Abstract
In the past 20 years, our understanding of the pathophysiology and management options among patients with gastric varices (GV) has changed significantly. GV are the most common cause of upper gastrointestinal bleeding in patients with portal hypertension after esophageal varices (EV) and generally have more severe bleeding than EV. In the United States, the majority of GV patients have underlying portal hypertension rather than splenic vein thrombosis. The widely used classifications are the Sarin Endoscopic Classification and the Japanese Vascular Classifications. The former is based on the endoscopic appearance and location of the varices, while the Japanese classification is based on the underlying vascular anatomy. In this article, the authors address the current concepts of classification, epidemiology, pathophysiology, and emerging management options of gastric varices. They describe the stepwise approach to patients with gastric varices, including the different available modalities, and the pearls, pitfalls, and stop-gap measures useful in managing patients with gastric variceal bleed.
Keywords: Gastric varices, portal hypertension, liver cirrhosis, BRTO, TIPS, splenorenal shunt, cyanoacrylate
Gastric varices (GV) are the most common cause of upper gastrointestinal (UGI) bleeding in patients with portal hypertension after esophageal varices (EV). Bleeding from GV is generally more severe and is associated with higher morbidity, transfusion requirements and mortality than EV.1 If all types of GV are included, the frequency of bleeding is lower than EV,2,3 but if ‘high risk’ GV are considered, the frequency of bleeding is as high as EV. Unfortunately, the approach to prevention of first bleed and rebleed from GV has remained empirical in the absence of large randomized trials.
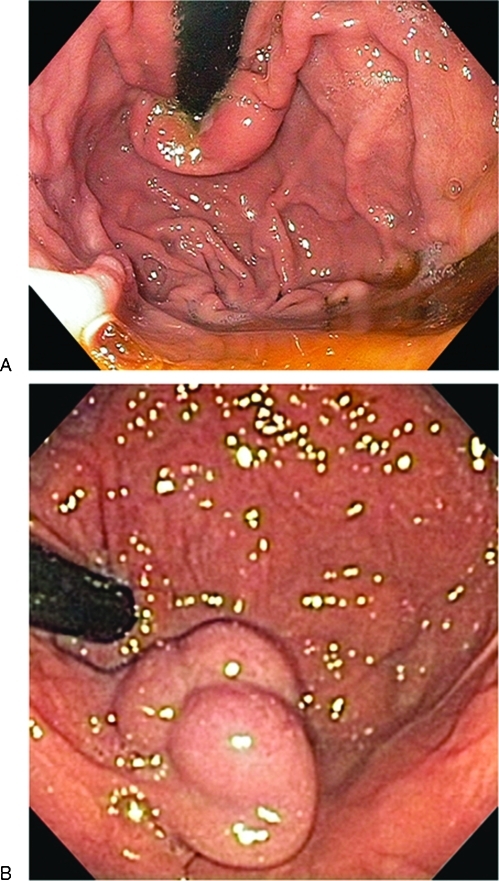
Much has changed in the past 20 years in our understanding of the pathophysiology and management options among patients with gastric varices. In the United States, the majority of GV patients have underlying portal hypertension rather than splenic vein thrombosis, although exclusion of the latter remains an essential early step in the evaluation. Especially problematic are varices that occur in the fundal area of the stomach. Fundal varices may present as serpiginous obviously vascular structures or sometimes as polypoid masses occasionally resembling a cluster of grapes (Fig. 1). From our experience in a typical tertiary care center, fundal varices are encountered at a rate of ∼1–2 cases per month. These range in severity from acute, active hemorrhage to prior recurrent episodes of bleeding to incidentally discovered varices sometimes in patients with previously unknown liver disease. Rarely, their somewhat polypoid appearance has led to an errant biopsy in patients not previously known to have liver disease (Fig. 1).
Figure 1.
(A,B) Endoscopic appearance of gastric varices. (A) Demonstrate a type 2 gastro-esophageal varices (GOV) in the cardia, while (B) shows an isolated gastric varix (TGV-1) type 1 in the fundus.
Below, we have addressed current concepts of classification, epidemiology, pathophysiology, and emerging management options of gastric varices. To our knowledge none of the management strategies discussed carry U.S. Food and Drug Administration (FDA) approval with the exception of the Sangstaken-Blakemore tube. Clearly, this is an area in need of carefully planned research in both classification and optimal management triage.
CLASSIFICATION OF GASTRIC VARICES
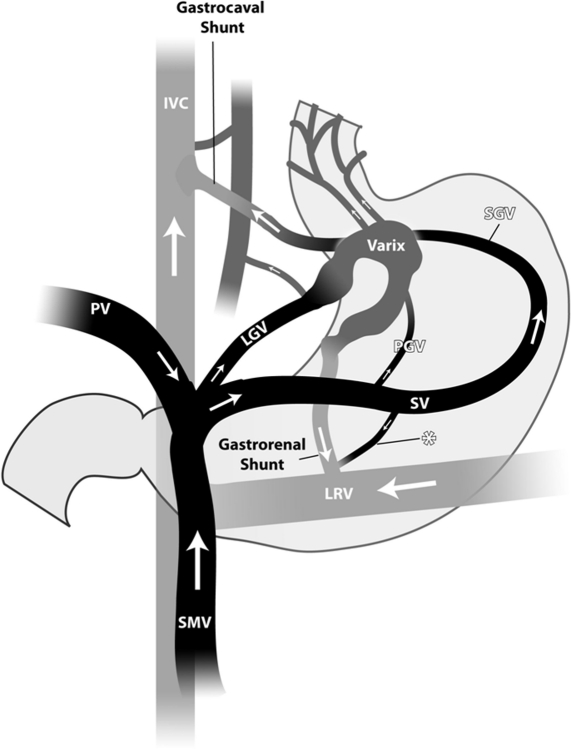
The most comprehensive classification of gastric varices divides them into those arising from isolated splenic vein thrombosis (SVT) and those arising from portal hypertension (cirrhotic or noncirrhotic). Gastric varices (GV) in the setting of SVT usually develop in the setting of pancreatitis or local neoplasm in the absence of portal venous hypertension. In our experience, GV arising due to SVT are much less common than GV due to portal hypertension.3 GV due to SVT usually arise from the short gastric veins running from the hilum of the spleen to the greater curvature aspect of the stomach rather than through spleno- or gastrorenal shunts common with portal hypertensive fundal varices (see below; Fig. 2). In addition, SVT-associated GV tend to be multiple and thus sometimes difficult to manage endoscopically due to recurrence of bleeding in alternative short gastric connections. Splenectomy can directly resolve the problem especially in those with pancreatitis, but the presence of underlying neoplasm often precludes effective surgery. Although the patient's history may provide important clues to the underlying disease such as prior bouts of pancreatitis or known GI (pancreatic or gastric) malignancy, early imaging of the splenic vein is helpful in the management of these patients especially when there is uncertainty regarding the presence of liver disease.
Figure 2.
Anatomy of the portal circulation: Gastric varices due to splenic vein thrombosis tend to arise from the short gastric veins running from the hilum of the spleen to the greater curvature aspect of the stomach rather than through spleno- or gastrorenal shunts common with portal hypertensive fundal varices. IVC, inferior vena cava; PV, portal vein; SMV, superior mesenteric vein; LGV, left gastric vein; LRV, left renal vein; PGV, posterior gastric vein; SGV, short gastric vein. (Courtesy of Dr. Saher Sabri.)
Other helpful classification systems, usually applied to patients with liver disease, include the Sarin Endoscopic Classification, which is based on the endoscopic appearance and location of the varices and the Japanese Vascular Classification system, which is based on underlying vascular anatomy.
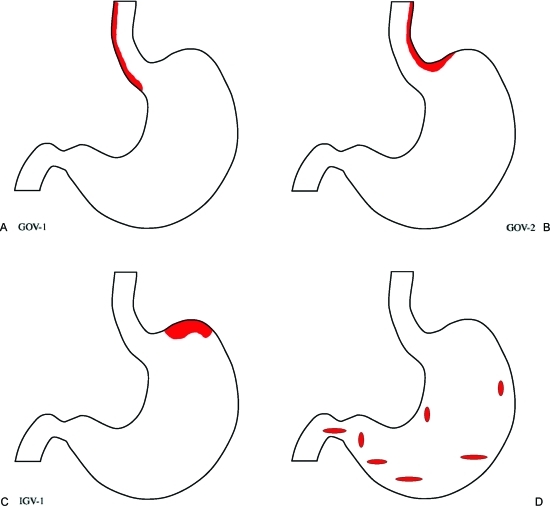
The Sarin classification is especially useful in describing the distribution of varices in the distal esophagus and stomach evident by endoscopic examination (Fig. 3).1,3,4,5 According to this classification, fundal varices are included in two groups: type 2 gastroesophageal varices (GOV 2) when the esophageal and fundal varices are present in continuity over the cardia, which might include type 1 isolated gastric varices (IGV 1) that are usually isolated gastric fundal varices. Type 1 gastroesophageal varices (GOV 1) are typically a continuation of esophageal varices into the lesser curvature varices. Type 2 isolated gastric varices (IGV 2) are gastric varices at ectopic sites in the stomach outside the cardiofundal region or the first part of the duodenum.
Figure 3.
Schematic diagram of Sarin's Classification of Gastric Varices: Fundal varices are included in two of the groups. (A) Type 1 gastroesophageal varices (GOV 1) are typically a continuation of esophageal varices into the lesser curvature varices. (B) Type 2 gastroesophageal varices (GOV 2) when the esophageal and fundal varices are present in continuity over the cardia, (C) which might include type 1 isolated gastric varices (IGV 1) that are usually isolated gastric fundal varices. (D) Type 2 isolated gastric varices (IGV 2) are gastric varices at ectopic sites in the stomach outside the cardiofundal region or the first part of the duodenum.
Although the Sarin classification scheme offers a useful perspective, it does not address the underlying vascular anatomy. Fundal gastric varices usually arise in part or in total from spleno- or gastrorenal shunts, so-called left-sided portal hypertension. This type of gastric varix often lacks direct vascular continuity with coexisting esophageal varices. Studies from Japan have clearly defined this peculiar anatomy and subsequently classified these varices depending on the underlying tributary vessels.5 Type 1 (fundal) gastric varices have a single dominant feeding channel arising from the splenic vein and empty into the left renal vein via the gastric cardia and/or fundus. Type 2 vessels follow a similar course to the left renal vein, but with multiple feeding tributaries (Fig. 2). Although it is likely that intermediate patterns exist, these two dominant patterns are helpful both conceptually and practically with implications for therapeutic alternatives (see below).
EPIDEMIOLOGY AND NATURAL HISTORY OF GASTRIC VARICES
Accurate incidence and prevalence figures of all types of gastric varices are difficult to determine. Most prior studies have focused on portal hypertension-related gastric varices rather than SVT-related GV. Less is known about cancer-related SVT, but in patients with chronic pancreatitis radiographically evident SVT occurs in ∼20% of patients, although bleeding from associated gastric varices is lower (estimated at ∼4% risk).6,7 This relatively low risk is consistent with our prior study where SVT accounted for only 10% of GV bleeding over ∼7 years.4
It is estimated that 30% of all cirrhosis patients develop variceal bleeding overall and that ∼10–20% of these are gastric.8,9 Gastric variceal bleeding is in general more severe and associated with a worse outcome compared with esophageal varices.9 Fundal varices (Sarin Class GOV 2 or IGV 1) have been noted to be less common than lesser curvature varices (GOV 1),10 but fundal varices accounted for over 80% of patients with bleeding GV in our series.9 This difference may well be due to referral bias, but probably indicates the greater challenge of dealing with bleeding fundal varices
Nonbleeding cardiofundal varices are sometimes encountered during screening endoscopy. The subsequent risk of bleeding from incidentally discovered fundal varices in cirrhosis patients is estimated to be 16%, 36%, and 44% at 1 year, 3 years, and 5 years, respectively.11 The size of these varices, the presence of surface red marks and the severity of underlying liver disease (Child-Pugh class) are predictive of bleeding. This raises the issue of prophylactic intervention for incidentally discovered gastric varices, although this question has not yet been fully answered: very little data exists to determine the risk and benefit ratio of preventive intervention.
MANAGEMENT OF BLEEDING GASTRIC (CARDIOFUNDAL) VARICES
Initial management of GV bleeding involves several diagnostic and therapeutic considerations. Basic measures common to GI bleeding, in general, such as establishment of intravenous access and airway control if the patient is unstable are beyond the focus of this article, but overaggressive volume resuscitation should be avoided as it can exacerbate portal hypertension (discussed below). The diagnosis of cirrhosis may be suspected by prior evaluation or through historical, physical, and laboratory findings; this diagnosis is not always apparent initially. In patients with known or suspected portal hypertension, medical therapy also usually includes antiportal hypertension medications and antibiotic prophylaxis.
Conventional Endoscopic Approaches
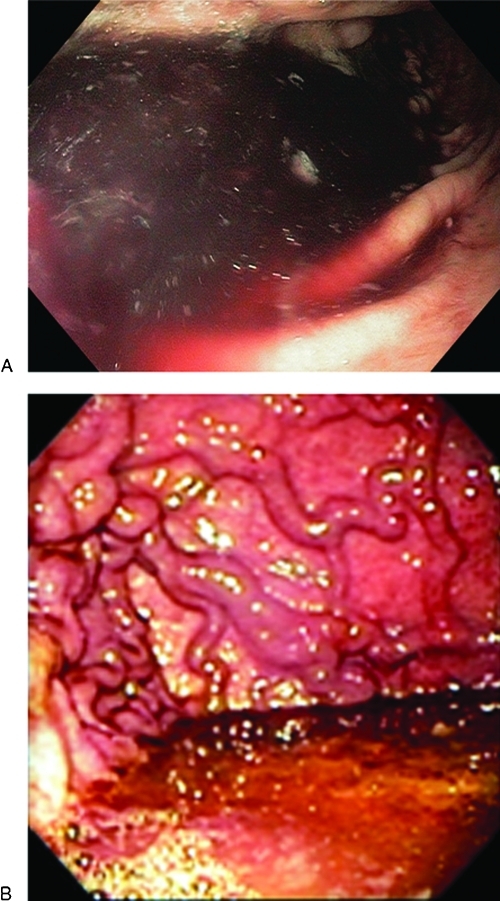
Once stabilized, upper endoscopic examination is usually undertaken as a routine early measure to evaluate upper GI bleeding. It is essential to adequately clear residual blood and clot to identify cardiofundal gastric varices especially when alternative causes of GI bleeding are not evident. Lavage through a typical small-bore nasogastric tube can help, but this is often insufficient and it is common to encounter a clot mixed in the fundus that can obscure the source (Fig. 4). Several strategies can be utilized. If the patient is stable, the procedure may be terminated and administration of prokinetic agents undertaken to promote gastric emptying while also radiographically imaging the portal vasculature and planning a follow-up endoscopy and/or radiologic intervention. However, it is often advisable to undertake clearance of a fundal clot using a large-bore lavage tube (Edlich or Ewald Lavage tubes). Alternatively, newer clot evacuation devices adaptable to the endoscope suction port are sometimes helpful.
Figure 4.
A clot mixed with a bloody pool in the fundus, which can obscure the source.
Encountering a high-risk GV with recent bleeding or an actively bleeding fundal varix presents a difficult challenge. Conventional endoscopic approaches using sclerosants or banding may be options, although prior studies have demonstrated a relatively high failure rate for acute control and an early rebleeding rate with conventional sclerotherapy.12 Jutabha et al reported a comparative trial of sclerotherapy versus banding plus sclerotherapy in acute fundal variceal bleeders.13 The study was limited by the very small number of subjects enrolled in each group (6 vs 11), but they showed a high rebleeding rate in both groups (33% vs 45%, not significant). This and similar studies demonstrated the significant morbidity associated with such endoscopic techniques and enforced the need for alternative approaches. Although not evident by this study, if banding of these vessels is undertaken, we recommend that it be accompanied by concomitant sclerosis to reduce the risk of catastrophic rebleeding should the band prematurely dissociate from these relatively large and deeper channels.
OTHER TEMPORARY MEASURES
The current conventional approaches poses significant limitations, in addition to the limited availability of alternatives such as cyanoacrylates (see below), temporary measures may be necessary to stabilize the patient especially if active bleeding is encountered. Intragastric balloons (e.g., Sangstaken-Blakemore or Minnesota tubes) are sometime utilized to tamponade these bleeding varices via type of different methods of traction.
The experience with the intragastric tubes has diminished over the years, so we offer several practical points. The gastric balloon needs to be tested to ensure its integrity, we recommend placing the tube in ice to stiffen it and facilitate its passage. Patients are usually intubated for oral passage and various stylets can be employed. Some do recommend passing the tube under direct vision employing a rat-tooth forceps passed through the endoscope to clutch the distal portion of the tube in place. Once in the stomach, a small amount of air (typically 50 cc) is inflated into the gastric balloon. Then, visualization with an x-ray centered over the xiphoid process to confirm intragastric placement, is recommended. Upon confirmation of the gastric balloon position, air is inflated (the volume of air varies by balloon brand) and traction is applied to effect tamponade, followed by confirmatory x-ray. We usually recommend an orthopedic trapeze bar holder for traction, and a one-liter saline bag rather than a helmet due to problem with airway access, pressure sores, and helmet removal if there is significant edema. The gastric balloon is managed with volume rather than manometric devices and if present, there is usually no need for inflation of the esophageal balloon. Nonetheless, these devices are only temporizing and typically require subsequent disposition to more definitive therapeutic approach. Alternative temporary measures may include recently reported hemoclip placement14 (although visualization may be challenging) and possibly procoagulant administration as discussed below.
VOLUME, PLASMA, AND PROCOAGULANTS
Animal and human studies have established that volume expansion increases portal vein pressure.15,16,17 Variceal bleeding is predominantly portal pressure driven, thus it is evident that minimizing portal pressure is the key objective in managing these patients and similarly avoidance of overaggressive volume resuscitation is warranted. Blood pressure lower than normal is therefore acceptable and relatively lower hematocrit target with packed red blood cell transfusions are the goal. This correlation has led to the target hematocrit level of 21% from the Baveno IV conference on variceal bleeding,18 whereas optimization of platelet function suggests a target of 25% for hematocrit (due to flow rheology and effective platelet margination).19 Renal support may be required for volume control. From the liver transplant experience, maintaining low volumes with its associated low portal pressures played a significant role in controlling bleeding during transplantation.20
Because the conventional dose of 2 units of frozen plasma only replace ∼10% of clotting factors and may unfavorably affect portal pressure, the vigilant use of plasma is warranted.21,22 We recommend avoiding target levels of international normalized ratio (INR) due to its marked limitations in cirrhosis including poor interlaboratory reproducibility and to the pathophysiology of variceal bleeding that is driven primarily by pressure changes as opposed to hemostatic defects.23,24 In contrast, more data exist to support platelet administration in this setting.25 Although the rupture of a varix is driven by conventional vascular physics, effective hemostasis usually involves components of the clotting system based on the significance of the nipple or platelet plug sign as a high-risk mark that consists of a “white clot” made of platelets and fibrin.26 In cirrhosis, platelet levels of around 56,000/mL or higher are associated with adequate thrombin generation.25 These results, though awaiting further clinical translational research, at least serve as a rational basis for target levels of circulating platelets.
The efficacy of synthetic procoagulants in the setting of cirrhosis remains uncertain. Recombinant activated factor VII (rFVIIa) had very limited efficacy in esophageal variceal bleeding outcomes among Child-Pugh C patients using a 42-day mortality composite endpoint with a relatively high dose.27 Due to its high cost and the existing data on rFVIIa, we would recommend its consideration only as a latent rescue measure when there is uncontrollable bleeding and by augmenting coagulation clot stabilization might be promoted. Of note, rFVIIa effect depends on sufficient circulating fibrinogen with target levels of 120 mg/dL or more that might necessitate the administration of cryoprecipitate. Other agents include DDAVP (desmopressin acetate, a synthetic analogue of the natural hormone arginine vasopressin), which was shown in a controlled trial to be ineffective in variceal bleeding .28 Further controlled studies comparing these and other treatment modalities are required before synthetic procoagulants can be universally recommended. In the design of studies using synthetic procoagulants, INR should be used with caution given its limitations as a predictor of hemostasis.
TIPS FOR CARDIOFUNDAL VARICEAL BLEEDING
Given the vascular anatomy of cardiofundal varices (see above and Fig. 2), it is not surprising that a conventional TIPS (transvenous intrahepatic portosystemic shunt), although very effective for esophageal varices, is rather challenging for many gastric varices.29 Stent occlusion plays a part in some rebleeding of cardiofundal varices after TIPS placement; however, rebleeding can result from failure of patent intrahepatic shunting to adequately diminish flow in varices arising from the distal splenic vein of the left portal system.30 Although the portal venous gradient is strongly predictive in esophageal variceal bleeding, it has a diminished relationship with gastric variceal bleeding, which is an indicator of the different sort of plumbing associated with cardiofundal varices in the left portal system.31 This has led to the recommendation that a TIPS in this setting should be accompanied by bland embolization of the gastric varices via a trans-TIPS technique despite the fact that vascular access for effective embolization is often limited (depending on the vascular feeding channels). Also, the patency of both a TIPS and a splenorenal shunt most likely will increase the risk of severe post-TIPS encephalopathy.32 Lastly, patients with higher MELD scores and decompensated cirrhosis may not tolerate TIPS.33 These concerns have led to the development of alternative approaches such as BRTO (balloon-occluded retrograde transvenous obliteration) by interventional radiology as discussed below. TIPSs are described in detail elsewhere in this issue of Seminars in Interventional Radiology.
CYANOACRYLATES AND GASTRIC VARICEAL BLEEDING
Cyanoacrylates are a family of compounds that has had a long history of use as a hemostatic agent. These agents encompass a common basic monomeric structure that consists of a reactive cyano (nitrile) group and an alkoxycarbonyl group of variable carbon chain length from which the chemical name derives (octyl-, butyl-, etc.) and which governs the properties of the commercially available forms.
Cyanoacrylate use emerged in Germany in the 1980s as a hemostatic agent for gastric variceal bleeding.34 Over the past 30 years cyanoacrylate injection has been established in many parts of the world as the primary means of achieving gastric varix obturation, while its use in the United States continues to be limited. In the meantime, several series of comparative and controlled trials have emerged including our own U.S.-based cohort of 92 patients of whom 66 had cardiofundal varices.4,35
Cyanoacrylate studies have had significant differences including the type of agent used and the variation in the use of contrast (e.g., lipiodol is utilized to slow polymerization). These variations warrant consideration in interpreting the literature. In an extended 2-year follow-up, a randomized controlled trial (RCT) with cyanoacrylate (enbucrilate mixed 1:1 with lipiodol) versus band ligation, Tan et al demonstrated a 27% rebleed rate in the cyanoacrylate group versus 63% rebleeding in the ligation group with no difference in long-term survival.36 In another RCT, Lo et al showed a 1-year rebleeding rate of 15% with cyanoacrylate (enbucrilate mixed 1:3 with lipiodol) versus 60% in the band ligation group with a significant survival advantage for the cyanoacrylate group, for the studied period.37 In a cohort study comparing cyanoacrylate (enbucrilate mixed with lipiodol 1:1.5) versus a TIPS, Mahadeva et al demonstrated similar rebleeding rates, with a slight (nonsignificant) advantage to a TIPS.38 In addition, Mahadeva et al showed long-term survival between the groups, but with a 50% cost reduction in the cyanoacrylate group, which was similar to our own cost analysis comparing these interventions.38,39,40 In contrast to the above study, our cohort comparison study did not detect a difference in rebleeding (∼15%) or survival at one year (∼70%) between cyanoacrylate (enbucrilate mixed with ethiodol 1:1) versus a TIPS, but showed increased morbidity mostly due to hepatic encephalopathy that was significantly higher in the TIPS group.40 In a more recent RCT of cyanoacrylate versus a TIPS, Lo et al reported a higher rebleeding rate from gastric varices in the cyanoacrylate group (38%) versus the TIPS group (11%) with a median follow-up of 33 months, although there was no difference in survival rate.41
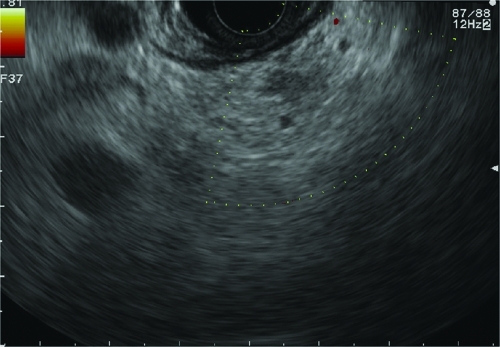
We recommend the following practical considerations including some methodologic techniques. Among these is the use of the short sigmoidoscope, as this tremendously facilitates maneuvering in the fundus because of its inherent deflection properties; it also decreases the impact of potential scope damage, which is very rare with careful cleaning with an acetone wipe and an endoscopic brush to remove any residual following the glue procedure. Certain injector needle hubs can interact with the cyanoacrylate and thus require in vitro testing to ensure compatibility of the plastic. In addition, we recommend a follow-up endoscopy to assess for residual varices and possible retreatment; however, appropriate intervals of follow-up have not been critically assessed and vary in the literature. This usually depends on the adequacy of the initial treatment and thus will vary between several days to several weeks. Endoscopic ultrasound (Fig. 5) can be very useful to assess for variceal flow occlusion and is likely superior to commonly employed blunt probe palpation. Finally, many experts have called for further multicenter RCTs of cyanoacrylate for gastric varices; nevertheless, the absence of a patentable substance limits industry interest as well as the relatively small number of patients will likely require approval as an orphan device if these multicenter RCTs were to happen. Additional endoscopic approaches have been reported, including thrombin injection and endoloop ligation. Intravariceal injection of thrombin has been shown to be effective in the control gastric variceal bleeding with a relatively good success rate of up to 75–100% and a low rebleeding rate of 7–25%.42,43,44,45
Figure 5.
Endoscopic ultrasound with color flow Doppler of obliterated gastric varix postcyanoacrylate glue injection. (Courtesy of Dr. Bryan Sauer.)
BRTO (BALLOON-OCCLUDED RETROGRADE TRANSVENOUS OBLITERATION)
Cardiofundal gastric varices usually have unique vascular anatomy (spontaneous splenorenal or gastrorenal shunts), thus an approach via the femoral vein and left renal vein is feasible and allows for transvenous obliteration (Fig. 2). BRTO was initially reported from Japan in the 1990s,46,47 this technique has enjoyed increasing utilization worldwide, with a few centers in the United States. Detailed discussions of BRTO are described elsewhere in this issue.
Successful occlusion of the splenorenal shunt with BRTO is associated with increased portal pressure flow to the liver in most patients.48 To our knowledge, this phenomenon has not been studied in patients with cyanoacrylate obturation, but may be less likely depending on the magnitude of the occlusion. In BRTO, this phenomenon can be associated with slightly increasing spleen size, exacerbation of ascites, gallbladder wall and intestinal edema, and variably with exacerbation of esophageal varix size.49,50 These were not found to be clinically significant in our initial experience with 16 patients, although we have observed transient bacteremia associated with longer balloon occlusion times in one patient who required extensive collateral embolization.51
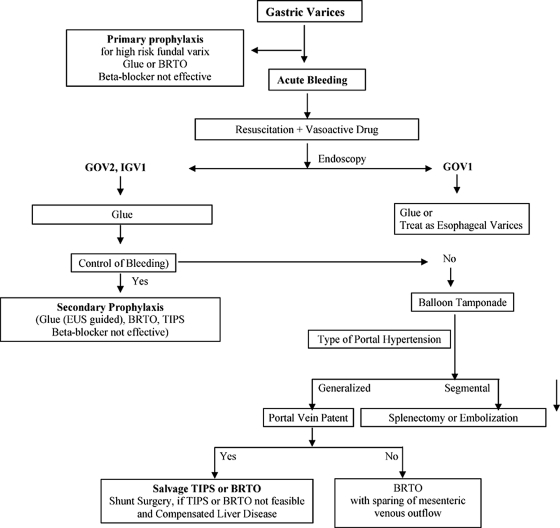
Follow-up strategies or BRTO have yet to be fully determined, but, as with cyanoacrylate therapy, we have found endoscopic ultrasound to be helpful in confirming occlusion of the cardiofundal varices. Presence of residual flow in the varices can be treated with cyanoacrylate with presumably even less risk of systemic embolization due to partial occlusion of the outflow track. Because of the reported possibility of exacerbating esophageal varices, periodic endoscopic examination is recommended. See Fig. 6 for a recommended algorithm for the management of gastric varices.52,53
Figure 6.
Algorithm for the management of gastric varices.
OTHER CONSEQUENCES OF CARDIOFUNDAL VARICES
Due to the physiology of the spontaneous splenorenal or gastrorenal shunt, it can be expected that these shunts can function like a TIPS or surgical shunt. In our center we have experienced several patients with nonbleeding cardiofundal varices who present with recurring bouts of severe encephalopathy and minimal or no ascites consistent with portal decompression through such shunts. These patients may respond to occlusion of these shunts utilizing the BRTO approach, although as noted above, this approach would warrant close observation in such patients for side-effects related to exacerbation of the portal venous gradient.54,55
SUMMARY
Gastric varices (GV) are the most common cause of UGI bleeding in patients with portal hypertension after esophageal varices. In the United States the majority of GV patients have underlying portal hypertension rather than splenic vein thrombosis. Treatment of these patients is usually complex and requires a team approach with defined stepwise management.
DISCLOSURE
Dr. SH Caldwell has disclosed financial support for research from CL Behring; the first author has no financial disclosure or otherwise relating to this manuscript.
ACKNOWLEDGMENTS
We thank Reem Asseri for the schematics of the gastric varices, and Elizabeth Hespenheide, R.N. and Divyesh Sejpal, M.D. for their initial work on the cyanoacrylate studies.
References
- Sarin S K, Kumar A. Gastric varices: profile, classification, and management. Am J Gastroenterol. 1989;84(10):1244–1249. [PubMed] [Google Scholar]
- Stray N, Jacobsen C D, Rosseland A. Injection sclerotherapy of bleeding oesophageal and gastric varices using a flexible endoscope. Acta Med Scand. 1982;211(1-2):125–129. doi: 10.1111/j.0954-6820.1982.tb01912.x. [DOI] [PubMed] [Google Scholar]
- Sarin S K, Lahoti D, Saxena S P, Murthy N S, Makwana U K. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16(6):1343–1349. doi: 10.1002/hep.1840160607. [DOI] [PubMed] [Google Scholar]
- Caldwell S H, Hespenheide E E, Greenwald B D, Northup P G, Patrie J T. Enbucrilate for gastric varices: extended experience in 92 patients. Aliment Pharmacol Ther. 2007;26(1):49–59. doi: 10.1111/j.1365-2036.2007.03351.x. [DOI] [PubMed] [Google Scholar]
- Arakawa M, Masuzaki T, Okuda K. Pathology of fundic varices of the stomach and rupture. J Gastroenterol Hepatol. 2002;17(10):1064–1069. doi: 10.1046/j.1440-1746.2002.02855.x. [DOI] [PubMed] [Google Scholar]
- Agarwal A K, Raj Kumar K, Agarwal S, Singh S. Significance of splenic vein thrombosis in chronic pancreatitis. Am J Surg. 2008;196(2):149–154. doi: 10.1016/j.amjsurg.2007.07.039. [DOI] [PubMed] [Google Scholar]
- Heider T R, Azeem S, Galanko J A, Behrns K E. The natural history of pancreatitis-induced splenic vein thrombosis. Ann Surg. 2004;239(6):876–880. discussion 880–882. doi: 10.1097/01.sla.0000128685.74686.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan B M, Stockbrugger R W, Ryan J M. A pathophysiologic, gastroenterologic, and radiologic approach to the management of gastric varices. Gastroenterology. 2004;126(4):1175–1189. doi: 10.1053/j.gastro.2004.01.058. [DOI] [PubMed] [Google Scholar]
- Trudeau W, Prindiville T. Endoscopic injection sclerosis in bleeding gastric varices. Gastrointest Endosc. 1986;32(4):264–268. doi: 10.1016/s0016-5107(86)71843-9. [DOI] [PubMed] [Google Scholar]
- Chey W D, Elta G H. Natural history of gastric varices. Gastroenterology. 1993;105(2):599–602. doi: 10.1016/0016-5085(93)90743-v. [DOI] [PubMed] [Google Scholar]
- Kim T, Shijo H, Kokawa H, et al. Risk factors for hemorrhage from gastric fundal varices. Hepatology. 1997;25(2):307–312. doi: 10.1053/jhep.1997.v25.pm0009021939. [DOI] [PubMed] [Google Scholar]
- Sarin S K. Long-term follow-up of gastric variceal sclerotherapy: an eleven-year experience. Gastrointest Endosc. 1997;46(1):8–14. doi: 10.1016/s0016-5107(97)70202-5. [DOI] [PubMed] [Google Scholar]
- Jutabha R, Jensen D M, Kovacs T OG, et al. Initial results of combination banding and sclerotherapy compared to sclerotherapy alone for bleeding gastric varices. Gastrointest Endosc. 1998;47:AB86. [Google Scholar]
- Scapa E. Treating gastrointestinal bleeding with endoscopic hemoclips. Surg Laparosc Endosc. 1997;7(2):94–96. [PubMed] [Google Scholar]
- Kravetz D, Bosch J, Arderiu M, Pilar Pizcueta M, Rodés J. Hemodynamic effects of blood volume restitution following a hemorrhage in rats with portal hypertension due to cirrhosis of the liver: influence of the extent of portal-systemic shunting. Hepatology. 1989;9(6):808–814. doi: 10.1002/hep.1840090603. [DOI] [PubMed] [Google Scholar]
- Castañeda B, Morales J, Lionetti R, et al. Effects of blood volume restitution following a portal hypertensive-related bleeding in anesthetized cirrhotic rats. Hepatology. 2001;33(4):821–825. doi: 10.1053/jhep.2001.23437. [DOI] [PubMed] [Google Scholar]
- Villanueva C, Ortiz J, Miñana J, et al. Somatostatin treatment and risk stratification by continuous portal pressure monitoring during acute variceal bleeding. Gastroenterology. 2001;121(1):110–117. doi: 10.1053/gast.2001.25536. [DOI] [PubMed] [Google Scholar]
- de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43(1):167–176. doi: 10.1016/j.jhep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Gabriel D A, Li X, Monroe DMIII, III, Roberts H R. Recombinant human factor VIIa (rFVIIa) can activate factor FIX on activated platelets. J Thromb Haemost. 2004;2(10):1816–1822. doi: 10.1111/j.1538-7836.2004.01015.x. [DOI] [PubMed] [Google Scholar]
- Porte R J, Molenaar I Q, Begliomini B, et al. EMSALT Study Group Aprotinin and transfusion requirements in orthotopic liver transplantation: a multicentre randomised double-blind study. Lancet. 2000;355(9212):1303–1309. doi: 10.1016/s0140-6736(00)02111-5. [DOI] [PubMed] [Google Scholar]
- Westerkamp A C, Lisman T, Porte R J. How to minimize blood loss during liver surgery in patients with cirrhosis. HPB (Oxford) 2009;11(6):453–458. doi: 10.1111/j.1477-2574.2009.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef W I, Salazar F D, Dasarathy S, Beddow T, Mullen K D. Role of fresh frozen plasma infusion in correction of coagulopathy of chronic liver disease: a dual phase study. Am J Gastroenterol. 2003;98(6):1391–1394. doi: 10.1111/j.1572-0241.2003.07467.x. [DOI] [PubMed] [Google Scholar]
- Porte R J, Lisman T, Tripodi A, Caldwell S H, Trotter J F, Coagulation in Liver Disease Study Group The international normalized ratio (INR) in the MELD score: problems and solutions. Am J Transplant. 2010;10(6):1349–1353. doi: 10.1111/j.1600-6143.2010.03064.x. [DOI] [PubMed] [Google Scholar]
- Trotter J F, Olson J, Lefkowitz J, Smith A D, Arjal R, Kenison J. Changes in international normalized ratio (INR) and model for endstage liver disease (MELD) based on selection of clinical laboratory. Am J Transplant. 2007;7(6):1624–1628. doi: 10.1111/j.1600-6143.2007.01822.x. [DOI] [PubMed] [Google Scholar]
- Tripodi A, Primignani M, Chantarangkul V, et al. Thrombin generation in patients with cirrhosis: the role of platelets. Hepatology. 2006;44(2):440–445. doi: 10.1002/hep.21266. [DOI] [PubMed] [Google Scholar]
- Hou M C, Lin H C, Kuo B I, Lee F Y, Schmidt C M, Lee S D. Clinical implications of the white nipple sign and its role in the diagnosis of esophageal variceal hemorrhage. Am J Gastroenterol. 1996;91(10):2103–2109. [PubMed] [Google Scholar]
- Bosch J, Thabut D, Albillos A, et al. International Study Group on rFVIIa in UGI Hemorrhage Recombinant factor VIIa for variceal bleeding in patients with advanced cirrhosis: a randomized, controlled trial. Hepatology. 2008;47(5):1604–1614. doi: 10.1002/hep.22216. [DOI] [PubMed] [Google Scholar]
- de Franchis R, Arcidiacono P G, Carpinelli L, et al. Randomized controlled trial of desmopressin plus terlipressin vs. terlipressin alone for the treatment of acute variceal hemorrhage in cirrhotic patients: a multicenter, double-blind study. New Italian Endoscopic Club. Hepatology. 1993;18(5):1102–1107. doi: 10.1016/0270-9139(93)90464-x. [DOI] [PubMed] [Google Scholar]
- Sanyal A J, Freedman A M, Luketic V A, et al. Transjugular intrahepatic portosystemic shunts compared with endoscopic sclerotherapy for the prevention of recurrent variceal hemorrhage. A randomized, controlled trial. Ann Intern Med. 1997;126(11):849–857. doi: 10.7326/0003-4819-126-11-199706010-00001. [DOI] [PubMed] [Google Scholar]
- Barange K, Péron J M, Imani K, et al. Transjugular intrahepatic portosystemic shunt in the treatment of refractory bleeding from ruptured gastric varices. Hepatology. 1999;30(5):1139–1143. doi: 10.1002/hep.510300523. [DOI] [PubMed] [Google Scholar]
- Tripathi D, Therapondos G, Jackson E, Redhead D N, Hayes P C. The role of the transjugular intrahepatic portosystemic stent shunt (TIPSS) in the management of bleeding gastric varices: clinical and haemodynamic correlations. Gut. 2002;51(2):270–274. doi: 10.1136/gut.51.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson J M, Boyer T D, Kutner M H, et al. DIVERT Study Group Distal splenorenal shunt versus transjugular intrahepatic portal systematic shunt for variceal bleeding: a randomized trial. Gastroenterology. 2006;130(6):1643–1651. doi: 10.1053/j.gastro.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Ferral H, Gamboa P, Postoak D W, et al. Survival after elective transjugular intrahepatic portosystemic shunt creation: prediction with model for end-stage liver disease score. Radiology. 2004;231(1):231–236. doi: 10.1148/radiol.2311030967. [DOI] [PubMed] [Google Scholar]
- Soehendra N, Grimm H, Nam V C, Berger B. N-butyl-2-cyanoacrylate: a supplement to endoscopic sclerotherapy. Endoscopy. 1987;19(6):221–224. doi: 10.1055/s-2007-1018288. [DOI] [PubMed] [Google Scholar]
- Rengstorff D S, Binmoeller K F. A pilot study of 2-octyl cyanoacrylate injection for treatment of gastric fundal varices in humans. Gastrointest Endosc. 2004;59(4):553–558. doi: 10.1016/s0016-5107(03)02865-7. [DOI] [PubMed] [Google Scholar]
- Tan P C, Hou M C, Lin H C, et al. A randomized trial of endoscopic treatment of acute gastric variceal hemorrhage: N-butyl-2-cyanoacrylate injection versus band ligation. Hepatology. 2006;43(4):690–697. doi: 10.1002/hep.21145. [DOI] [PubMed] [Google Scholar]
- Lo G H, Lai K H, Cheng J S, Chen M H, Chiang H T. A prospective, randomized trial of butyl cyanoacrylate injection versus band ligation in the management of bleeding gastric varices. Hepatology. 2001;33(5):1060–1064. doi: 10.1053/jhep.2001.24116. [DOI] [PubMed] [Google Scholar]
- Mahadeva S, Bellamy M C, Kessel D, Davies M H, Millson C E. Cost-effectiveness of N-butyl-2-cyanoacrylate (histoacryl) glue injections versus transjugular intrahepatic portosystemic shunt in the management of acute gastric variceal bleeding. Am J Gastroenterol. 2003;98(12):2688–2693. doi: 10.1111/j.1572-0241.2003.08769.x. [DOI] [PubMed] [Google Scholar]
- Greenwald B D, Caldwell S H, Hespenheide E E, et al. N-2-butyl-cyanoacrylate for bleeding gastric varices: a United States pilot study and cost analysis. Am J Gastroenterol. 2003;98(9):1982–1988. doi: 10.1111/j.1572-0241.2003.07637.x. [DOI] [PubMed] [Google Scholar]
- Procaccini N J, Al-Osaimi A M, Northup P, Argo C, Caldwell S H. Endoscopic cyanoacrylate versus transjugular intrahepatic portosystemic shunt for gastric variceal bleeding: a single-center U.S. analysis. Gastrointest Endosc. 2009;70(5):881–887. doi: 10.1016/j.gie.2009.03.1169. [DOI] [PubMed] [Google Scholar]
- Lo G H, Liang H L, Chen W C, et al. A prospective, randomized controlled trial of transjugular intrahepatic portosystemic shunt versus cyanoacrylate injection in the prevention of gastric variceal rebleeding. Endoscopy. 2007;39(8):679–685. doi: 10.1055/s-2007-966591. [DOI] [PubMed] [Google Scholar]
- Williams S G, Peters R A, Westaby D. Thrombin—an effective treatment for gastric variceal haemorrhage. Gut. 1994;35(9):1287–1289. doi: 10.1136/gut.35.9.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przemioslo R T, McNair A, Williams R. Thrombin is effective in arresting bleeding from gastric variceal hemorrhage. Dig Dis Sci. 1999;44(4):778–781. doi: 10.1023/a:1026626212129. [DOI] [PubMed] [Google Scholar]
- Yang W L, Tripathi D, Therapondos G, Todd A, Hayes P C. Endoscopic use of human thrombin in bleeding gastric varices. Am J Gastroenterol. 2002;97(6):1381–1385. doi: 10.1111/j.1572-0241.2002.05776.x. [DOI] [PubMed] [Google Scholar]
- Ramesh J, Limdi J K, Sharma V, Makin A J. The use of thrombin injections in the management of bleeding gastric varices: a single-center experience. Gastrointest Endosc. 2008;68(5):877–882. doi: 10.1016/j.gie.2008.02.065. [DOI] [PubMed] [Google Scholar]
- Kanagawa H, Mima S, Kouyama H, Gotoh K, Uchida T, Okuda K. Treatment of gastric fundal varices by balloon-occluded retrograde transvenous obliteration. J Gastroenterol Hepatol. 1996;11(1):51–58. doi: 10.1111/j.1440-1746.1996.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Hamamoto N, Nomura T, et al. Balloon-occluded retrograde transvenous obliteration of high risk gastric fundal varices. Am J Gastroenterol. 1999;94(3):643–649. doi: 10.1111/j.1572-0241.1999.00928.x. [DOI] [PubMed] [Google Scholar]
- Tanihata H, Minamiguchi H, Sato M, et al. Changes in portal systemic pressure gradient after balloon-occluded retrograde transvenous obliteration of gastric varices and aggravation of esophageal varices. Cardiovasc Intervent Radiol. 2009;32(6):1209–1216. doi: 10.1007/s00270-009-9679-3. [DOI] [PubMed] [Google Scholar]
- Cho S K, Shin S W, Do Y S, et al. Development of thrombus in the major systemic and portal veins after balloon-occluded retrograde transvenous obliteration for treating gastric variceal bleeding: its frequency and outcome evaluation with CT. J Vasc Interv Radiol. 2008;19(4):529–538. doi: 10.1016/j.jvir.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Cho S K, Shin S W, Yoo E Y, et al. The short-term effects of balloon-occluded retrograde transvenous obliteration, for treating gastric variceal bleeding, on portal hypertensive changes: a CT evaluation. Korean J Radiol. 2007;8(6):520–530. doi: 10.3348/kjr.2007.8.6.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri S S, Swee W, Turba U C, et al. Bleeding gastric varices obliteration with balloon-occluded retrograde transvenous obliteration using sodium tetradecyl sulfate foam. J Vasc Interv Radiol. 2011;22(3):309–316. quiz 316. doi: 10.1016/j.jvir.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Sarin S K, Mishra S R. Endoscopic therapy for gastric varices. Clin Liver Dis. 2010;14(2):263–279. doi: 10.1016/j.cld.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Irani S, Kowdley K, Kozarek R. Gastric varices: an updated review of management. J Clin Gastroenterol. 2011;45(2):133–148. doi: 10.1097/MCG.0b013e3181fbe249. [DOI] [PubMed] [Google Scholar]
- Sakurabayashi S, Sezai S, Yamamoto Y, Hirano M, Oka H. Embolization of portal-systemic shunts in cirrhotic patients with chronic recurrent hepatic encephalopathy. Cardiovasc Intervent Radiol. 1997;20(2):120–124. doi: 10.1007/s002709900118. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Hirota S, Sugimura K. Long-term results of balloon-occluded retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy. J Vasc Interv Radiol. 2001;12(3):327–336. doi: 10.1016/s1051-0443(07)61912-5. [DOI] [PubMed] [Google Scholar]