Anatomic classification. Radiographics. 2003;23(4):911–920. doi: 10.1148/rg.234025044. [DOI] [PubMed] [Google Scholar]
- Kiyosue H, Mori H, Matsumoto S, Yamada Y, Hori Y, Okino Y. Transcatheter obliteration of gastric varices: Part 2. Strategy and techniques based on hemodynamic features. Radiographics. 2003;23(4):921–937. discussion 937. doi: 10.1148/rg.234025135. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Hirota S, Sugimoto K, Matsumoto S, Zamora C A, Sugimura K. “Downgrading” of gastric varices with multiple collateral veins in balloon-occluded retrograde transvenous obliteration. J Vasc Interv Radiol. 2005;16(10):1379–1383. doi: 10.1097/01.RVI.0000175336.05823.eb. [DOI] [PubMed] [Google Scholar]
- Demagny A. Comparative study into the efficacy of a sclerosant product in the form of liquid or foam in echo-guided of arches of the long and short saphenous veins. Phlebologie. 2002;55:133–137. [Google Scholar]
- Coleridge Smith P. Sclerotherapy and foam sclerotherapy for varicose veins. Phlebology. 2009;24(6):260–269. doi: 10.1258/phleb.2009.009050. [DOI] [PubMed] [Google Scholar]
- Gandini R, Chiocchi M, Konda D, Pampana E, Fabiano S, Simonetti G. Transcatheter foam sclerotherapy of symptomatic female varicocele with sodium-tetradecyl-sulfate foam. Cardiovasc Intervent Radiol. 2008;31(4):778–784. doi: 10.1007/s00270-007-9264-6. [DOI] [PubMed] [Google Scholar]
- Gandini R, Konda D, Reale C A, et al. Male varicocele: transcatheter foam sclerotherapy with sodium tetradecyl sulfate—outcome in 244 patients. Radiology. 2008;246(2):612–618. doi: 10.1148/radiol.2462061295. [DOI] [PubMed] [Google Scholar]
- Tan K T, Kirby J, Rajan D K, Hayeems E, Beecroft J R, Simons M E. Percutaneous sodium tetradecyl sulfate sclerotherapy for peripheral venous vascular malformations: a single-center experience. J Vasc Interv Radiol. 2007;18(3):343–351. doi: 10.1016/j.jvir.2006.12.735. [DOI] [PubMed] [Google Scholar]
- Yamaki T, Nozaki M, Sakurai H, Takeuchi M, Soejima K, Kono T. Prospective randomized efficacy of ultrasound-guided foam sclerotherapy compared with ultrasound-guided liquid sclerotherapy in the treatment of symptomatic venous malformations. J Vasc Surg. 2008;47(3):578–584. doi: 10.1016/j.jvs.2007.11.026. [DOI] [PubMed] [Google Scholar]
- Sabri S S, Swee W, Turba U C, et al. Bleeding gastric varices obliteration with balloon-occluded retrograde transvenous obliteration using sodium tetradecyl sulfate foam. J Vasc Interv Radiol. 2011;22(3):309–316. quiz 316. doi: 10.1016/j.jvir.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Koizumi J, Hashimoto T, Myojin K, et al. C-arm CT-guided foam sclerotherapy for the treatment of gastric varices. J Vasc Interv Radiol. 2010;21(10):1583–1587. doi: 10.1016/j.jvir.2010.05.029. [DOI] [PubMed] [Google Scholar]
- Orsini C, Brotto M. Immediate pathologic effects on the vein wall of foam sclerotherapy. Dermatol Surg. 2007;33(10):1250–1254. doi: 10.1111/j.1524-4725.2007.33261.x. [DOI] [PubMed] [Google Scholar]
- Ceulen R P, Sommer A, Vernooy K. Microembolism during foam sclerotherapy of varicose veins. N Engl J Med. 2008;358(14):1525–1526. doi: 10.1056/NEJMc0707265. [DOI] [PubMed] [Google Scholar]
- Forlee M V, Grouden M, Moore D J, Shanik G. Stroke after varicose vein foam injection sclerotherapy. J Vasc Surg. 2006;43(1):162–164. doi: 10.1016/j.jvs.2005.09.032. [DOI] [PubMed] [Google Scholar]
- Park S J, Chung J W, Kim H C, Jae H J, Park J H. The prevalence, risk factors, and clinical outcome of balloon rupture in balloon-occluded retrograde transvenous obliteration of gastric varices. J Vasc Interv Radiol. 2010;21(4):503–507. doi: 10.1016/j.jvir.2009.11.023. [DOI] [PubMed] [Google Scholar]