Abstract
The majority of patients undergoing balloon retrograde transvenous obliteration (BRTO) are decompensated cirrhotic for either bleeding gastric varices (GV) or hepatic encephalopathy. These patients will require close follow-up and assessments pre- and post-BRTO including clinical, laboratory, endoscopic, and imaging evaluations. It is essential that clinicians are aware of the potential benefits and complications that may result from BRTO. These complications may include fever, chest or epigastric pain, hemoglobinuria, transient hypertension, nausea or vomiting, and many more. These complications usually resolve within the first 10 days. Laboratory abnormalities are transient and uncommon. Radiologic and endoscopic follow-up are required including computed tomography (CT), magnetic resonance imaging (MRI), routine upper endoscopy and endoscopic ultrasound (EUS), which are detailed in this review. Patients undergoing BRTO are usually complicated and will require a team approach. This team should include the hepatologist, endoscopist, and interventional radiologist. Understanding and open dialogue are essential in the management of post-BRTO patients. The authors review the possible benefits, potential complications, and the evaluation tools needed to improve outcomes.
Keywords: Gastric varices, portal hypertension, liver cirrhosis, BRTO, TIPS, splenorenal shunt, EUS
Patients undergoing balloon retrograde transvenous obliteration (BRTO) for bleeding gastric varices (GV) will require close follow-up and assessments including clinical, laboratory, endoscopic, and imaging tools. It is essential that clinicians are appraised of these needed assessments post-BRTO to prevent complications and deliver critical quality care. The majority of patients undergoing BRTO are cirrhotics, which will require a team approach. This team is composed of the hepatologist, endoscopist, and interventional radiologist. Open dialogue and collaboration is essential in the management of post-BRTO patients.
CLINICAL ASSESSMENT
Patients undergoing BRTO are usually cirrhotics who had decompensation with bleeding varices or hepatic encephalopathy (HE).1,2,3,4,5,6 Studies have demonstrated improvement of the hepatic functional reserve, increased portal blood flow, reduction of gastric variceal hemorrhage, and improvement in impaired glucose tolerance testing after BRTO.6,7,8,9,10,11,12,13,14 Recent studies have shown improvement or stability of hepatic function via Child-Pugh (CP) score and MELD (Model of End-Stage Liver Disease).7,15 On the other hand, most of these patients are cirrhotics, thus detailed attention to the patient' liver and renal function pre- and post-BRTO is essential. Patients with fluid overload (including ascites, lower extremity edema and/or hepatic hydrothorax[HH]), and esophageal varices will need close monitoring, as they may worsen post-BRTO in up to 40% of patients.7,16 In the immediate post-BRTO the following complications has been noted and were transient; fever (33%), chest or epigastric pain (56%), hemoglobinuria (49%), transient hypertension (35%), nausea or vomiting (21%), gastric ulcers (9%), and hemorrhagic portal hypertensive gastropathy (2%).7,16 In addition, less common complications have been observed in the first 7–10 days including pleural effusion (HH: 12%), and pulmonary infarction (2%), which usually resolves within the first 10 days.7,16 Thus, a routine check of blood gas analysis and chest roentgenogram is recommended in all patients in the first 24 hours post-BRTO.16 Lactate dehydrogenase, aspartate aminotransferase, and bilirubin increase in the first couple of days, but return to normal and are thought due to intravascular hemolysis.16,17 Albumin, total bilirubin, and protime have been shown to improve over time in patients who have undergone BRTO.7,8 Renal function might be affected, which could be related to several factors including diuretic use (for ascites or fluid overload), acute vascular necrosis (resulting from hemodynamic instability postvariceal bleed or ethanolamine oleate or other sclerosant agents), contrast-induced nephropathy or hepatorenal syndrome.16,17 In 5–10% of patients undergoing BRTO, TIPS is performed either simultaneously or subsequently before or after BRTO; thus TIPS-specific complications should be anticipated, and prevented and corrected as clinically indicated.
RADIOLOGIC ASSESSMENT
Imaging of gastric varices post-BRTO can be performed using contrast-enhanced computed tomography (CECT) or contrast-enhanced magnetic resonance imaging (CEMRI). The timing of obtaining the follow-up cross-sectional imaging has been variable in the literature with the most common practice to obtain follow-up imaging with CECT or CEMRI at 1, 3, 6, and 12 months and then every 6 months or annually.5,18 It is also important to obtain postprocedural cross-sectional imaging and/or endoscopic ultrasound (EUS) imaging of the varices before the patient is discharged from the hospital or within the first week to confirm obliteration of the varices.19 Cross-sectional imaging is important in documenting lack of enhancement of the gastric varices, confirm patency of the splenic vein, main portal vein, and intrahepatic branches, as well as document patency of the left renal vein and rule out any retroperitoneal bleeding or infectious complications.
Post-BRTO Follow-Up with CECT
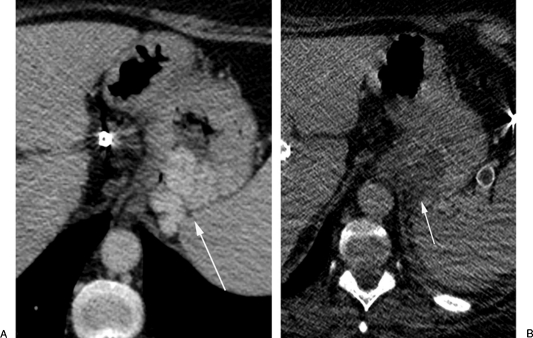
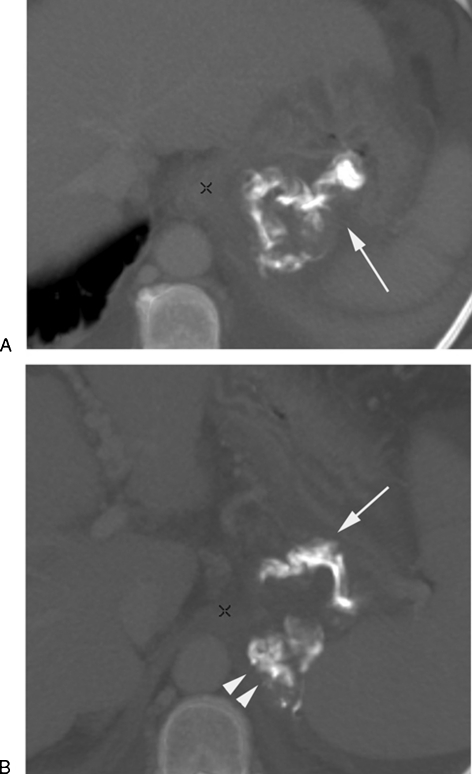
It is recommended to obtain noncontrast and portal venous phase contrast CT images to better evaluate the gastric varices post-BRTO. Depending on the sclerosing agent used, the appearance of the gastric varices may vary. Ethanolamine oleate (EO; Oldamin, Aska Pharmaceutical, Tokyo, Japan), the most commonly used agent, is usually mixed with iodinated contrast material during the procedure and is usually absorbed by the time of the follow-up CT. The varices filled with EO would appear isodense on precontrast images and show no enhancement on postcontrast images. Similarly the varices will have the same appearance if sodium tetradecol sulfate (Sotradecol, AngioDynamics, Inc., Queensbury, NY), polidocanol (Polidocasklerol, ZERIA Pharmaceutical, Tokyo, Japan) or n-butyl-2-cyanoacrylate (NBCA) where used as sclerosing agents with iodinated contrast (Fig. 1). However, if any of the agents are mixed with lipiodol (Ethiodol, Savage Laboratories, Melville, NY), which is added to enhance visualization of the foam forms of the sclerosant,19 the stagnant sclerosing mixture usually appears hyperdense on postprocedural images, especially the short-term follow-up images (Fig. 2) and show no enhancement on postprocedural images. However, due to the difficulty of visualizing enhancement due to the presence of the hyperdense lipiodol, CEMRI may be a better follow-up tool for BRTO procedures utilizing lipiodol as an image-enhancing agent.
Figure 1.
A computed tomography (CT) follow-up study post-BRTO with Sotradecol mixed with iodinated contrast. (A) Axial CT scan shows enhancing gastric fundal varices (arrow). (B) Axial CT scan 4-weeks post-BRTO showing isodense appearance of the varices (arrow) with no enhancement.
Figure 2.
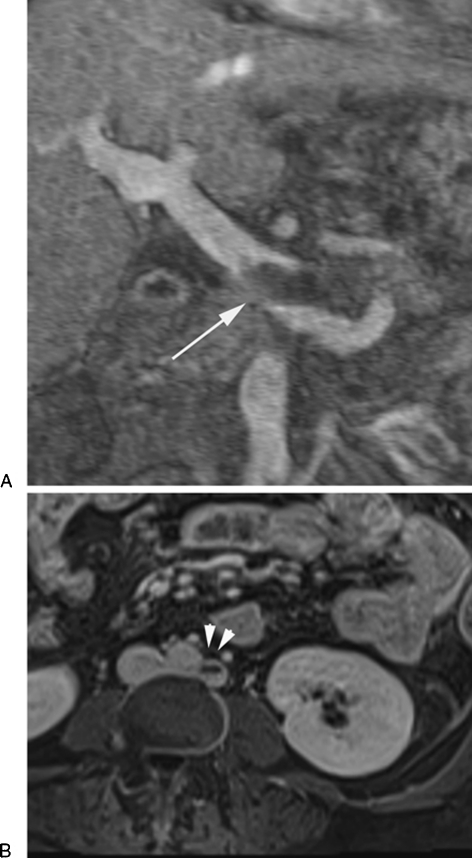
A computed tomography (CT) follow-up study post-BRTO with Sotradecol mixed with lipiodol. Axial CT scan 1-week post BRTO shows (A) the hyperdense sclerosing material (arrow) within the gastric varices and (B) within the afferent posterior gastric vein (arrow) and the draining gastrorenal shunt (arrowheads).
Post-BRTO Follow-Up with CEMRI
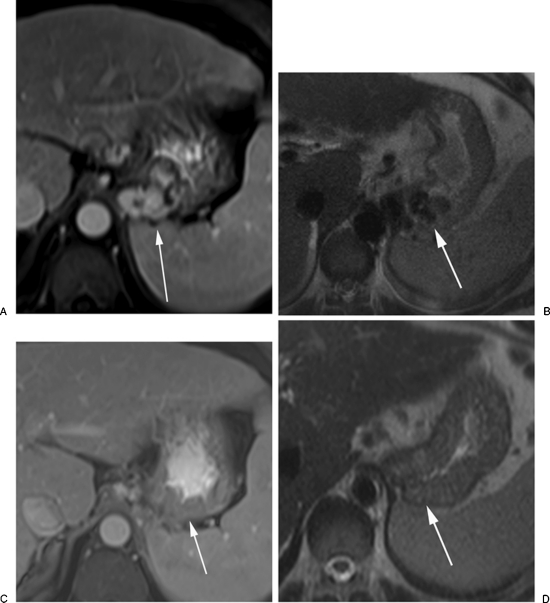
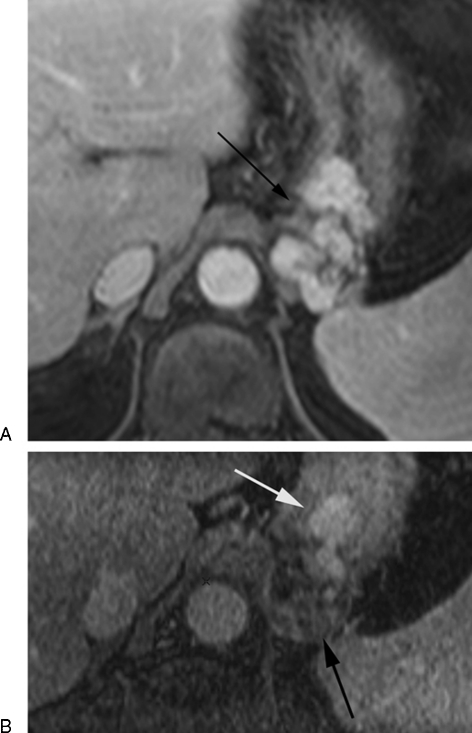
CEMRI with T1-weighted, T2-weighted imaged and dynamic contrast-enhanced images is ideal for follow-up of BRTO when lipiodol is utilized during the procedure. The gastric varices may show a hyperintense signal on precontrast T1 images, which can be attributed to retained lipiodol or blood products in thrombosed varices. The varices may appear more isointense on T2-weighted images and show no enhancement on dynamic postcontrast images regardless of the sclerosing agent used (Fig. 3). Residual enhancement within the varices indicated incomplete obliteration, which could be secondary to insufficient amount of sclerosing agent used or due to residual filling of the varices through a second afferent pathway that was not completely embolized during the procedure and usually require a second BRTO procedure (Fig. 4). Complications such as renal vein or portal vein thrombosis can also be seen on contrast-enhanced sequences (Fig. 5).
Figure 3.
Magnetic resonance imaging (MRI) follow-up study post-BRTO. (A) Axial contrast-enhanced MRI scan showing enhancing gastric fundal varices (arrow). (B) Axial T2-weighted images show the gastric varices as flow voids, 4-weeks post-BRTO. (C) Contrast-enhanced image shows lack of enhancement of gastric varices (arrow) indicating obliteration. (D) The varices now appear isointense and decompressed (arrow) on T2-weighted images.
Figure 4.
Partial obliteration of gastric varices. (A) Axial contrast-enhanced magnetic resonance image showing enhancing gastric fundal varices (arrow). (B) 4 Weeks post-BRTO images show lack of enhancement of the posterior cluster of gastric varices (black arrow) indicating obliteration, while the more anterior cluster continue to enhance indicating patency (white arrow).
Figure 5.
Complication post-BRTO. Coronal (A) and axial (B) contrast-enhanced magnetic resonance images post-BRTO show partial thrombosis of main portal vein (arrow) and left retroaortic renal vein (arrowheads). The patient remained asymptomatic during the follow-up period of 24 months.
ENDOSCOPIC ASSESMENT
Routine upper endoscopy might be indicated to assess obliteration of gastric varices and/or worsening of esophageal varices. During upper endoscopy, band ligation of the esophageal varices might be required. Here we will describe EUS as a tool in assessing gastric variceal obliteration post-BRTO, as well as banding of esophageal varices in the same setting.
EUS Background
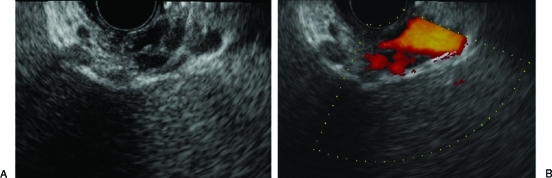
Endoscopic ultrasonography (EUS) uses sound waves to visualize luminal, intramural, as well as extramural lesions of the gastrointestinal tract. Structures that are fluid filled, such as varices, are often dark and therefore described as anechoic (Figure 6). This technology has the added advantage of Doppler, which allows the assessment of flow in vascular structures. These attributes make EUS a potentially valuable tool for the assessment of esophageal and gastric varices.
Figure 6.
Patent gastric varices on (A) endoscopic ultrasound B-mode and (B) color flow Doppler. Note the hypoechoic nature of the gastric varices and evidence of flow on color flow Doppler.
The first study that described EUS for the study of blood flow in varices was in 1983.20 Subsequently, conventional EUS was used in 198621 to image esophagogastric varices in patients with portal hypertension, including three posttreatment cases. The authors concluded, “Endoscopic ultrasonography will probably become a fundamental technique in the study of portal hypertension and esophageal varices, before and after therapy.” There have since been numerous studies on EUS in the study of gastric varices.22,23,24,25,26,27,28
It is important for the endosonographist to have a good understanding of portal hypertension and to differentiate submucosal vessels as true varices that are seen endoscopically, and differentiating these from dilated veins outside of the wall of the lumen, which are not visible by conventional endoscopy.29 There also needs to be good communication between the endoscopic and hepatologist, to assure that the information requested is being obtained.
With advances in medicine, and treatment breakthroughs in the management of gastric varices, the applications of EUS have naturally extended to the evaluation of gastric varices after treatment.
Use of Endoscopic Ultrasound in Posttherapy Assessment
EUS for the evaluation of gastric varices posttreatment was first described in 1995 after cyanoacrylate glue injection.25 After cyanoacrylate gluing, probe-based EUS with color flow Doppler (CFD) was used and demonstrated no significant flow immediately following glue injection in a small study of 13 patients. After 4 weeks, those with persistent variceal occlusion on EUS (i.e., no flow on repeat EUS) had a lower rate of recurrent bleeding (0/4 vs 2/3;).25
In another study, EUS was repeated biweekly with subsequent cyanoacrylate glue injection until complete gastric variceal obliteration. When compared with “on demand” gluing of gastric varices, those with close EUS follow-up had reduced late recurrence of bleeding and a trend for improved survival.30 One limitation of this study is that EUS was not performed with Doppler and therefore blood flow could not be assessed. Another study reported 48% (n = 31) of patients who underwent gastric variceal gluing received EUS for follow-up. In this study, there was no difference in the rebleeding rate when comparing those with and without EUS,31 although interpretation is cautioned due to a few episodes of rebleeding (n = 5). Additionally, EUS-guided treatment approaches, where real-time assessment of occlusion can be performed, have also been reported.32,33
Overall, EUS is accurate and helpful in posttherapy follow-up of gastric varices. In particular, when Doppler is used, vascular flow can be identified and further therapy directed if needed.
Use of Endoscopic Ultrasound in Post-BRTO Assessment
To date, no study has reported EUS for follow-up after BRTO. In our experience, EUS shortly after BRTO demonstrates an isoechoic filling of the gastric varices as compared with the pretreatment anechoic nature of the varices (Figs. 6, 7, 8). We routinely use EUS to assess gastric varices for flow 1–2 days following BRTO and at 6 months to assess for obliteration.
Figure 7.
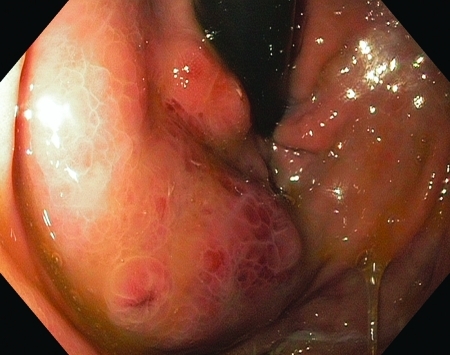
Endoscopic appearance of gastric varices post-BRTO.
Figure 8.
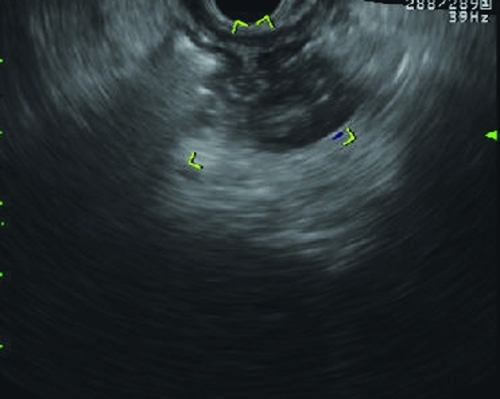
Gastric varices under endoscopic ultrasound with color flow Doppler post-BRTO. Notice the more hypoechoic filling of the varix (as opposed to the anechoic filling) and lack of flow on Doppler.
From 2008–2010 we performed 23 EUS procedures for post-BRTO follow-up and demonstrated no flow in 18 of 23 of the gastric varices. In the remaining four individuals with flow, three underwent subsequent therapy (two glue, one repeat BRTO) and two were managed conservatively (unpublished data).
Technique of Endoscopic Ultrasound Post-BRTO
When performing post-BRTO evaluation of gastric varices, we started with a standard upper endoscopy. This allows visualization of the gastric varices with a forward-viewing endoscope prior to ultrasonography. This was helpful in identifying the number and location of gastric varices. In our experience, the gastric varices have a more scaly erythematous or mosaic mucosal appearance when comparing pre- to post-BRTO images (Fig. 7); similar findings have been previously reported.16
After standard upper endoscopy, we typically use a diagnostic linear echoendoscope or a newer generation electronic radial echoendoscope (GF-UC140P and GF-UE160 respectively) as these allow for CFD. It is important to note that mechanical radial echoendoscopes and many EUS probes do not allow for Doppler, and therefore we have not used these. After the echoendoscope is intubated into the stomach, the varix is visualized in B mode with and without Doppler. Because the varix is filled with material, it often has a more isoechoic appearance rather than an anechoic lesion. When color flow Doppler is applied, flow in the varix can be further assessed.
CONCLUSION
It is crucial to understand the possible clinical, laboratory, and radiologic complications of balloon-occluded retrograde transvenous obliteration (BRTO), and the needed assessment as we have discussed here.
ACKNOWLEDGMENTS
Dr. BG Sauer and Dr. SS Sabri contributed equally to this manuscript.
References
- Kanagawa H, Mima S, Kouyama H, Gotoh K, Uchida T, Okuda K. Treatment of gastric fundal varices by balloon-occluded retrograde transvenous obliteration. J Gastroenterol Hepatol. 1996;11(1):51–58. doi: 10.1111/j.1440-1746.1996.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Koito K, Namieno T, Nagakawa T, Morita K. Balloon-occluded retrograde transvenous obliteration for gastric varices with gastrorenal or gastrocaval collaterals. AJR Am J Roentgenol. 1996;167(5):1317–1320. doi: 10.2214/ajr.167.5.8911204. [DOI] [PubMed] [Google Scholar]
- Kitamoto M, Imamura M, Kamada K, et al. Balloon-occluded retrograde transvenous obliteration of gastric fundal varices with hemorrhage. AJR Am J Roentgenol. 2002;178(5):1167–1174. doi: 10.2214/ajr.178.5.1781167. [DOI] [PubMed] [Google Scholar]
- Sakurabayashi S, Sezai S, Yamamoto Y, Hirano M, Oka H. Embolization of portal-systemic shunts in cirrhotic patients with chronic recurrent hepatic encephalopathy. Cardiovasc Intervent Radiol. 1997;20(2):120–124. doi: 10.1007/s002709900118. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Hirota S, Sugimura K. Long-term results of balloon-occluded retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy. J Vasc Interv Radiol. 2001;12(3):327–336. doi: 10.1016/s1051-0443(07)61912-5. [DOI] [PubMed] [Google Scholar]
- Tanabe N, Ishii M, Sato Y, et al. Effects of collateral vessel occlusion on oral glucose tolerance test in liver cirrhosis. Dig Dis Sci. 2000;45(3):581–586. doi: 10.1023/a:1005461611262. [DOI] [PubMed] [Google Scholar]
- Kumamoto M, Toyonaga A, Inoue H, et al. Long-term results of balloon-occluded retrograde transvenous obliteration for gastric fundal varices: hepatic deterioration links to portosystemic shunt syndrome. J Gastroenterol Hepatol. 2010;25(6):1129–1135. doi: 10.1111/j.1440-1746.2010.06262.x. [DOI] [PubMed] [Google Scholar]
- Takuma Y, Nouso K, Makino Y, Saito S, Shiratori Y. Prophylactic balloon-occluded retrograde transvenous obliteration for gastric varices in compensated cirrhosis. Clin Gastroenterol Hepatol. 2005;3(12):1245–1252. doi: 10.1016/s1542-3565(05)00744-5. [DOI] [PubMed] [Google Scholar]
- Nakano R, Iwao T, Oho K, Toyonaga A, Tanikawa K. Splanchnic hemodynamic pattern and liver function in patients with cirrhosis and esophageal or gastric varices. Am J Gastroenterol. 1997;92(11):2085–2089. [PubMed] [Google Scholar]
- Choi Y H, Yoon C J, Park J H, Chung J W, Kwon J W, Choi G M. Balloon-occluded retrograde transvenous obliteration for gastric variceal bleeding: its feasibility compared with transjugular intrahepatic portosystemic shunt. Korean J Radiol. 2003;4(2):109–116. doi: 10.3348/kjr.2003.4.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Uematsu T, Nishigaki Y, Sugihara J, Tomita E, Moriwaki H. Therapeutic effect of balloon-occluded retrograde transvenous obliteration on portal-systemic encephalopathy in patients with liver cirrhosis. Intern Med. 2001;40(8):688–691. doi: 10.2169/internalmedicine.40.688. [DOI] [PubMed] [Google Scholar]
- Akahane T, Iwasaki T, Kobayashi N, et al. Changes in liver function parameters after occlusion of gastrorenal shunts with balloon-occluded retrograde transvenous obliteration. Am J Gastroenterol. 1997;92(6):1026–1030. [PubMed] [Google Scholar]
- Miyamoto Y, Oho K, Kumamoto M, Toyonaga A, Sata M. Balloon-occluded retrograde transvenous obliteration improves liver function in patients with cirrhosis and portal hypertension. J Gastroenterol Hepatol. 2003;18(8):934–942. doi: 10.1046/j.1440-1746.2003.03087.x. [DOI] [PubMed] [Google Scholar]
- Cardoso J E, Gautreau C, Jeyaraj P R, et al. Augmentation of portal blood flow improves function of human cirrhotic liver. Hepatology. 1994;19(2):375–380. [PubMed] [Google Scholar]
- Saad W, Darwish W, Anderson , et al. The effect of balloon-occluded retrograde transvenous obliteration (BRTO) on the model of end-stage liver disease (MELD) score. (Abst.) J Vasc Interv Radiol. 2011;22(35):S34–35. [Google Scholar]
- Shimoda R, Horiuchi K, Hagiwara S, et al. Short-term complications of retrograde transvenous obliteration of gastric varices in patients with portal hypertension: effects of obliteration of major portosystemic shunts. Abdom Imaging. 2005;30(3):306–313. doi: 10.1007/s00261-004-0270-8. [DOI] [PubMed] [Google Scholar]
- Wada H, Hashizume M, Yamaga H, Kitano S, Sugimachi K. Hemodynamic and morphological changes in the dog kidney after injection of 5% ethanolamine oleate into the superior vena cava. Eur Surg Res. 1990;22(2):63–70. doi: 10.1159/000129084. [DOI] [PubMed] [Google Scholar]
- Hiraga N, Aikata H, Takaki S, et al. The long-term outcome of patients with bleeding gastric varices after balloon-occluded retrograde transvenous obliteration. J Gastroenterol. 2007;42(8):663–672. doi: 10.1007/s00535-007-2077-1. [DOI] [PubMed] [Google Scholar]
- Sabri S S, Swee W, Turba U C, et al. Bleeding gastric varices obliteration with balloon-occluded retrograde transvenous obliteration using sodium tetradecyl sulfate foam. J Vasc Interv Radiol. 2011;22(3):309–316. quiz 316. doi: 10.1016/j.jvir.2010.11.022. [DOI] [PubMed] [Google Scholar]
- McCormack T, Martin T, Smallwood R H, Robinson P, Walton L, Johnson A G. Doppler ultrasound probe for assessment of blood-flow in oesophageal varices. Lancet. 1983;1(8326 Pt 1):677–678. doi: 10.1016/s0140-6736(83)91971-2. [DOI] [PubMed] [Google Scholar]
- Caletti G C, Bolondi L, Zani L, Brocchi E, Guizzardi G, Labò G. Detection of portal hypertension and esophageal varices by means of endoscopic ultrasonography. Scand J Gastroenterol Suppl. 1986;123:74–77. doi: 10.3109/00365528609091866. [DOI] [PubMed] [Google Scholar]
- Burtin P, Calès P, Oberti F, et al. Endoscopic ultrasonographic signs of portal hypertension in cirrhosis. Gastrointest Endosc. 1996;44(3):257–261. doi: 10.1016/s0016-5107(96)70161-x. [DOI] [PubMed] [Google Scholar]
- Caletti G, Brocchi E, Baraldini M, Ferrari A, Gibilaro M, Barbara L. Assessment of portal hypertension by endoscopic ultrasonography. Gastrointest Endosc. 1990;36(2, Suppl):S21–S27. doi: 10.1016/s0016-5107(90)71011-5. [DOI] [PubMed] [Google Scholar]
- Choudhuri G, Dhiman R K, Agarwal D K. Endosonographic evaluation of the venous anatomy around the gastro-esophageal junction in patients with portal hypertension. Hepatogastroenterology. 1996;43(11):1250–1255. [PubMed] [Google Scholar]
- Iwase H, Suga S, Morise K, Kuroiwa A, Yamaguchi T, Horiuchi Y. Color Doppler endoscopic ultrasonography for the evaluation of gastric varices and endoscopic obliteration with cyanoacrylate glue. Gastrointest Endosc. 1995;41(2):150–154. doi: 10.1016/s0016-5107(05)80599-1. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Nakamura T, Kida H, Seno H, Okazaki K, Chiba T. Catheter US probe EUS evaluation of gastric cardia and perigastric vascular structures to predict esophageal variceal recurrence. Gastrointest Endosc. 2002;55(2):197–203. doi: 10.1067/mge.2002.121338. [DOI] [PubMed] [Google Scholar]
- Lo G H, Lai K H, Cheng J S, Huang R L, Wang S J, Chiang H T. Prevalence of paraesophageal varices and gastric varices in patients achieving variceal obliteration by banding ligation and by injection sclerotherapy. Gastrointest Endosc. 1999;49(4 Pt 1):428–436. doi: 10.1016/s0016-5107(99)70038-6. [DOI] [PubMed] [Google Scholar]
- Wong R C, Farooq F T, Chak A. Endoscopic Doppler US probe for the diagnosis of gastric varices (with videos) Gastrointest Endosc. 2007;65(3):491–496. doi: 10.1016/j.gie.2006.11.017. [DOI] [PubMed] [Google Scholar]
- El-Saadany M, Jalil S, Irisawa A, Shibukawa G, Ohira H, Bhutani M S. EUS for portal hypertension: a comprehensive and critical appraisal of clinical and experimental indications. Endoscopy. 2008;40(8):690–696. doi: 10.1055/s-2008-1077400. [DOI] [PubMed] [Google Scholar]
- Lee Y T, Chan F K, Ng E K, et al. EUS-guided injection of cyanoacrylate for bleeding gastric varices. Gastrointest Endosc. 2000;52(2):168–174. doi: 10.1067/mge.2000.107911. [DOI] [PubMed] [Google Scholar]
- Rajoriya N, Forrest E H, Gray J, et al. Long-term follow-up of endoscopic Histoacryl glue injection for the management of gastric variceal bleeding. QJM. 2011;104(1):41–47. doi: 10.1093/qjmed/hcq161. [DOI] [PubMed] [Google Scholar]
- Romero-Castro R, Pellicer-Bautista F, Giovannini M, et al. Endoscopic ultrasound (EUS)-guided coil embolization therapy in gastric varices. Endoscopy. 2010;42(Suppl 2):E35–E36. doi: 10.1055/s-0029-1215261. [DOI] [PubMed] [Google Scholar]
- Romero-Castro R, Pellicer-Bautista F J, Jimenez-Saenz M, et al. EUS-guided injection of cyanoacrylate in perforating feeding veins in gastric varices: results in 5 cases. Gastrointest Endosc. 2007;66(2):402–407. doi: 10.1016/j.gie.2007.03.008. [DOI] [PubMed] [Google Scholar]