Abstract
First described in 1955 by Goodwin et al as a minimally invasive treatment for urinary obstruction causing marked hydronephrosis, percutaneous nephrostomy (PCN) placement quickly found use in a wide variety of clinical indications in both dilated and nondilated systems. Although the advancement of modern endourological techniques has led to a decline in the indications for primary nephrostomy placement, PCNs still play an important role in the treatment of multiple urologic conditions. In this article, the indications, placement, and postprocedure management of percutaneous nephrostomy drainage are described.
Keywords: Nephrostomy, interventional radiology, kidney, hydronephrosis, nephroureterostomy
First described in 1955 by Goodwin et al as a minimally invasive treatment for urinary obstruction causing marked hydronephrosis, percutaneous nephrostomy (PCN) placement quickly found use in a wide variety of clinical indications in both dilated and nondilated systems. Although the advancement of modern endourological techniques has led to a decline in the indications for primary nephrostomy placement, PCNs still play an important role in the treatment of multiple urologic conditions. In this article, we will describe the indications, placement, and postprocedure management of percutaneous nephrostomy drainage.
INDICATIONS
There are four broad indications for the placement of a PCN. These are (1) relief of urinary obstruction, (2) diagnostic testing, (3) access for therapeutic interventions, and (4) urinary diversion (Table 1). To determine the appropriateness of nephrostomy placement, familiarity with the clinical presentation, diagnostic work-up, and typical management of each specific indication is essential. In addition, because nephrostomy placement is often a second-line therapy to retrograde endourological techniques, which typically have a lower associated morbidity, it is important to be familiar with the typical situations in which endourological approaches fail.
Table 1.
Indications
| A. Indications include, but are not limited to |
| 1. Relief of urinary obstruction |
| a. Urosepsis or suspected infection |
| b. Acute renal failure |
| c. Intractable pain |
| 2. Urinary diversion |
| a. Hemorrhagic cystitis |
| b. Traumatic or iatrogenic ureteral injury |
| c. Inflammatory or malignant urinary fistula |
| 3. Access for endourologic procedure |
| a. Stone removal |
| b. Dilatation or stenting of a ureteral stricture |
| c. Endopyelotomy |
| d. Foreign body retrieval (e.g., fractured stent) |
| e. Ureteral occlusion for urinary fistula |
| f. Tumor fulguration |
| g. Delivery of medications and chemotherapy |
| h. Biopsy of a urothelial lesion |
| 4. Diagnostic testing |
| a. Antegrade pyelography |
| b. Ureteral perfusion (Whitaker test) |
Relief of Urinary Obstruction
Relief of urinary obstruction represents the most common indication for PCN placement representing 85 to 90% of patients in several large series1. The three most common causes of renal obstruction in adults are urinary stones, malignancy, and iatrogenic benign stricture. In one large series, 26% of all nephrostomy tubes were placed because of calculus disease and 61% due to malignancy.2
The clinical presentation of the patient with urinary obstruction varies depending on the etiology, location, degree, and timing of the obstruction. The typical symptom is flank pain. In general, the more rapidly developing and complete an obstruction, the greater the acute distension of the renal capsule, and the more intense the feeling of pain. An abrasive process irritating the sensitive urothelium, such as a stone will also produce flank pain. However, a slowly developing partial obstruction, such as due to malignancy, may be painless and only incidentally discovered on imaging. Abnormal laboratory values are an insensitive sign of early renal obstruction. Plasma creatinine concentration is rarely elevated in the setting of a normally functioning contralateral kidney, although bilateral renal obstruction can produce the constellation of acute renal failure with a distal renal tubular metabolic acidosis and hyperkalemia. Ultimately, however, when there is clinical suspicion for renal obstruction, imaging is the most sensitive diagnostic method.
The imaging diagnosis of renal obstruction can be made with ultrasound, computed tomography (CT), nuclear medicine, or magnetic resonance imaging (MRI). Ultrasound is usually the first choice due to relative availability, minimal risk, and high sensitivity for detecting a dilated collecting system. However, compared with other imaging methods it is not as effective in determining the etiology and location of obstruction. In very large patients with renal impairment, noncontrast CT can effectively detect hydronephrosis and has the added benefit of being highly sensitive for the detection of obstructing stones. In patients with normal renal function, contrast-enhanced CT has a very high success rate for detecting and identifying the cause of hydronephrosis.3 For uroenteric diversions, MR urography can be helpful in delineating the anatomy.4 It should be noted that early renal obstruction does not always have associated hydronephrosis particularly when the collecting system is relatively noncompliant or there is poor underlying renal function and reduced urine production. Conversely, hydronephrosis can occur in the absence of obstruction when there is (1) a high rate of urine production such as in pregnancy or after overhydration (particularly when there is a mild stenosis that becomes flow limiting only at abnormally high urine flow rates), (2) persistent residual hydronephrosis after a chronic obstruction has been relieved, (3) significant persistent ureteral reflux producing megaureter such as in the setting of a uroenteric conduit. In situations where the ultrasound or CT diagnosis of obstruction is indeterminate, diuretic renography with technetium 99m-MAG3 can be helpful. Obstructed systems typically have a continuously rising renogram both before and after diuretic administration, whereas unobstructed systems have a postdiuretic T1/2 time of collecting system emptying of less than 10 to 15 minutes. If the patient's underlying renal function is very poor, the diuretic response is diminished and a false- positive finding for mechanical obstruction may be seen.
In general, because an uninfected obstructed kidney is not acutely threatened, nephrostomy placement is an urgent rather than emergent procedure. Clinical data in humans suggests that complete recovery of the glomerular filtration rate (GFR) can be expected with one week of complete obstruction with very little recovery seen after 12 weeks of complete obstruction.5,6 Complete or partial obstruction of urine flow leads to elevated urinary pressure with associated afferent arteriolar vasoconstriction causing marked reduction in glomerular blood flow. Over time, chronic obstruction leads to permanent progressive functional loss through a combination of ischemic or disuse-induced tubular injury as well as inflammation and interstitial renal fibrosis7,8 Although in a rat model complete unilateral ureteral obstruction for 24 hours led to 15% of nephrons being nonfunctional on examination 2 months later, the GFR of the kidney was preserved due to compensatory hypertrophy and hyperfiltration of the remaining nephrons.9 Ultimately, however, the rate of irreversible obstructive injury is a multifactorial process influenced by the degree, level, and duration of the obstruction, as well as by the presence of infection.8 In a clinical situation, it is difficult to predict how much renal function will be recoverable in an individual patient; thus, a therapeutic trial of nephrostomy drainage may be indicated before judging a kidney to be irreversibly damaged.
In patients with imaging findings of bilateral obstruction, the kidney that has been obstructed for a shorter time, or has more parenchyma should be selected for the nephrostomy placement. Bilateral nephrostomy drainage is rarely necessary, and should be reserved for patients with obstruction due to treatable malignant conditions such as lymphoma, benign disease, and in patients with suspected bilateral infection of the obstructed urinary tract.
An infected, obstructed kidney requires emergent drainage because of the high risk of mortality from urosepsis as well as the risk of rapid permanent deterioration of renal function. Although it has been a commonly accepted dogma that PCN placement is the only acceptable first-line therapy in this situation, it should be noted that in the setting of pyonephrosis secondary to a ureteral stone, emergent PCN and retrograde stenting have been shown in a randomized control trial to have equivalent patient outcomes.10,11 For this reason, the Joint Guidelines of the American and European Urological Associations recommend either procedure in the emergent situation. The type of drainage should be determined by local technical expertise and availability as well as patient-specific factors.12 A morbidly obese or coagulopathic patient with a minimally dilated collecting system that is poorly visualized by ultrasound may be better served by retrograde ureteroscopic stent placement.
Diagnostic Testing
Occasionally, despite clinical and noninvasive imaging findings, the diagnosis of ureteral obstruction may still be uncertain. Nephrostomy placement may be helpful in confirming the diagnosis of obstruction via an antegrade nephrostogram, a therapeutic trial of drainage, or a perfusion-pressure flow study.13
The perfusion-pressure flow study, or Whitaker test, was first described in 1973 as a diagnostic tool for differentiating the unobstructed from obstructed, dilated upper urinary tract.14 Contrast is infused at a steady rate through the nephrostomy while the pressure gradient between the renal pelvis and the bladder is measured. The infusion rate is initially 0 mL/min and is incrementally increased to a maximum of 20 mL/min if there is no rise in the pressure gradient. A gradient greater than 22 mm H2O or less than 15 mm H2O suggests obstructed or unobstructed systems, respectively. A gradient between 15 to 22 mm H2O is considered indeterminate. Currently, the availability of less-invasive diagnostic methods such as diuretic renography has replaced the Whitaker test in most circumstances. However, in the setting of equivocal or discordant renography results, some authors still consider the Whitaker test to be a useful complement. In an analysis of 143 patients with suspected upper urinary tract obstruction who had undergone diuretic renography and perfusion-pressure flow measurements, Lupton et al found the Whitaker test to be helpful in determining management in 84% of cases and accurate in predicting the outcome of therapy in 77%. The perfusion-pressure flow study was particularly helpful when the results of renography were equivocal, there was poor function of the obstructed kidney, or there were symptoms ipsilateral to the dilated kidney with a diuretic renogram suggesting no obstruction.15 Despite this optimistic recent report, we have not found a Whitaker test to be necessary or useful in our own clinical practice.
Urinary Diversion
PCN placement is sometimes requested to provide urine diversion in the setting of ureteral leak, urinary fistula, and hemorrhagic cystitis. Eighty percent of ureteral injuries are due to iatrogenic causes, particularly pelvic surgery.16 Injuries also commonly arise from penetrating trauma, most commonly bullets, and less commonly, blunt trauma in the setting of sudden deceleration. Although early surgical treatment is preferred, particularly when the injury is discovered intraoperatively, this is often not possible because of delayed diagnosis (patients with iatrogenic ureteral injuries typically present 2 to 3 weeks after surgery) or associated injuries requiring more-urgent attention. Treatment with retrograde stenting may be attempted17 (Fig. 1); however, PCN placement with antegrade ureteric stenting is generally preferred because of its greater ability to divert urine from the area of injury.
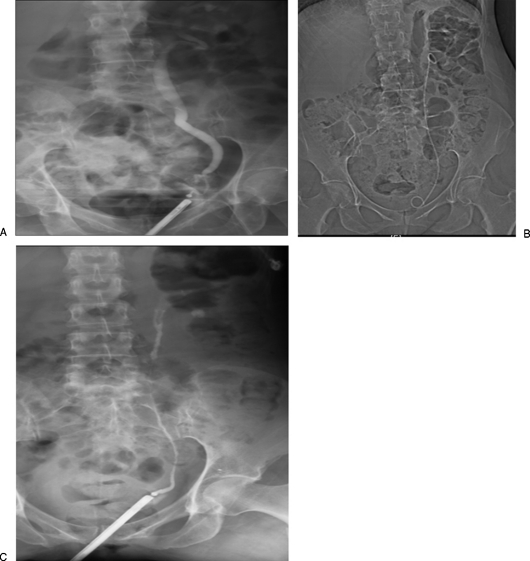
Figure 1.
Left pelvic ureteral injury during hysterectomy managed by retrograde ureteral stenting. (A) A 55-year-old woman, 4 weeks postvaginal hysterectomy, with vaginal leakage of urine. Left retrograde ureterogram demonstrates extravasation from the left pelvic ureter. (B) Internalized double pigtail ureteral stent was successfully placed with cystoscopic guidance. (C) Six weeks later, retrograde ureterogram shows complete healing of the ureter. Subsequent imaging showed no stricture or obstruction.
As with ureteral injury, urinary fistulas are most commonly caused by prior pelvic surgery with hysterectomies accounting for ∼75% of cases. Pelvic radiation therapy and malignancy are the two other leading etiologies. Most commonly, fistulas are vesicovaginal and ureterovaginal fistulas; however, ureterocutaneous and ureteroenteric fistulas can also occur. Patients present with the continuous leakage of urine from the vagina or the skin. Confirmation of the site of origin of the fistula can be made by cystoscopy and retrograde pyelography. For ureteral fistulas, the first choice of treatment is generally retrograde ureteral stent placement. However, when there is continued leakage PCN placement may be necessary. Vesicular fistulas may be initially managed with bilateral percutaneous nephrostomies but nephrostomies alone do not provide complete cessation of urine drainage into the urinary bladder. Ureteral occlusion techniques are variably successful in preventing antegrade flow of urine. Surgical treatment is usually necessary, but is deferred until endoscopic and percutaneous management have failed.18
Hemorrhagic cystitis causing persistent hematuria most commonly occurs in the oncology population secondary to bladder epithelial and vascular damage by cyclophosphamide therapy or radiation therapy. Symptoms may occur months to years after therapy. Cystoscopically directed fulguration of the bleeding sites in the bladder and bladder irrigation with astringent intravesicular agents are the usual management strategies. To limit the promoting effect of urine urokinase on bleeding, bilateral PCNs may be placed in refractory cases.19
Access for Therapeutic Interventions
PCN access may be necessary for the management of renal stones, percutaneous therapy of upper track urothelial cancer, and the extraction of fragmented ureteral stents that cannot be removed through a retrograde approach. Of these, therapy for stones is the most common indication for providing PCN access.
Extracorporeal shock wave lithotripsy (ESWL) is the treatment of choice for renal and lumbar ureteral calculi that are clinically problematic, but cannot pass spontaneously. However, when stones are larger than 2 cm in size (which includes all staghorn calculi), associated with urinary tract obstruction (as in patients with ureteral strictures or ureteropelvic junction obstruction), occur in patients with urinary diversions, or are composed of cystine, ESWL is less effective in producing a stone-free state.20 In patients who are not eligible for or have failed ESWL, percutaneous nephrolithotomy (PCNL) is an effective alternative. Although the nephroscopy and stone fragmentation is usually performed by urologists, radiologists create the initial percutaneous nephrostomy access in close to 90% of cases.21 When performing a PCNL, the PCN tract is serially dilated to a diameter of up to 30 French (Fr), a 30-Fr sheath placed, and the stones then fragmented with high-frequency ultrasound or laser lithotripsy. The stone fragments are retrieved under direct nephroscopic guidance with forceps or three-prong grabbers.
Depending on their size and location, ureteral stones are treated with medical expectant management, retrograde ureteroscopy, or ESWL. Percutaneous antegrade access may be requested for large, impacted proximal ureteral stones (>2 cm) as these have lower complete stone removal rates by ESWL and may be impassable by retrograde ureteroscopy.12
Other Indications for Percutaneous Nephrostomy
Because of the lower morbidity associated with retrograde endourological techniques, PCN placement is typically reserved for when retrograde approaches are unsuccessful or unfeasible. Specific situations in which retrograde approaches have a high failure rate include uroenteric diversions, renal transplants, and external malignant obstruction.
URINARY DIVERSION
Uroenteric diversions are commonly performed after cystectomy for invasive bladder cancer. Regardless of diversion type, surgical mobilization of the ureter can lead to obstructive strictures in 2 to 10% of patients.22 Most commonly, this involves the ureteroenteral anastomosis, and is either a technical complication or due to ischemia related to compromise of the vascular supply of the distal ureter during surgery. Less commonly, obstruction can be secondary to external compression, stones, or stenosis of the enteric conduit. Cannulating an obstructed uroenteric anastomosis can be difficult through the conduit or the pouch and success rates are very variable, ranging from 14 to 56%. Percutaneous techniques play an important role in this patient population.23,24
The evaluation of hydronephrosis in this population is complicated by the fact that the uroenteric anastomosis can be constructed to be either freely refluxing or antirefluxing. A freely refluxing anastomosis may allow proximal collecting system dilatation without obstruction (significant collecting system dilation in a nonobstructed system usually occurs many years after the urinary diversion surgery), whereas an antirefluxing anastomosis will prevent reflux at the expense of a greater incidence of anastomotic obstruction. Thus, when evaluating upper-tract dilatation, it is important to know the surgical technique used.25
There are three broad categories of uroenteric diversion. In an ileal conduit urinary diversion, the ureters are implanted onto the proximal end of an isolated 15 to 20 cm of distal ileum through which urine flows into a stoma, usually located in the right lower quadrant. An ileal conduit is most commonly created with a freely refluxing uroenteric anastomosis; it has normal and active peristalsis. This allows urine to reflux into the upper collecting system and produce calyceal dilatation despite the absence of obstruction. Upper-tract dilatation has been reported in 34% of patients with ileal conduits within 5 years of conduit creation.26 Obstruction and reflux can be distinguished by a loopogram, in which contrast is injected via the stoma to see whether it refluxes up the ureter. If no reflux is seen, renal ultrasound or a cross-sectional imaging study is helpful in evaluating for upper-tract hydronephrosis to indicate obstruction (Fig. 2).
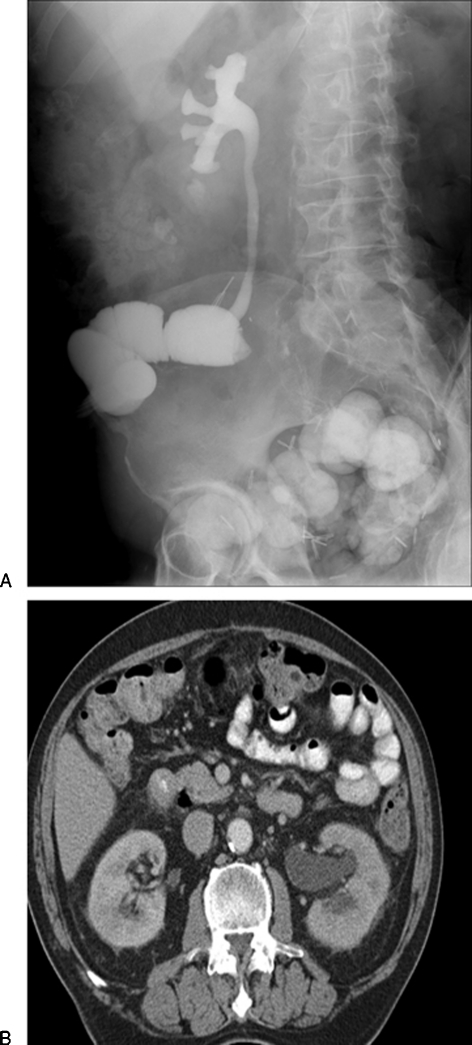
Figure 2.
Anastomotic stricture at left uretero-ileal anastomosis in a 62-year-old man. (A) Loopogram demonstrates free reflux into the right ureter and collecting system, which are normal in appearance. There is no reflux into the left ureter, which is highly suspicious for an anastomotic stricture. These tend to be related to benign fibrosis and are not usually related to urothelial tumor recurrence. (B) A computed tomography (CT) scan demonstrates left hydronephrosis. Other images demonstrated ureteral dilation to the ureteroileal anastomosis. The patient underwent left percutaneous nephrostomy and balloon dilation of the anastomotic stricture.
Continent diversions can be either of the cutaneous variety with a cutaneous stoma through which the pouch is catheterized and emptied, or orthotopic, where the pouch is anastomosed to the urethra and the patient voids by increasing abdominal pressure (the Credé maneuver).
The Indiana pouch is the prototypical continent cutaneous diversion where the cecum and ascending colon are detubularized and fashioned into a reservoir. The terminal ileum is usually brought to the skin as the catheterizing stoma for pouch emptying. In continent orthotopic reconstructions, the most common of which is the Studer pouch, a neobladder is fashioned from a segment of detubularized ileum, and then connected to the native urethra.27
RENAL TRANSPLANTS
Following renal transplantation, urinary obstruction and leak are among the most frequent complications. Overall, urinary tract complications are estimated to occur in 3 to 10% of transplants, with most series reporting a 3 to 5% incidence of obstruction and a 2 to 5% incidence of urine leak.28,29
Distal ureteral ischemia is the most common underlying etiology for most cases of obstruction. Because the distal transplant ureter receives blood only from an arterial branch descending from the renal hilum, it is prone to ischemia secondary to surgical manipulation. The most common site of obstruction is at the ureteroneocystostomy site. Other causes of renal transplant ureteral obstruction include kinking, compression by an external mass or fluid collection, calculi, and postsurgical fibrosis or adhesions.30 The typical clinical presentation of transplant obstruction consists of a rising serum creatinine, decreasing urine output, and hydronephrosis. However, significant hydronephrosis may not be observed when the obstruction is acute, the collecting system is not distensible due to chronic graft fibrotic change, or there is poor underlying graft function secondary to acute tubular necrosis or rejection. In addition, graft denervation and decreased collecting system tone can result in mild persistent hydronephrosis in the absence of obstruction.31 In ambiguous cases, radionuclide scintigraphy may be helpful; however, if graft uptake of radiotracer is poor, then antegrade pyelography may ultimately be necessary. Although retrograde ureteroscopy and pyelography can sometimes be attempted to evaluate suspected renal transplant urinary obstruction, postsurgical anatomic considerations often make this difficult. In transplants created with a ureteroureterostomy or ureteropyelostomy (anastomosis of native ureter of recipient to the renal pelvis or ureter of the renal transplant), the anatomy of the distal ureter and ureterovesical junction is normal and thus retrograde access is easily performed. However, most commonly, renal transplant urine drainage is through a ureteroneocystostomy at the bladder dome and the distal 2 to 3 cm of the transplant ureter is buried within a submucosal bladder wall tunnel.28 This type of anastomosis can be very difficult to access with retrograde ureteroscopy, particularly in the setting of a distal ureteral stricture.32 Antegrade pyelography and nephrostomy tube drainage play a crucial role in these cases.
Patients with renal transplant leak may present with swelling and pain, leakage of fluid from the surgical incision, rising creatinine, or an ultrasound showing a perinephric anechoic collection in association with hydronephrosis. Presentation typically occurs 2 to 3 weeks after transplantation, and virtually always by 5 to 6 weeks posttransplant.33,34 Differentiation of urinoma from lymphocele can be made with either renal scintigraphy showing abnormal perinephric radionuclide uptake or aspiration and analysis of the fluid showing a creatinine level similar to that of urine. If the leak arises from the bladder (easily diagnosed with either a fluoroscopic or CT cystogram), it may sometimes be successfully treated with Foley catheter placement alone. However, most commonly the leak arises from the distal ureter or ureteroneocystostomy, and is secondary to ureteral ischemia. Initial treatment is generally by nephroureteral stent placement.
MALIGNANT OBSTRUCTION
Percutaneous drainage is often requested for ureteral obstruction due to malignancy. Malignant urinary obstruction can be secondary to extrinsic tumor compression, direct tumor invasion, or an intrinsic genitourinary (GU) malignancy. One large study reported malignant obstruction as the indication for over 60% of all nephrostomies.2 Although retrograde stenting often is attempted, it has a high failure rate. This is because when there is involvement of the UVJ or distal ureter, as can commonly occur in prostate, bladder, or cervical cancer, retrograde cannulation of the ureter can be difficult or impossible. Furthermore, when the ureteral obstruction is due to external compression, ureteral stents often function poorly. One study noted an ∼50% failure rate of ureteral stents placed for obstruction due to external compression as compared with a 0% failure rate when obstruction was due to intrinsic disease. Urine drainage in a patient with ureteral obstruction and a ureteral stent is due to drainage through the lumen of the stent as well as wicking of urine around the stent. In an individual patient, the relative roles of the two mechanisms of drainage can be very variable. With high-grade external ureteral obstruction, there is probably no urine wicking around the stent, leading to stent malfunction.35
Prior to performing percutaneous drainage for the treatment of obstruction from malignancy, consideration should be given to both the patient's long-term prognosis and the negative impact a nephrostomy may have on quality of life. Several large retrospective series have noted the poor survival of patients undergoing drainage for malignant ureteral obstruction, ranging from 96 days to 6.8 months.36,37,38 In patients with advanced malignancy, a 6-month survival of only 2% was reported.38 For this reason, several authors recommend drainage only in patients who are symptomatic or have stable tumors or continued therapeutic options (particularly when nephrotoxic drugs such as cisplatin are needed).39 Consideration should also be given to the necessity of bilateral drainage in patients with bilateral malignant obstruction. One retrospective study in patients with bilateral malignant obstruction noted normalization of blood urea levels in 88% of patients, regardless of whether unilateral or bilateral nephrostomy drainage was performed.40 One approach is to first drain the symptomatic or greater functioning kidney. The contralateral kidney is drained only when there is suspected infection, or when renal function does not improve enough to administer the appropriate chemotherapy.41
CONTRAINDICATIONS
There are no absolute contraindications to percutaneous nephrostomy.1 A patient with renal obstruction and associated severe life-threatening electrolyte or metabolic disorder such as hyperkalemia with significant electrocardiogram changes (particularly if K >7) or severe metabolic acidosis should undergo emergent dialysis prior to nephrostomy placement as dialysis is faster and more reliable at alleviating the life threat than is drainage. If possible, a severe coagulopathy should be corrected because this has been associated with an increased risk of bleeding complications; however, if the coagulopathy is secondary to urosepsis it may be refractory to correction without emergent drainage.2
PREPROCEDURAL EVALUATION AND PATIENT PREPARATION
Preprocedural workup consists of confirming the indication for the nephrostomy placement, assessing the risk of sedation and the procedure, and when possible, minimizing any factors contributing to increased risk. All available cross-sectional imaging should be reviewed to assess for any anatomic factors (described below), which may make the procedure more difficult or risky.
Anesthesia Assessment
Assessment of airway and anesthesia risk is of particular importance prior to nephrostomy placement. Because patients are placed in a prone position, proper positioning and monitoring of the airway during the procedure will be difficult. The prone position will also make it difficult to quickly gain control of the airway in the event of a respiratory emergency. For this reason, there should be a lower threshold for requesting anesthesia support in patients who may be of moderate to high risk of intraprocedural respiratory compromise. In patients at high risk for respiratory compromise in the prone position, a supine oblique position may be considered for the procedure. Routine exchange of nephrostomy tubes can usually be performed with minimal discomfort and with no sedation or only light sedation.
Coagulation Status
The Society of Interventional Radiology (SIR) guidelines for the periprocedural management of coagulation categorizes nephrostomy placement as a procedure with “significant bleeding risk, which is difficult to detect or control42.” Farrell and Hicks noted that bleeding complications requiring transfusion occurred in 2% of patients with normal coagulation parameters and 4% of patients with a coagulopathy.2 Although there is little evidence to support specific threshold values, the SIR consensus document recommends routine preprocedural testing of international normalized ratio (INR) and platelet counts in all patients and of a partial thromboplastin time (PTT) in those receiving intravenous (IV) unfractionated heparin. Correction of coagulation abnormalities is recommended for INR and PTT values greater than 1.5 and platelet counts less than 50,000. Unfractionated heparin, which has a half-life of 60 minutes, should be stopped ∼2 to 3 hours prior to the procedure. Coumadin should be stopped ∼5 days prior to the procedure or corrected with vitamin K or fresh frozen plasma (FFP) administration. When possible, such as for elective procedures, fractionated heparin such as enoxaparin should be withheld for 24 hours and antiplatelet agents, such as Plavix and aspirin, should be held for 5 days prior to the procedure.
Preprocedural Antibiotics
Because percutaneous interventions of the GU tract are considered, at best, clean-contaminated procedures, but are most often contaminated or dirty procedures as defined by the SIR Guidelines on Antibiotic Prophylaxis, routine antibiotic prophylaxis is recommended.43 The most common organisms within the GU tract are gram-negative rods such as E. Coli, Proteus, Klebsiella, and Enterococcus. In the absence of specific culture results, such as those from a positive urine culture, single dose broad-spectrum prophylactic antibiotic coverage is usually administered such as ceftriaxone alone or ampicillin with gentamycin. With patients with an allergy to penicillin, one can use vancomycin alone or clindamycin plus an aminoglycoside.28 Antibiotic prophylaxis is most effective when started within 1 hour prior to the procedure, with the incidence of infectious complications increasing significantly when prophylaxis is given perioperatively or greater than 2 hours prior to the procedure.44 There is also an increased risk of infectious complications in patients with indwelling catheters, stones, uroenteric anastomosis, bacteriuria, and prior manipulation. In patients with one of these risk factors, Cochran et al demonstrated a reduction in sepsis risk from 50% to 9% when prophylactic antibiotics were given.45 The greatest risk of infectious complications is seen in patients with clinical signs of infection. In these patients, the goal of antibiotics is treatment rather than prophylaxis. Broad-spectrum empiric IV antibiotic treatment should be started prior to the procedure and continued after the procedure, with modifications based on the results of culture and sensitivity testing of urine obtained during the nephrostomy placement.
Periprocedural Monitoring
Intraprocedural continuous electrocardiogram and transcutaneous oximetry monitoring is standard with analgesia provided by moderate IV conscious sedation combined with local anesthesia. Postprocedure monitoring of vital signs is essential to detect early signs of significant bleeding or sepsis. Although nephrostomy placement is most often performed as an inpatient procedure, several reports, reaching diverging conclusions, have described outpatient nephrostomy placement in selected patients. Cochran et al restricted outpatient nephrostomies to 55 patients free of signs of infection, hypertension, and coagulopathy; however, they ultimately needed to admit 25% of patients, most commonly due to signs of sepsis.45 Gray et al selected a low-risk subset of 48 patients without hypertension, untreated urinary tract infection (UTI), coagulopathy and staghorn calculi, monitored these patients for 4 to 6 hours postprocedure, and admitted only 6% because of issues related to PCN placement.46
TECHNIQUE
Anatomy
An understanding of renal and retroperitoneal anatomy is essential to safe nephrostomy tube placement. With the patient supine, the upper and lower poles of each kidney are at approximately T12 and L3, respectively, with the renal hila positioned ∼5 cm lateral to the spinous process of T1. The right kidney is usually 1 to 2 cm inferior to the left kidney; when the patient is prone, both kidneys move slightly cranially. In the coronal and sagittal planes, the long axis of each kidney is angled obliquely, parallel to the psoas muscles with the lower poles lateral and slightly anterior to the upper poles.47 Nephrostomy placement can potentially injure five surrounding structures: the pleura, diaphragm, colon, spleen, and liver. In practice, pleural and diaphragm injury is by far the most common, with colon injury occasionally reported and spleen or liver injury rarely reported.
The pleura extends to approximately the 9th rib at the mid-axillary line, 10th rib at the posterior axillary line, 11th rib at the scapulary line, and the lower margin of the 12th rib at the paravertebral line.48 Thus, the risk of pleural transgression is minimized when nephrostomy placement is below the 12th rib. Analyzing CT images of patients in the prone position, Hopper and Yates estimated the risk of pleural transgression for PCNs placed in 11th intercostal space to be ∼29% on the right and 14% on the left.49 The diaphragm arises posteriorly from the transverse process of L1 and the tips of the posterior 10th through 12th ribs. Punctures above the 12th rib will usually pass through the diaphragm.
Approximately 5% of prone patients will have a retrorenal colon at the level of the lower renal poles.50 This is most common in thin patients with little intraabdominal fat. A review of any available cross-sectional imaging before the procedure can help identify this variant. If cross-sectional imaging is not available, the colon can usually be identified on fluoroscopy during the procedure as it is usually distended with air when patients are prone.
Although calyceal anatomy can vary significantly, typically, the calyces of the renal collecting system are arranged in anterior and posterior rows that are oriented approximately orthogonal to each other. Because the normal kidney is rotated with the medial margin ventral to the more dorsal lateral margin, the anterior calyces typically extend laterally in the coronal plane while the posterior calyces extend posterolaterally at approximately a 20 to 30 degree angle from the sagittal plane.51,52
Within the renal vascular pedicle, the main renal artery is situated posterior to the main renal vein and anterior to the renal pelvis. It typically bifurcates into anterior and posterior divisions. Typically, the anterior division has 3 or 4 segmental branch vessels that supply the anterior two thirds of the kidney; the posterior division has a single segmental branch supplying the posterior third. Within the renal sinus, these segmental arteries divide into interlobar arteries that enter the renal parenchyma, run along the border of the medullary pyramids within the septa of Bertin, and divide into the arcuate arteries that arc along the base of the pyramids while giving off interlobular arteries that supply the renal cortex. The junction of the anterior and posterior vascular territories, located in the posterolateral aspect of the kidney, is a zone of relative avascularity, containing only very small arterial vessels.48 The long axis of the posterior calyx is usually aligned with this avascular zone and thus entry along this axis, at an angle 20 to 30 degrees from the sagittal plane, has the least risk of significant arterial injury. In practical terms though, identification of this avascular plane is very difficult. Catheter placement through a calyx, rather than through an infundibulum or directly into the renal pelvis runs the least risk of vascular injury and attempts should be made to achieve catheter placement through a calyx, particularly if PCNL or other large-bore catheter placement is being considered.
One-Stick Technique
In the one-stick technique the intention is to make a single, accurate needle pass into a posterior mid or lower pole calyx under ultrasound guidance, and utilize the same needle access for both for opacification of the collecting system and placement of the nephrostomy tube. With the availability of high-quality ultrasound, this is by far the most commonly used technique. It is best suited for kidneys with well-dilated collecting systems, easily visualized with ultrasound. The patient is ideally prone, although the lateral oblique position may sometimes be necessary in patients with an enteric conduit.
The kidney is first visualized with ultrasound, typically in a sagittal plane such that the lower, mid, and upper pole posterior calyces can be visualized in the field of view. To achieve this, the longitudinal axis of the ultrasound probe will usually be angled parallel to the psoas muscle, aligned with the renal axis. The target calyx depends on the intended purpose of the nephrostomy tube. Although lower pole calyces have the lowest risk of complications, interpolar calyces may provide greater mechanical advantage for future placement of a ureteral stent. If nephrostomy placement is for treatment of focal small-volume stone disease then puncture of the stone-containing calyx is desirable. However, in the more typical setting of complex large-volume stone disease, nephrostomy placement into an upper or lower pole calyx may allow easier access to the entire collecting system during PCNL.
Next, the skin entry point is chosen. This is ideally below the 12th rib, to minimize the risk of transpleural complications. Too medial an entry point will lead to more pain as the nephrostomy tube passes through the paraspinal muscles and make the tube more prone to kinking when the patient is supine. Too lateral an entry point increases the risk of colonic transgression. Injury to a retrorenal colon can be minimized by briefly surveying the intended area of nephrostomy tube placement with fluoroscopy. The retrorenal colon is usually distended with air in the prone position.
If the target calyx is well visualized, an 18-gauge trocar needle or a 21-guage Chiba needle can be used to make the puncture, entering the skin at an angle of 20 to 30 degrees to the sagittal plane and ideally traversing the renal fornix to enter a calyx. PCN traversal of the infundibula or renal pelvis is associated with a higher risk of injury to the interlobar vessels and major segmental branches of the renal artery and vein53as mentioned above. The advantages of the 18-gauge needle are that it tracks straighter and easier through the soft tissues and is better visualized with ultrasound. It also allows direct insertion of a 0.035-inch guidewire over which a nephrostomy tube can be placed. Although the larger gauge might be expected to have an increased risk of significant bleeding complications, this was not borne out in a small randomized controlled trial.54 Instead, use of an 18-gauge needle was noted to require fewer punctures of the renal capsule and a shorter procedure time. This is particularly true for PCN for stone disease, where there tends to be pericapsular fibrosis, which can deflect a smaller gauge needle and prevent accurate placement of the needle into the desired calyx.
Once the needle is inserted into the calyx, urine is aspirated both to send to the laboratory for culture and sensitivity and to help decompress the system. Contrast equal in amount to the aspirate is used to opacify the collecting system. Overdistension of the collecting system should be avoided as it increases the risk of sepsis by forcing infected urine into the venous system. If contrast injection confirms puncture of an appropriate calyx, a stiff 0.035-inch wire can be advanced through the 18-gauge needle and guided into either the proximal ureter or an upper pole calyx. If a 21-gauge needle was used this can be converted to an 0.035-inch wire using one of several commonly used access systems such an Accustick (Boston Scientific, Natick, MA) or Neff set (Cook Medical, Bloomington, IN). Once the 0.035-inch wire is appropriately positioned, an 8- or 10-Fr self-retaining nephrostomy catheter can be placed.
Two-Stick Technique
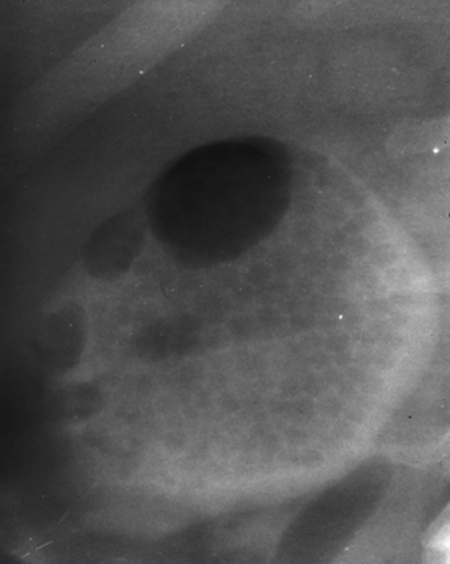
In this technique the first needle puncture of the collecting system is used only to opacify the collecting system with contrast to allow for a definitive “second-stick” needle entry into an appropriate posterior calyx under fluoroscopy. This technique is particularly useful for patients in whom the needle or individual calyces cannot be clearly visualized by ultrasound due to patient body habitus or collecting system nondilation. Because the initial 21- or 22-gauge needle stick is not converted to a nephrostomy, the renal pelvis can be directly targeted with minimal risk of bleeding or injury to adjacent organs. The simplest path is usually perpendicularly through the skin directly over the renal pelvis. The collecting system is then opacified with contrast. Because the dense contrast will often preferentially fill the anterior calyces, seen tangentially in the anteroposterior (AP) projection, a small amount of air or carbon dioxide can be subsequently injected through the needle to opacify the posterior calyces. The buoyant gas ascends into the posterior calyces, seen end-on in the AP projection (Fig. 3). Once a target posterior calyx is chosen, a clamp is used to mark the skin entry site for the definitive “second-stick,” ideally under the 12th rib along the posterior axillary line. A 0.018- or 0.035-inch wire is advanced into the collecting system and a nephrostomy tube is placed as outlined in the single-stick method.
Figure 3.
Patient with ureteropelvic junction obstruction underwent percutaneous nephrostomy (PCN) for planned percutaneous endopyelotomy. CO2 was injected through a retrograde ureteral catheter (not shown). Because the patient was prone, the CO2 rose to delineate the posterior calyces. An interpolar calyx was subsequently punctured.
One-Stick Technique Using Fluoroscopy
The fluoroscopic one-stick technique is helpful when direct access into a stone-containing calyx is needed for PCN, or if an opaque staghorn calculus can be used as a fluoroscopic target. With the patient prone, fluoroscopy is first used to confirm that the stone is visible. A needle is then used to puncture the stone-containing calyx as described above. Because the stone may obstruct wire advancement into the calyx, the combination of an 18-gauge needle and a glide-wire often work best. The 18-gauge needle can be used to dig into and displace the calyceal stone to allow the glide-wire to pass between the stone and the calyceal wall. Once the glide-wire has been advanced sufficiently it can be exchanged for a stiffer wire using a 4-French catheter to allow for nephrostomy placement.
Nondilated Systems
In addition to the two-stick technique, two other methods are helpful for nephrostomy placement in nondilated or poorly visualized systems. If the patient has normal renal function, 50 to 100 mL of IV contrast can be administered to allow for fluoroscopic visualization of the collecting system during the excretory phase and definitive calyceal puncture can then be performed. As the collecting system is only opacified for a limited time and the contrast opacifies the more dependent anterior calyces, some follow IV contrast injection with a modified two-stick method. Once the collecting system has been opacified by excretion of IV contrast, a direct puncture is made into the renal pelvis to allow for subsequent direct opacification of the collecting system with contrast and CO2 when performing the more technically challenging second stick into the target renal calyx.55 Another aid for collecting system visualization is ureteroscopic placement of a retrograde catheter prior to PCN placement. The catheter can then be used to directly opacify the collecting system with contrast, air, or CO2 (Fig. 3). This is particularly helpful when elective PCN access is requested for PCN in a patient with a nondilated collecting system and nonopaque stones.
COMPLICATIONS
Most series report combined major and minor complication rates of PCN placement of ∼10% with a mortality rate of 0.05 to 0.3%.1,52 The major complications can be divided into three types, injury to adjacent structures, severe bleeding, or severe infection/sepsis.
Injury to adjacent organs, most commonly the pleura or colon, is very uncommon when careful attention is paid to patient anatomy and preprocedural planning as described earlier. Three large series consisting of over 1600 patients reported only two cases of transcolonic nephrostomy tube placement and one pneumothorax.2,56,57 The overall reported incidence of colonic perforation is less than 0.2% of cases.1 It is most common in the setting of a retrorenal colon, which occurs more often on the left side in patients with little intraabdominal fat. The few case series in the literature report variable signs and symptoms to alert one to this complication; most patients develop fever and some present with gastrointestinal bleed or drainage of gas and feces from the PCN. If unrecognized, PCN traversal of the colon can lead to nephrocolic or colocutaneous fistula and associated abscess (Fig. 4). Peritonitis is uncommon as most injuries are retroperitoneal. Conservative nonoperative management is generally successful.57,58,59 El-Nahas et al reported on a strategy of double-J stent placement to ensure antegrade urine flow followed by nephrostomy withdrawal and placement of a pericolonic drain. Patients were placed on bowel rest with total parenteral nutrition (TPN) for 7 days and antibiotic coverage for colonic flora. Thirteen of 15 patients were successfully managed in this manner. The two failures were due to persistent enterocutaneous fistula requiring surgical treatment.60
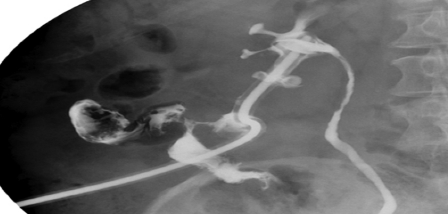
Figure 4.
Nephrocolic fistula following percutaneous nephrolithotomy (PCNL). Nephrostogram through a nephrostomy catheter after PCNL for a large staghorn calculus demonstrates opacification of the ascending colon through the nephrostomy track. The patient was asymptomatic. The fistula was managed conservatively: the patient was placed on a low residue diet, a double pigtail internalized stent was placed, and the nephrostomy catheter was retracted into the perinephric space. The fistula closed in 10 days.
The reported incidence of pleural complications in PCN placement is 0.1 to 0.2% and can consist of a pneumothorax, hemothorax, or nephropleural fistula.1 As noted earlier, the risk of pleural complications increases significantly with intercostal PCN placement as may sometimes be necessary for upper pole access for PCNL. For this reason, a postprocedure chest x-ray is recommended after intercostal intervention. The initial management of pleural transgression is directed at treating the resulting pneumothorax or pleural fluid, if clinically significant, usually with chest-tube placement. Authors differ with regard to management of the transpleural PCN, with some advocating removal only after enough time has passed for pericatheter tract formation and others removing the PCN as soon as it is no longer necessary. It is important, however, that antegrade urinary flow has been restored prior to tube removal to prevent formation of a nephropleural fistula.
Transient minor bleeding after nephrostomy tube placement is very common, occurring in up to 95% of cases. Often this is due to small vessel or venous bleeding. Severe postprocedure bleeding requiring transfusion or other intervention is reported to occur in 1 to 4% of patients.1 This can take the form of hematuria or retroperitoneal bleeding. Persistent gross hematuria more than 3 to 5 days after PCN placement may indicate severe arterial injury requiring treatment. If there is a significant drop in hemoglobin (HGB) discordant with the degree of hematuria, an expanding retroperitoneal hematoma should be suspected, particularly in the setting of increasing flank pain. Small retroperitoneal hematomas not requiring treatment have been reported in up to 13% of patients imaged with CT after nephrostomy placement.61 The workup and treatment of suspected arterial injury consists of a renal angiogram followed by subselective coil embolization of disrupted vascular branches. If the initial renal angiogram does not demonstrate a vascular injury, such as an arteriovenous fistula, pseudoaneurysm, or arterial transection, the angiogram should be repeated after removal of the nephrostomy tube over a wire. If there is still no evidence of arterial injury, and accessory renal arteries have been excluded, venous bleeding may be the source. This can sometimes be treated by tamponade with a larger diameter nephrostomy tube or balloon catheter.
Transient low-grade fever is common after PCN placement, with one study reporting a 100% incidence in 160 patients receiving emergency PCN placement.56 A second study consisting of patients receiving nonemergent PCN placement as an outpatient procedure noted fevers and chills without hypotension to occur in 21% of patients.45 Whether this always represents a microbial response or is sometimes a response to inflammatory mediators released by the procedure is debated.62 Progression to septic shock, with fevers, chills and hypotension, is less common and reported to occur in 1 to 3% of all patients and 7 to 9% of patients with pyonephrosis.63 Because urosepsis has a high mortality risk, prevention is key. If purulent-appearing urine is unexpectedly discovered during PCN placement, the urine should be sent for culture and sensitivity and further manipulations minimized until several days of drainage has occurred. All patients with known pyonephrosis should be initially treated with pre and postprocedure broad-spectrum empiric antibiotic coverage followed by specific coverage based on culture and sensitivity results of urine obtained during nephrostomy placement. Despite these precautions, urosepsis may still occur in the immediate postprocedure period. Initial treatment consists of supportive therapy with stabilization and maintenance of blood pressure with intravenous fluid and vasoactive substances to maintain a mean blood pressure greater than 65 mm Hg and less than 90 mm Hg, oxygenation, and early initiation of broad spectrum empiric microbial therapy (e.g., antipseudomonal third-generation cephalosporin + aminoglycoside (AG) or a carbapenem).64,65 Transfer to an intensive care unit for higher level monitoring and management should be made as soon as possible.
RESULTS
In the setting of dilated, obstructed collecting systems, successful PCN placement is achieved in 98 to 99% of patients. As might be expected, a lower success rate of 85 to 90% has been reported for PCN placement in nondilated systems or for complex stone disease.63 After PCN placement in patients with azotemia secondary to obstruction, renal function has been noted to normalize in two-thirds of patients within 15 days, with a mean of 7.7 days.51 After PCN placement in patients with pyonephrosis, fever and flank pain usually improve within 24 to 48 hours.66
POSTPROCEDURE NEPHROSTOMY TUBE MANAGEMENT
Over time, urine crystal deposition can lead to encrustation and tube obstruction. This occurs to a variable degree in each patient and is not significantly affected by frequency of catheter flushing between tube exchanges. Only improved hydration has been shown to reduce the encrustation rate. To prevent silent tube obstruction, routine PCN exchange every 3 months is recommended.
Occasionally, catheters can become dislodged. The incidence ranges from less than 1% in the early postplacement period to 11 to 30% in the subsequent months.2,56 If nephrostomy tubes are dislodged or obstructed, patients are scheduled for replacement during normal daytime working hours unless they develop recurrent fever and flank pain, in which case they are instructed to report to the ER. Emergent nephrostomy tube replacement may be necessary when the clinical situation suggests an infected, obstructed system.
References
- Ramchandani P, Cardella J F, Grassi C J, et al. Society of Interventional Radiology Standards of Practice Committee Quality improvement guidelines for percutaneous nephrostomy. J Vasc Interv Radiol. 2003;14(9 Pt 2):S277–S281. [PubMed] [Google Scholar]
- Farrell T A, Hicks M E. A review of radiologically guided percutaneous nephrostomies in 303 patients. J Vasc Interv Radiol. 1997;8(5):769–774. doi: 10.1016/s1051-0443(97)70658-4. [DOI] [PubMed] [Google Scholar]
- El-Ghar M E, Shokeir A A, El-Diasty T A, Refaie H F, Gad H M, El-Dein A B. Contrast enhanced spiral computerized tomography in patients with chronic obstructive uropathy and normal serum creatinine: a single session for anatomical and functional assessment. J Urol. 2004;172(3):985–988. doi: 10.1097/01.ju.0000135368.77589.7c. [DOI] [PubMed] [Google Scholar]
- Zielonko J, Studniarek M, Markuszewski M. MR urography of obstructive uropathy: diagnostic value of the method in selected clinical groups. Eur Radiol. 2003;13(4):802–809. doi: 10.1007/s00330-002-1550-8. [DOI] [PubMed] [Google Scholar]
- Better O S, Arieff A I, Massry S G, Kleeman C R, Maxwell M H. Studies on renal function after relief of complete unilateral ureteral obstruction of three months' duration in man. Am J Med. 1973;54(2):234–240. doi: 10.1016/0002-9343(73)90228-3. [DOI] [PubMed] [Google Scholar]
- Sacks S H, Aparicio S A, Bevan A, Oliver D O, Will E J, Davison A M. Late renal failure due to prostatic outflow obstruction: a preventable disease. BMJ. 1989;298(6667):156–159. doi: 10.1136/bmj.298.6667.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahr S. Pathophysiology of obstructive nephropathy. Kidney Int. 1983;23(2):414–426. doi: 10.1038/ki.1983.36. [DOI] [PubMed] [Google Scholar]
- Vaughan E D, Jr, Marion D, Poppas D P, Felsen D. Pathophysiology of unilateral ureteral obstruction: studies from Charlottesville to New York. J Urol. 2004;172(6 Pt 2):2563–2569. doi: 10.1097/01.ju.0000144286.53562.95. [DOI] [PubMed] [Google Scholar]
- Bander S J, Buerkert J E, Martin D, Klahr S. Long-term effects of 24-hr unilateral ureteral obstruction on renal function in the rat. Kidney Int. 1985;28(4):614–620. doi: 10.1038/ki.1985.173. [DOI] [PubMed] [Google Scholar]
- Pearle M S, Pierce H L, Miller G L, et al. Optimal method of urgent decompression of the collecting system for obstruction and infection due to ureteral calculi. J Urol. 1998;160(4):1260–1264. [PubMed] [Google Scholar]
- Ramsey S, Robertson A, Ablett M J, Meddings R N, Hollins G W, Little B. Evidence-based drainage of infected hydronephrosis secondary to ureteric calculi. J Endourol. 2010;24(2):185–189. doi: 10.1089/end.2009.0361. [DOI] [PubMed] [Google Scholar]
- Preminger G M, Tiselius H G, Assimos D G, et al. EAU/AUA Nephrolithiasis Guideline Panel 2007 guideline for the management of ureteral calculi. J Urol. 2007;178(6):2418–2434. doi: 10.1016/j.juro.2007.09.107. [DOI] [PubMed] [Google Scholar]
- Sperling H, Becker G, Heemann U, Lümmen G, Philipp T, Rübben H. The Whitaker test, a useful tool in renal grafts? Urology. 2000;56(1):49–52. doi: 10.1016/s0090-4295(00)00541-0. [DOI] [PubMed] [Google Scholar]
- Whitaker R H. Methods of assessing obstruction in dilated ureters. Br J Urol. 1973;45(1):15–22. [PubMed] [Google Scholar]
- Lupton E W, George N J. The Whitaker test: 35 years on. BJU Int. 2010;105(1):94–100. doi: 10.1111/j.1464-410X.2009.08609.x. [DOI] [PubMed] [Google Scholar]
- Png J C, Chapple C R. Principles of ureteric reconstruction. Curr Opin Urol. 2000;10(3):207–212. doi: 10.1097/00042307-200005000-00004. [DOI] [PubMed] [Google Scholar]
- Brandes S, Coburn M, Armenakas N, McAninch J. Diagnosis and management of ureteric injury: an evidence-based analysis. BJU Int. 2004;94(3):277–289. doi: 10.1111/j.1464-410X.2004.04978.x. [DOI] [PubMed] [Google Scholar]
- Avritscher R, Madoff D C, Ramirez P T, et al. Fistulas of the lower urinary tract: percutaneous approaches for the management of a difficult clinical entity. Radiographics. 2004;24(Suppl 1):S217–S236. doi: 10.1148/rg.24si045508. [DOI] [PubMed] [Google Scholar]
- Zagoria R J, Hodge R G, Dyer R B, Routh W D. Percutaneous nephrostomy for treatment of intractable hemorrhagic cystitis. J Urol. 1993;149(6):1449–1451. doi: 10.1016/s0022-5347(17)36412-1. [DOI] [PubMed] [Google Scholar]
- Samplaski M K, Irwin B H, Desai M. Less-invasive ways to remove stones from the kidneys and ureters. Cleve Clin J Med. 2009;76(10):592–598. doi: 10.3949/ccjm.76a.09014. [DOI] [PubMed] [Google Scholar]
- Bird V G, Fallon B, Winfield H N. Practice patterns in the treatment of large renal stones. J Endourol. 2003;17(6):355–363. doi: 10.1089/089277903767923119. [DOI] [PubMed] [Google Scholar]
- Tal R, Sivan B, Kedar D, Baniel J. Management of benign ureteral strictures following radical cystectomy and urinary diversion for bladder cancer. J Urol. 2007;178(2):538–542. doi: 10.1016/j.juro.2007.03.142. [DOI] [PubMed] [Google Scholar]
- Tal R, Bachar G N, Baniel J, Belenky A. External-internal nephro-uretero-ileal stents in patients with an ileal conduit: long-term results. Urology. 2004;63(3):438–441. doi: 10.1016/j.urology.2003.09.062. [DOI] [PubMed] [Google Scholar]
- Hyams E S, Winer A G, Shah O. Retrograde ureteral and renal access in patients with urinary diversion. Urology. 2009;74(1):47–50. doi: 10.1016/j.urology.2009.02.050. [DOI] [PubMed] [Google Scholar]
- Hautmann R E, Abol-Enein H, Hafez K, et al. World Health Organization (WHO) Consensus Conference on Bladder Cancer Urinary diversion. Urology. 2007;69(1, Suppl):17–49. doi: 10.1016/j.urology.2006.05.058. [DOI] [PubMed] [Google Scholar]
- Singh G, Wilkinson J M, Thomas D G. Supravesical diversion for incontinence: a long-term follow-up. Br J Urol. 1997;79(3):348–353. doi: 10.1046/j.1464-410x.1997.01007.x. [DOI] [PubMed] [Google Scholar]
- Ghoneim M A, Osman Y. Uretero-intestinal anastomosis in low-pressure reservoirs: refluxing or antirefluxing? BJU Int. 2007;100(6):1229–1233. doi: 10.1111/j.1464-410X.2007.07052.x. [DOI] [PubMed] [Google Scholar]
- Kayler L, Kang D, Molmenti E, Howard R. Kidney transplant ureteroneocystostomy techniques and complications: review of the literature. Transplant Proc. 2010;42(5):1413–1420. doi: 10.1016/j.transproceed.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Shoskes D A, Hanbury D, Cranston D, Morris P J. Urological complications in 1,000 consecutive renal transplant recipients. J Urol. 1995;153(1):18–21. doi: 10.1097/00005392-199501000-00008. [DOI] [PubMed] [Google Scholar]
- Akbar S A, Jafri S Z, Amendola M A, Madrazo B L, Salem R, Bis K G. Complications of renal transplantation. Radiographics. 2005;25(5):1335–1356. doi: 10.1148/rg.255045133. [DOI] [PubMed] [Google Scholar]
- Langer J, Jones L. Sonographic evaluation of the renal transplant. Ultrasound Clin. 2007;2(1):73–88. [Google Scholar]
- Basiri A, Simforoosh N, Nikoobakht M, Hosseini Moghaddam M M. The role of ureteroscopy in the treatment of renal transplantation complications. Urol J. 2004;1(1):27–31. [PubMed] [Google Scholar]
- Bhagat V J, Gordon R L, Osorio R W, et al. Ureteral obstructions and leaks after renal transplantation: outcome of percutaneous antegrade ureteral stent placement in 44 patients. Radiology. 1998;209(1):159–167. doi: 10.1148/radiology.209.1.9769827. [DOI] [PubMed] [Google Scholar]
- Fontaine A B, Nijjar A, Rangaraj R. Update on the use of percutaneous nephrostomy/balloon dilation for the treatment of renal transplant leak/obstruction. J Vasc Interv Radiol. 1997;8(4):649–653. doi: 10.1016/s1051-0443(97)70625-0. [DOI] [PubMed] [Google Scholar]
- Docimo S G, Dewolf W C. High failure rate of indwelling ureteral stents in patients with extrinsic obstruction: experience at 2 institutions. J Urol. 1989;142(2 Pt 1):277–279. doi: 10.1016/s0022-5347(17)38729-3. [DOI] [PubMed] [Google Scholar]
- Shekarriz B, Shekarriz H, Upadhyay J, et al. Outcome of palliative urinary diversion in the treatment of advanced malignancies. Cancer. 1999;85(4):998–1003. doi: 10.1002/(sici)1097-0142(19990215)85:4<998::aid-cncr30>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Wong L M, Cleeve L K, Milner A D, Pitman A G. Malignant ureteral obstruction: outcomes after intervention. Have things changed? J Urol. 2007;178(1):178–183. discussion 183. doi: 10.1016/j.juro.2007.03.026. [DOI] [PubMed] [Google Scholar]
- Ishioka J, Kageyama Y, Inoue M, Higashi Y, Kihara K. Prognostic model for predicting survival after palliative urinary diversion for ureteral obstruction: analysis of 140 cases. J Urol. 2008;180(2):618–621. discussion 621. doi: 10.1016/j.juro.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Kouba E, Wallen E M, Pruthi R S. Management of ureteral obstruction due to advanced malignancy: optimizing therapeutic and palliative outcomes. J Urol. 2008;180(2):444–450. doi: 10.1016/j.juro.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Chapman M E, Reid J H. Use of percutaneous nephrostomy in malignant ureteric obstruction. Br J Radiol. 1991;64(760):318–320. doi: 10.1259/0007-1285-64-760-318. [DOI] [PubMed] [Google Scholar]
- Millward S F. Percutaneous nephrostomy: a practical approach. J Vasc Interv Radiol. 2000;11(8):955–964. doi: 10.1016/s1051-0443(07)61322-0. [DOI] [PubMed] [Google Scholar]
- Malloy P C, Grassi C J, Kundu S, et al. Standards of Practice Committee with Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Endorsement Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2009;20(7, Suppl):S240–S249. doi: 10.1016/j.jvir.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Venkatesan A M, Kundu S, Sacks D, et al. Society of Interventional Radiology Standards of Practice Committee Practice guidelines for adult antibiotic prophylaxis during vascular and interventional radiology procedures. [corrected] J Vasc Interv Radiol. 2010;21(11):1611–1630. quiz 1631. doi: 10.1016/j.jvir.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Classen D C, Evans R S, Pestotnik S L, Horn S D, Menlove R L, Burke J P. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326(5):281–286. doi: 10.1056/NEJM199201303260501. [DOI] [PubMed] [Google Scholar]
- Cochran S T, Barbaric Z L, Lee J J, Kashfian P. Percutaneous nephrostomy tube placement: an outpatient procedure? Radiology. 1991;179(3):843–847. doi: 10.1148/radiology.179.3.2028003. [DOI] [PubMed] [Google Scholar]
- Gray R R, So C B, McLoughlin R F, Pugash R A, Saliken J C, Macklin N I. Outpatient percutaneous nephrostomy. Radiology. 1996;198(1):85–88. doi: 10.1148/radiology.198.1.8539411. [DOI] [PubMed] [Google Scholar]
- Papanicolaou N. Renal anatomy relevant to percutaneous interventions. Semin Intervent Radiol. 1995;12:163–172. [Google Scholar]
- Snell R S. Clinical Anatomy by Regions. 8th ed. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- Hopper K D, Yakes W F. The posterior intercostal approach for percutaneous renal procedures: risk of puncturing the lung, spleen, and liver as determined by CT. AJR Am J Roentgenol. 1990;154(1):115–117. doi: 10.2214/ajr.154.1.2104692. [DOI] [PubMed] [Google Scholar]
- Hopper K D, Sherman J L, Luethke J M, Ghaed N. The retrorenal colon in the supine and prone patient. Radiology. 1987;162(2):443–446. doi: 10.1148/radiology.162.2.3797658. [DOI] [PubMed] [Google Scholar]
- Pappas P, Stravodimos K G, Mitropoulos D, et al. Role of percutaneous urinary diversion in malignant and benign obstructive uropathy. J Endourol. 2000;14(5):401–405. doi: 10.1089/end.2000.14.401. [DOI] [PubMed] [Google Scholar]
- Zagoria R J, Dyer R B. Do's and don't's of percutaneous nephrostomy. Acad Radiol. 1999;6(6):370–377. doi: 10.1016/s1076-6332(99)80233-5. [DOI] [PubMed] [Google Scholar]
- Sampaio F J, Zanier J F, Aragão A H, Favorito L A. Intrarenal access: 3-dimensional anatomical study. J Urol. 1992;148(6):1769–1773. doi: 10.1016/s0022-5347(17)37024-6. [DOI] [PubMed] [Google Scholar]
- Clark T W, Abraham R J, Flemming B K. Is routine micropuncture access necessary for percutaneous nephrostomy? A randomized trial. Can Assoc Radiol J. 2002;53(2):87–91. [PubMed] [Google Scholar]
- Patel U, Hussain F F. Percutaneous nephrostomy of nondilated renal collecting systems with fluoroscopic guidance: technique and results. Radiology. 2004;233(1):226–233. doi: 10.1148/radiol.2331031342. [DOI] [PubMed] [Google Scholar]
- Lee W J, Patel U, Patel S, Pillari G P. Emergency percutaneous nephrostomy: results and complications. J Vasc Interv Radiol. 1994;5(1):135–139. doi: 10.1016/s1051-0443(94)71470-6. [DOI] [PubMed] [Google Scholar]
- LeRoy A J, Williams H J, Jr, Bender C E, Segura J W, Patterson D E, Benson R C. Colon perforation following percutaneous nephrostomy and renal calculus removal. Radiology. 1985;155(1):83–85. doi: 10.1148/radiology.155.1.3975424. [DOI] [PubMed] [Google Scholar]
- Traxer O. Management of injury to the bowel during percutaneous stone removal. J Endourol. 2009;23(10):1777–1780. doi: 10.1089/end.2009.1553. [DOI] [PubMed] [Google Scholar]
- Gerspach J M, Bellman G C, Stoller M L, Fugelso P. Conservative management of colon injury following percutaneous renal surgery. Urology. 1997;49(6):831–836. doi: 10.1016/s0090-4295(97)00237-9. [DOI] [PubMed] [Google Scholar]
- El-Nahas A R, Shokeir A A, El-Assmy A M, et al. Colonic perforation during percutaneous nephrolithotomy: study of risk factors. Urology. 2006;67(5):937–941. doi: 10.1016/j.urology.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Cronan J J, Dorfman G S, Amis E S, Denny D F., Jr Retroperitoneal hemorrhage after percutaneous nephrostomy. AJR Am J Roentgenol. 1985;144(4):801–803. doi: 10.2214/ajr.144.4.801. [DOI] [PubMed] [Google Scholar]
- Dogan H S, Guliyev F, Cetinkaya Y S, Sofikerim M, Ozden E, Sahin A. Importance of microbiological evaluation in management of infectious complications following percutaneous nephrolithotomy. Int Urol Nephrol. 2007;39(3):737–742. doi: 10.1007/s11255-006-9147-9. [DOI] [PubMed] [Google Scholar]
- Ramchandani P, Cardella J F, Grassi C J, et al. SCVIR Standards of Practice Committee Quality improvement guidelines for percutaneous nephrostomy. J Vasc Interv Radiol. 2001;12(11):1247–1251. doi: 10.1016/s1051-0443(07)61546-2. [DOI] [PubMed] [Google Scholar]
- Wagenlehner F M, Weidner W, Naber K G. Optimal management of urosepsis from the urological perspective. Int J Antimicrob Agents. 2007;30(5):390–397. doi: 10.1016/j.ijantimicag.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Funk D, Sebat F, Kumar A. A systems approach to the early recognition and rapid administration of best practice therapy in sepsis and septic shock. Curr Opin Crit Care. 2009;15(4):301–307. doi: 10.1097/MCC.0b013e32832e3825. [DOI] [PubMed] [Google Scholar]
- Camúñez F, Echenagusia A, Prieto M L, Salom P, Herranz F, Hernández C. Percutaneous nephrostomy in pyonephrosis. Urol Radiol. 1989;11(2):77–81. doi: 10.1007/BF02926481. [DOI] [PubMed] [Google Scholar]