Abstract
Renal cysts are a common imaging finding. Although most cysts never have symptoms, some cause pain, collecting system compression, hematuria, hypertension, and secondary infection. The mere presence of a cyst is not an indication for intervention, but treatment may be indicated in symptomatic patients or those with secondary obstruction. Urinomas generally are a contained collection of urine outside of the normal pathways where urine travels. As such, urinomas can arise anywhere from the upper abdomen down into the low pelvis and have a variety of etiologies. Ureteral obstruction with forniceal rupture and trauma (blunt, penetrating, or iatrogenic) are the most common causes of urinomas. When urinomas arise spontaneously, the likely cause varies with the patient's age. Blunt or penetrating trauma can cause perinephric urinomas by two mechanisms—direct disruption of the pelvis or collecting system or by degeneration of nonviable tissue. These urinomas are often perinephric, but can also occur in a subcapsular location. This review will discuss diagnosis, classification, and treatment of renal cysts and urinomas.
Keywords: Renal cysts, urinoma, interventional radiology, sclerosis, puncture
RENAL CYSTS
Renal cysts are a common routine imaging finding, and autopsy studies suggest that more than half of patients 50 years of age and older have at least one renal cyst.1 Although most cysts never cause symptoms, some cause pain, collecting system compression, hematuria, hypertension, and secondary infection. The mere presence of a cyst is not an indication for intervention, but treatment may be indicated in symptomatic patients or those with secondary obstruction. Understanding the imaging appearance and classification of renal cysts is an important initial step in providing full evaluation and appropriate management of renal cysts.
Before attempting interventional obliteration of a cystic renal lesion, it is important to determine that the lesion is not malignant; 15% of renal cell carcinomas are cystic on radiologic examination.2 When describing and determining management of cystic renal lesions, radiologists and urologists typically use a lesion's morphology and enhancement characteristics for categorization into one of five groups (I, II, IIF, III, and IV) according to the Bosniak renal cyst classification system, each category corresponding to a management recommendation.3
Category I lesions are benign simple cysts that measure water attenuation on computed tomography (CT) with a hairline thin wall. These lesions do not contain septa, calcification, or any solid component.3 Simple cysts account for 80 to 85% of all space-occupying lesions in the kidneys.4 Although the etiology of simple cysts is unclear, the age distribution indicates that they are acquired lesions. Pathologic studies have shown that cysts start as dilatations or diverticula of the tubules in the nephron and they are thought to arise from focal infarcts or inflammation.5,6
Parapelvic cysts are a subset of simple cysts that arise within the renal parenchyma adjacent to the renal sinus. Though they are rare in children, parapelvic cysts account for 5% of all renal cysts in adults.7 These may extend into the renal sinus and thus may not be completely surrounded by renal parenchyma as simple cortical cysts, but their etiology is probably similar. Parapelvic lesions can be interrogated with Duplex ultrasound to exclude a vascular mass like an aneurysm or vascular malformation.8,9 Parapelvic cysts are a distinct entity from peripelvic cysts, which are small, confluent cysts that arise primarily in the renal sinus. Peripelvic cysts are thought to represent a congenital embryologic remnant or sequelae of acquired lymphatic obstruction. They can usually be differentiated from parapelvic cysts on ultrasound by the presence of multiple thin, linear septations that extend radially from the renal hilum. Peripelvic cysts rarely cause symptoms that require treatment.10
Like category I lesions, category II lesions have no malignant potential and therefore require no further imaging. However, unlike category I lesions, category II lesions may contain a few hairline thin septa, fine calcification, or short-segment slightly thickened calcification within the wall or septa. Uniformly high attenuation cysts measuring < 3 cm are also included in this category.
Category IIF, III, and IV lesions vary widely in their risk of malignancy, with 5% of category IIF lesions, 50% of category III lesions, and over 80% of category IV lesions ultimately proving to be malignant.11 Category IIF lesions may contain multiple hairline thin septa or smooth, minimally thickened wall or septa, which may contain calcification that may be thick and nodular. However, by definition, these lesions do not demonstrate measureable enhancement. These lesions require follow-up imaging demonstrating stability to prove they are benign. Category III lesions demonstrate enhancing, thickened, irregular, or smooth walls or septa and require surgical removal due to their high potential for malignancy. Infected cysts may also enhance, but clinical symptoms and white blood cell count should distinguish these lesions from malignancies. Category IV lesions typically contain enhancing soft-tissue components adjacent to, but independent of, the wall or septum. These also require surgical removal due to their high likelihood of malignancy.3
CYST SYMPTOMS
Pain is the most common symptom associated with renal cysts and probably results from distention of the renal capsule, but may also be a secondary effect due to obstruction of the collecting system. Cysts cause some degree of collecting system obstruction in 2.5 to 16.0% of cases. Parapelvic cysts may obstruct the ureter or low pelvis, whereas peripheral cortical cysts can cause infundibular or calyceal obstruction.7,10
Cyst rupture can be a source of significant pain. Rupture can occur due to blunt trauma or increased intracyst pressure in the setting of intracyst hemorrhage or infection.12 Hematuria is seen in up to 84% of patients with cyst rupture, likely resulting from cyst rupture into an adjacent calyx.13 Less commonly, cysts can rupture through the renal capsule and cause a perirenal urinoma or hematoma.14,15,16
A relationship between simple renal cysts and hypertension has been suggested. Because the prevalence of hypertension and the prevalence, size, and number of simple cysts increase with age, it has been suggested that simple renal cysts may cause hypertension in some patients.17 One proposed mechanism is that a large cyst causes compression of the surrounding renal parenchyma and induces a hyperreninemic state; drainage of cysts has been shown to cure hyperreninemic hypertension in some cases.17,18 However, evidence that simple renal cysts are an important cause of hypertension is still lacking.
Secondary infection of cysts occurs in 2.5% of cases,19 and can result from either hematogenous spread of bacteria or from local extension from infected renal parenchyma or collecting system. The typical clinical presentation includes pain, leukocytosis, and fever. Pain caused by infected cysts can be severe enough to mimic an acute abdomen.19 Infected cysts are initially managed like an abscess by percutaneous drainage and intravenous antibiotics. Cyst aspiration can also be used to determine if a cyst is infected or not. If infected, cyst drainage is valuable because some antibiotics do not penetrate into cyst fluid even when the cyst wall is inflamed.20 Cyst drainage may be used to initiate therapy or as a diagnostic trial to see if it eliminates the symptoms.
Cysts occurring in the setting of polycystic kidneys or multicystic dysplastic kidneys are not usually treated with interventional techniques because distinguishing which cyst is causing symptoms can be difficult. Exceptions include when specific complications develop such as infection or pain from intracyst hemorrhage. Infection or intracyst hemorrhage can lead to increases in cyst size, attenuation, or wall enhancement on CT. 111Indium-labeled leukocyte scans have been used to help determine which cyst might be infected.21 Magnetic resonance imaging (MRI) may also help distinguish infected cysts by gadolinium enhancement of the infected cyst wall and internal septations.22
CYST PUNCTURE AND ASPIRATION
Treatment options for renal cysts include percutaneous and surgical methods, including percutaneous aspiration (with or without sclerosing agent), percutaneous marsupialization, and cyst unroofing (laparoscopic or open).23 Although laparoscopic decortication of peripheral and peripelvic cysts is safe, effective, and less invasive than open surgery, it remains costly and requires general anesthesia. Percutaneous aspiration of renal cysts with or without the use of a sclerosing agent has been shown to be a safe, well-tolerated, low-cost, and effective method for treatment.
Preparation for cyst puncture requires precautions similar to those taken before other interventional procedures. Bleeding history, coagulation parameters, and platelet count need to be checked. Any history of prior contrast reaction and any other medical condition that could be exacerbated by the procedure should be obtained.
The skin entry point for cyst puncture should be chosen similar to a nephrostomy access. For most cortical cysts, the puncture should be started around the lateral border of the major erector muscles of the back. A more lateral entry point increases the risk of injury to nontarget organs such as colon, liver, or spleen. A more medial entry point is acceptable if there is no plan to leave a drainage tube in place, as a medial drainage catheter is uncomfortable for the patient because it traverses more muscle mass, and it is more difficult to avoid lying on a medially positioned tube. However, for parapelvic cyst treatment, a more medial entry point may be warranted because entering along the lateral border of the erector muscles may cause the tract to skim the renal parenchyma.
Cyst puncture is generally guided by ultrasound, although fluoroscopy can alternatively be used if iodinated contrast was given and the cyst is large enough to distort the collecting system or cause a defect in the nephrogram. CT is rarely needed to guide cyst puncture, except when trying to treat a very small cyst, or in very obese patients in whom body habitus limits visualization of the lesion on sonography. CT guidance is also helpful when it is necessary to puncture one specific cyst in a patient with polycystic disease. A 21- or 22-gauge needle is generally sufficient for aspiration and a cystogram. If catheter drainage is planned, use of an 18-gauge needle may facilitate the procedure because 18-gauge needles will accept a larger 0.035- to 0.038-inch guidewire. Thus, one can avoid using a less-stiff 0.018-inch guidewire as well as the extra step of using a transition dilator to introduce the larger guidewire.
The cyst should be punctured as atraumatically as possible to decrease bleeding into the cyst. The fluid in a benign simple cyst should be clear and colorless to slightly yellow. A small amount of blood in the fluid that clears during the drainage indicates a traumatic tap. Bloody fluid that does not become more serous is suggestive of a tumor,24,25 although a clear aspirate does not exclude the possibility of cystic malignancy.26 The aspirated fluid may be sent for cytologic evaluation, which in some series has shown good sensitivity for malignancy.27 However, other authors have indicated that a negative cytology does not exclude malignancy.28,29 In fact, Kleist et al29 found that cytology failed to detect malignant cells in 9 of 11 cystic malignancies that they studied. Some authors also recommend analyzing the aspirated fluid for lipid content, which in cystic malignancies is approximately five times higher than that seen in benign cysts.29 However, both false-positive and false-negative lipid analyses are possible.26
After cyst aspiration, a cystogram should be performed using a single-contrast technique in which the cyst fluid is replaced with dilute contrast. Dilute contrast is used to avoid obscuring details of the cyst wall. Double-contrast techniques have also been described25 in which 50% of the aspirated volume of the cyst is replaced with air and 25% of the volume is replaced by contrast. The cyst walls in benign Bosniak category I and II cysts should be perfectly smooth. Although category II cysts may contain internal septations or lobulations, the cyst wall itself should remain smooth. Any irregularity of the wall raises the possibility of tumor. Interpretation of the cystogram may be more difficult when incomplete distention of the cyst or partial collapse from prior cyst rupture leads to some cyst wall irregularity. Organized or adherent thrombus from prior hemorrhage may also mimic malignancy. With the combination of cyst fluid analysis and cystogram, the accuracy of cyst aspiration for exclusion of malignancy has been reported to be around 95 to 98%.4,30 The accuracy of these techniques decreases in the setting of hemorrhagic cysts or highly septated cysts.
Simple needle aspiration of cysts is a safe procedure. Major complications are uncommon and occur in only 0.75 to 3.00% of cases.31,32 The most common complication is perirenal hemorrhage, which occurs in 0.18 to 0.30% of cases when 20- to 22-gauge needles are used. Other complications that have been reported include pneumothorax, arteriovenous fistula, infection, urinoma formation, and inadvertent puncture of adjacent bowel. Pneumothorax occurs most frequently with punctures of upper pole cysts, particularly in the left kidney. Aside from these immediate procedural complications, the only long-term problem that has been noted is tumor seeding along the tract after aspiration of a cystic malignancy. However, this is a rare complication, with only a few case reports in the literature.33,34 The problem with simple aspiration is the high recurrence rate because the epithelium of cysts can rapidly produce more fluid and refill the cyst.35 Recurrence of renal cysts after simple aspiration occurs in anywhere from 30 to 100% of cases in the series reported.36,37,38,39 For this same reason, simple catheter drainage is rarely used.
SCLEROSIS OF CYSTS
To decrease the incidence of cyst recurrence, sclerosis is utilized to destroy the secreting epithelial cells lining the cyst. Zerem et al randomized 92 patients with simple renal cysts to ultrasound-guided continuous catheter drainage with negative pressure or single-session alcohol sclerotherapy. At 24 months, complete resolution of the cyst occurred in 52% of continuous catheter drainage and only 28% of single-session sclerotherapy patients, with the probability of cyst disappearance in the sclerotherapy group significantly less if the cyst was > 500 mL. In cysts smaller than 500 mL, the likelihood of complete resolution was comparable in the two groups.23
Although sclerosis can be performed via the aspirating needle, sclerotherapy can generally be more safely and effectively performed after placement of a catheter within the cyst. Using a catheter has several advantages, one of which is performing delayed cystograms after initial drainage, which allows for detection of communication with the collecting system that may not have been apparent at the time of initial drainage. Subsequent cystograms also allow one to monitor the collapse of the cyst cavity, which helps determine the effectiveness of treatment and the volume of sclerosant needed on subsequent treatments. An additional benefit is the fibrous tract that forms around a drainage catheter is also beneficial in that sclerosant leaking from the cyst will drain along the tract rather than freely extravasate into the perirenal space.
Before placing a catheter, one should determine if the catheter material is stable in alcohol to avoid damaging the catheter's structural integrity. Both trocar and Seldinger techniques can be used to place catheters within renal cysts. Although the Seldinger technique is probably most common, it can be difficult to pass the catheter through the nondistended cyst wall if the cyst decompresses into the perirenal space during exchanges.
After catheter placement within the cyst, a thorough cystogram is performed to ensure that there is no communication with the collecting system. This is particularly important in cysts that have partially or completely ruptured because 52% of the time they rupture into the caliceal system. These communications may partially seal, allowing the cyst to redistend, but still maintain communication with the collecting system.13 Such a connection between the cyst and collecting system precludes use of a sclerosing agent to avoid introducing sclerosant into the collecting system.
If the cyst is infected, it should be drained until the infection clears before one initiates sclerosis. Sclerosis of an infected cyst could theoretically lead to loculation of infected material. When one is attempting to sclerose giant cysts, the cyst walls may adhere to themselves and form separate loculated compartments. This may require manipulating the catheter into the loculations or even placing an additional drainage catheter.
After complete drainage of the cyst fluid, replacement with contrast allows one to estimate the cyst volume to determine how much sclerosant to use. Extravasation of contrast into the perirenal space should be noted. If this occurs freely without overdistention of the cyst, it is advisable to continue catheter drainage until a fibrous tract forms around the catheter. The volume of sclerosant used should approximate 25 to 50% of the volume of the cyst estimated by the injection of contrast.
Multiple agents have been used to sclerose cysts, including glucose, phenol, Pantopaque, tetracycline, bismuth, Betadine, cyanoacrylate glue, sodium tetradecyl sulfate, hypertonic saline, ethanolamine oleate, acetic acid, and OK-432 (Picibanil; Chugai Pharmaceutical, Tokyo, Japan).38,40,41,42,43,44,45,46,47,48,49,50 Absolute alcohol is currently the most commonly used sclerosant. Bean39 showed experimentally that although the cyst wall epithelium becomes nonviable after 1 to 3 minutes of exposure to alcohol, it takes ∼ 4 to 12 hours for alcohol to penetrate the cyst capsule. Thus, this agent can safely defunctionalize the secreting cells of the cyst without affecting adjacent renal parenchyma. Alcohol also has the advantages of being inexpensive and readily available. Furthermore, a linear correlation between ethanol concentration and attenuation on CT allows for ethanol concentration monitoring during CT-guided sclerotherapy, which has been shown to result in improved success rates.51
Once the alcohol is instilled, the patient should be repositioned at intervals onto each side and onto both prone and supine positions to expose all portions of the cyst wall to the alcohol. Midway through the sclerosis session the alcohol should be aspirated and replaced with a fresh aliquot. The alcohol is left in the cyst for 15 to 20 minutes and the catheter is then placed back to drainage. Typically, repeat sclerosis is done in subsequent sessions until the drainage from the cyst becomes negligible. The optimal frequency of sclerosis has not been determined. Cysts with high output may need to be sclerosed daily in which case the patient can be taught to perform the sclerosis at home. Other sclerosis regimens have been advocated. Two series52,53 that utilized daily sclerosis for only 2 and 3 days, respectively, reported complete cyst resolution in 84% and 97% of cases. Single session drainage and sclerosis with ethanol has been reported and although good results can sometimes be achieved (Fig. 1), cyst resolution is usually seen in only 17 to 28% of cases.23,54,55 However, Akinci et al report an average cyst volume reduction of 93% at the end of one year.23
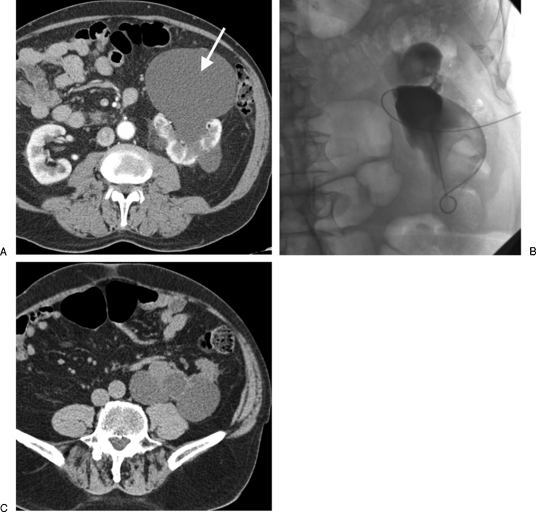
Figure 1.
(A) A 78-year-old man with pain and pressure caused by a large anterior left renal cyst (arrow). (B) A catheter was inserted and the cyst drained. This image shows the infolding of the cyst as a large volume of fluid was drained. The cyst was then sclerosed with alcohol that was later aspirated in a single session. Note the catheter was inserted from an anterior approach given the large size of the cyst, thus the orientation of this image. (C) Follow-up computed tomography 9 months later shows no recurrence of the anterior cyst that was sclerosed although the posterior cysts have grown in size.
Although cyst sclerosis can commonly cause some pain or fever, significant complications are infrequent. Bean39 reported microscopic hematuria in 2 of 29 patients (6.9%) but no major complications. Gelet et al43 on the other hand, reported infectious-type complications in 2 of 10 patients (20%) after Betadine sclerosis. One patient developed sepsis after the procedure, and one patient developed delayed infection of a residual cyst cavity 3 months after sclerosis. The more typical incidence of infectious complication is 0 to 0.5%.39,56 Zerem et al55 described symptoms and signs of patient intoxication in sclerosis of 8 of 46 cysts treated with single-session alcohol sclerotherapy. The maximal blood alcohol concentration in those patients was 73–120 mg/dL 3 hours after alcohol instillation, with symptom resolution in all patients within 24 hours. No other major complications have been described for peripheral cysts. Sclerosis of parapelvic cysts carries the additional theoretic risk of damage to adjacent hilar structures. Ureteral pelvic obstruction caused by sclerosis-induced fibrosis has been described.56
Alternative sclerosing agents have been used in an effort to avoid the pain some patients experience with alcohol sclerotherapy and eliminate the risk of leakage of alcohol from the cyst. The effectiveness of sclerotherapy appears to vary with the sclerosing agent used. Some authors advocate Betadine, also inexpensive and readily available, because it is less toxic than ethanol,44,57 whereas others have found Betadine sclerotherapy to be followed by a high rate of recurrence.58 Some authors advocate use of acetic acid, sodium tetradecyl sulfate, ethanolamine oleate, and OK-432 because they have similar efficacy in sclerosing renal cysts but cause less pain than alcohol.46,49,50 Hypertonic saline, though cost effective and readily available, is less effective than alcohol but is an option for patients preferring a less painful procedure.48 In a randomized clinical trial of 65 patients with 68 symptomatic renal cysts, Demir et al found similar efficacy between 95% ethanol and 3% sodium tetradecyl sulfate (complete ablation in 82% and 76%, partial regression of 9% and 6%, and failure of treatment in 9% and 6% in ethanol and sodium tetradecyl sulfate groups, respectively). However, pain caused by injection was significantly less for the sodium tetradecyl sulfate group.47 In our experience, alcohol sclerotherapy has not usually been associated with significant pain.
SURGICAL THERAPY OF CYSTS
Advantages of surgical management of cysts include potential cyst obliteration in one treatment as opposed to sequential treatments sometimes required for sclerotherapy, direct visual inspection of the cyst to more confidently exclude malignancy, and acquisition of a tissue specimen for pathologic examination. However, surgical management requires general anesthesia, hospitalization, and is more costly than percutaneous treatment. Furthermore, although a normal appearance at surgical inspection should exclude malignancy, there has been at least one case reported where disseminated renal cell carcinoma developed shortly after laparoscopic decortication of what appeared to be a simple cyst.59
Open surgical cyst unroofing is associated with a morbidity of 8 to 16%, and the hospital stay averages up to 9 days for this procedure.32,60 This has largely been supplanted by laparoscopic unroofing, which is much less invasive,60,61,62,63 but also requires hospitalization up to 3 days.64 Laparoscopic unroofing is done through either a transperitoneal or retroperitoneal approach depending on cyst location. After identifying the cyst laparoscopically, the procedure involves the following: The cyst fluid is aspirated and sent for cytology; the cyst wall is resected and sent for pathology; the remaining cyst wall is visually inspected and any masses are resected and submitted for frozen section; the base of the cyst is then fulgurated.
Parapelvic cysts are more difficult to manage laparoscopically as evidenced by longer operative time, greater blood loss, and longer hospital stays compared with peripheral cysts.65 Although urologists advocate that sclerosis is dangerous in the parapelvic region, laparoscopic management of parapelvic cysts can also be complicated by laceration or stricture of the renal pelvis or ureter. In the renal hilum, adjacent major arteries and veins may limit the resection of the cyst wall and distinguishing the cyst from the renal vein may be difficult enough to require the use of a laparoscopic ultrasound.66
Another surgical alternative is to marsupialize the cyst into the collecting system, with possible fulguration of the cyst wall to cause the cavity to close through scarring. Although this can be performed via a retrograde ureteroscopic approach,30 the more common access is via a percutaneous tract into the cyst,67 which requires placement of a 24- to 30-French (Fr) sheath to provide access large enough to accommodate the endoscope. Instead of marsupializing the cyst into the collecting system, this percutaneous technique can also be used to unroof the cyst into the retroperitoneum by resecting part of the cyst wall after pulling the sheath back.68
Despite the more invasive nature of endourologic management, complete cyst obliteration may not be possible in all cases (Fig. 2). One series reports complete obliteration in only 60% of cysts.43 Recurrence rates range from 3% (with no recurrent symptoms) up to 17% symptomatic recurrence.43,65 Complication rates range from 13 to 33%62,65 and include complications such as ureteral stricture, diaphragm injury, ileus, and large retroperitoneal hematoma.
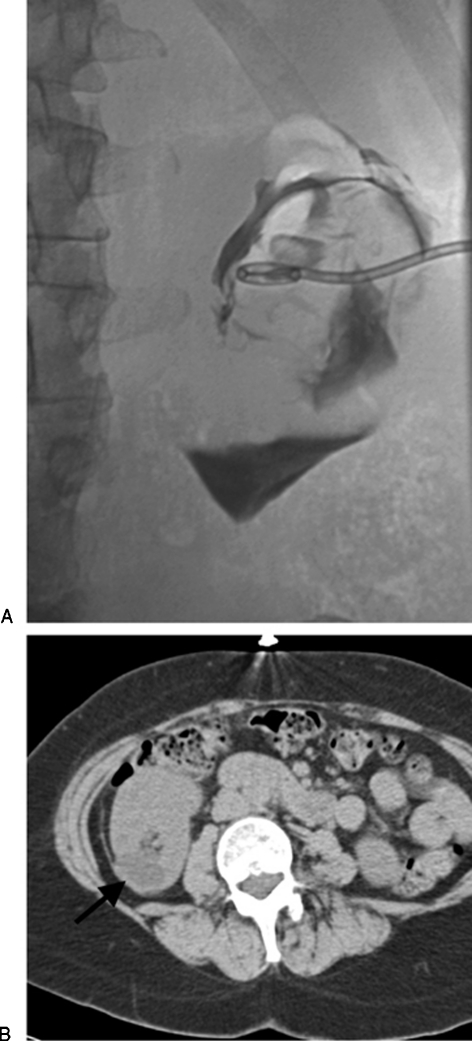
Figure 2.
(A) Cyst injection in this case was difficult to interpret as to whether a mass was present or this just represented folding of the cyst walls. For this reason, the patient underwent surgical unroofing of the cyst, which did prove to be benign. (B) Computed tomography done several months after the surgical unroofing shows persistent fluid (arrow) in the cyst bed despite the more aggressive surgical approach.
Although success rates of surgical therapy and percutaneous sclerosis are fairly comparable, sclerosis has several advantages. Sclerotherapy can easily be performed as an extension of an initial diagnostic procedure. Also, catheter placement and sclerosis are well-tolerated under local anesthesia alone, and unlike surgical techniques, do not require general anesthesia. Lack of operating room, anesthesia, and hospitalization costs makes percutaneous sclerotherapy more economical. Sclerosis is typically performed through a single 8- to 10-Fr catheter, significantly smaller than the 24- to 30-Fr size endourologic sheath. Furthermore, laparoscopic treatment requires four small incisions to accommodate the ports used for visualization and manipulation. Average procedure time required for laparoscopic or open surgical management is much longer than what is required for percutaneous catheter drainage and sclerotherapy. Thus, interventional techniques are much less expensive, less complex, and less invasive than surgical treatment of symptomatic renal cysts.
URINOMAS: ETIOLOGY
Urinomas generally are a contained collection of urine outside of the normal pathways where urine travels. As such, urinomas can arise anywhere from the upper abdomen down into the low pelvis and have a variety of etiologies. Ureteral obstruction with forniceal rupture and trauma (blunt, penetrating, or iatrogenic) are the most common causes of urinomas. When urinomas arise spontaneously, the likely cause varies with the patient's age. For example in neonates posterior urethral valves are a common cause and urinomas occur in as many as 15% of neonates with posterior urethral valves.69 In older children a congenital obstruction such as a ureteropelvic obstruction would be more likely than obstruction due to malignancy or ureteral calculi that one might see in an adult. Blunt or penetrating trauma can cause perinephric urinomas by two mechanisms: direct disruption of the pelvis or collecting system or by degeneration of nonviable tissue. These urinomas are often perinephric, but can also occur in a subcapsular location. Symptomatic urinomas are reported to occur in 17% of blunt renal trauma cases in children.70 Traumatic disruptions of the pelvis or ureter can initially go unrecognized.71 Rupture of a cortical cyst that communicates to the collecting system is another rare cause of urinoma.
Surgical damage to the kidneys, ureters, or bladder can result in urinomas as can leakage from an anastomosis that is technically inadequate or has dehisced. After renal transplantation, urine leak occurs in 1 to 5% of cases72 and can result from anastomotic dehiscence or ischemic necrosis of the distal ureter. The timing of the leak is a clue to the etiology. Technical error with dehiscence of the neoureterocystostomy is most likely if the leak occurs within the first week after transplant but later on ischemia of the ureter is more likely. Although less invasive, minimally invasive procedures can also be a potential cause of urinomas formation. They have been reported to occur after procedures such as percutaneous nephrostomy, stone basketing, cystoscopy, and extracorporeal shock wave lithotripsy.73,74,75 As minimally invasive procedures become more invasive, it is possible that urinomas may be seen more frequently. In one study, urinomas were reported in 3 out of 16 ablation-assisted laparoscopic partial nephrectomies.76
PRESENTATION AND DIAGNOSIS
Urinomas may cause sensations of pressure or pain due to their mass effect, but they may also be asymptomatic and discovered as an incidental finding during cross-sectional imaging. If the urinoma is large enough or positioned in a critical spot, it can cause obstruction of adjacent structures, such as ureters. For upper abdominal urinomas, the mass effect can cause respiratory difficulties if the urinoma puts pressure on the diaphragm. Urinomas can present in a delayed fashion, even weeks after the initial insult.76 If the urinoma is secondarily infected, fever or an elevated white blood cell count may herald the presence of the urinoma. This is particularly concerning in the renal transplant population because these patients are often immunosuppressed and an infection of this sort must be treated promptly. If the renal transplant patient is in the recent postoperative period, urine may leak out of the incision rather than forming a contained collection.
Both CT and ultrasound can readily define a fluid collection. Given the lower cost and slightly greater availability of ultrasound, this is often the first test. Typically, ultrasound merely demonstrates a fluid collection and cannot distinguish urinoma from seroma or abscess. However, Doppler visualization of a unidirectional jet of fluid into the cavity has been described as an indication that one is dealing with a urinoma.77 A caveat about ultrasound is that appearance can be deceiving in regards to the suitability for percutaneous drainage. With ultrasound, these collections may look like there are multiple loculations; however, these are often filmy and easily disrupted by passage of a guidewire into the urinoma. Therefore, the ultrasound appearance should not dissuade one from attempting drainage. Contrast-enhanced CT has an advantage that can increase the diagnostic specificity because urine flow into the fluid collection can be documented by increased attenuation in the collection on delayed images.78 CT can often also clearly pinpoint the source of the leak (Fig. 3). As mentioned earlier, urine may leak out of an incision; thus, imaging may not define an abnormal fluid collection. This is particularly true after renal transplantation where a definable urinoma is not present in 23 to 33% or ureteral leak cases.79,80
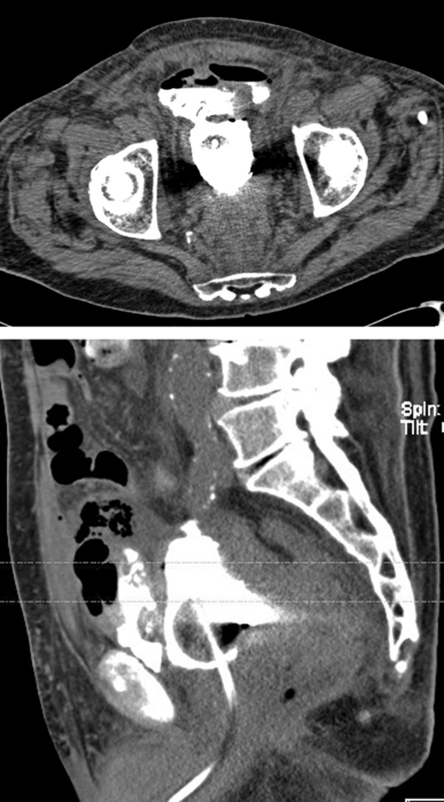
Figure 3.
(A,B) Axial and sagittal computed tomography cystogram images clearly show bladder rupture with extravasation into the anterior urinoma in this posttrauma patient.
Beyond identifying the urinoma itself, it is even more important to identify the source of the leak. Although contrast-enhanced CT may be able to identify the source of the leak, more-direct methods may be needed. Injection of contrast near the leak will likely better demonstrate the leak. So depending on the level of the leak, tests such as cystograms (or CT cystograms), retrograde ureterograms, or antegrade nephrostograms may be needed. In the renal transplant population, discovering the leak may still be difficult even with these more direct techniques. In these patients antegrade nephrostograms can fail to detect the leak in 17 to 43% of cases because of urinary obstruction frequent accompanies ischemic leakage from the ureter.79,80
Imaging alone is not always able to distinguish between a urinoma and other types of fluid collections. Aspiration of the fluid can make that distinction by allowing measurement of the creatinine level in the fluid and comparing it to the serum creatinine. Seroma fluid will have creatinine level comparable to the serum creatinine, but the creatinine in urinoma fluid will be significantly higher than serum creatinine. Aspiration for culture is also particularly useful in the renal transplant population because up to 60% or urinomas may be infected in these patients81; yet at the same time, they may not manifest symptoms of infection because of their immune suppression.
TREATMENT
Typically, the most important treatment is to deal with the underlying condition that led to the urinomas formation in the first place. Thus, nephrostomy and diversion or stenting is needed in cases caused by leakage without obstruction such as in traumatic injuries or postoperative anastomotic dehiscence. The diversion of urine away from the defect needs to be maintained until the urothelium heals over the hole. Diversion not only facilitates healing of the rent in the system, but also prevents further flow out into the urinoma (Fig. 4). Hence, if the urinoma is small, it may not need to be drained separately. The urine may reabsorb as long as further flow into the urinoma has been eliminated.
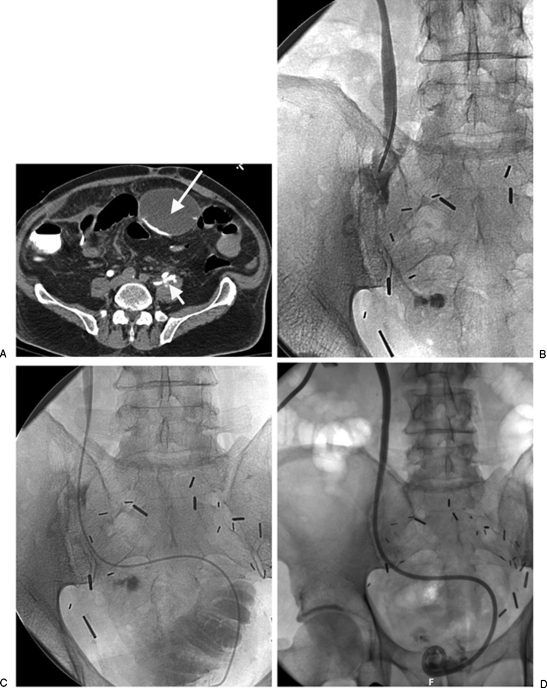
Figure 4.
(A) The patient is postcystoprostatectomy with ileal neobladder formation with leak from a ureteral injury. The computed tomography scan shows both local extravasation (short arrow) from the ureter as well as tracking of contrast into a more remote anterior urinoma (long arrow). (B) Direct injection in the ureter from a catheter introduced through a percutaneous nephrostomy show the periureteric extravasation. (C) An angiographic catheter was able to be manipulated past the ureteral leak into the neobladder. An internal-external nephroureteral catheter was then placed to provide diversionary drainage. (D) Follow-up nephrostogram just 2 weeks later shows no further extravasation from the ureter.
If the urinoma is caused by a ureteral transection or large tear, it may be difficult to get a stent across the area of injury because guidewires may tend to just pass out into the urinoma. In that setting a rendezvous technique can be used where guidewires from above and below are passed into the urinoma, one wire is exchanged for a snare, which is then used to grab the other wire and pull it out, thus yielding continuous wire access across the leak.82,83
In cases of renal trauma where the urine leak is caused by devitalized tissue, healing may not occur despite adequate drainage. In the past, this has been managed with surgical removal of the devitalized tissue. However, this can be managed less invasively by arterial embolization of the portion of renal parenchyma supplying the leaking region of kidney.84,85 This allows greater preservation of renal tissue than could be achieved with partial nephrectomy.
When obstruction of the urinary system causes the urinomas, the obstruction needs to be relieved. For benign, treatable conditions (such as posterior urethral valves), fixing the underlying problem may be all that is needed. However, when the obstruction cannot be easily fixed (as with malignant ureteral obstruction or intractable anastomotic stricture), then again nephrostomy or ureteral stenting is needed to provide drainage.
Percutaneous drainage of the urinoma itself is not always necessary because spontaneous reabsorption can occur if the leakage has stopped. Assuming the source of the urinoma can heal spontaneously, conservative management may be all that is required (Fig. 5). Pain medication and observation was all that was needed in two of three urinomas that developed after radiofrequency ablation-assisted laparoscopic partial nephrectomy.76 Urinoma drainage is indicated in several situations. Fever or leukocytosis, suggesting that the urinoma is infected is good reason to proceed to drainage. The urinoma may need to be drained to reduce pain or pressure on adjacent structures.
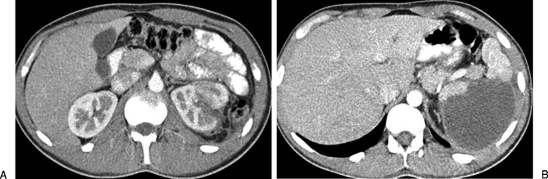
Figure 5.
(A) Computed tomography (CT) showing a left renal fracture in a patient who had a motor vehicle accident. (B) More cephalad slice of the CT showing a urinoma that resulted from the renal fracture. Despite the size of this urinoma, with conservative management the urinoma resolved without requiring drainage.
Needle aspiration of the urinomas may suffice if the underlying problem is sufficiently addressed.86,87 However, catheter drainage has advantages. A catheter can usually more completely drain the urinoma. Also having an indwelling catheter allows monitoring of output to determine if the underlying problem has been adequately treated. In addition, catheter placement maintains access into the collection until culture and fluid analysis has been completed. Drainage can be easily accomplished in most cases by ultrasound-guided placement of a Cope loop-type drain. Because these are often retroperitoneal in location, there is usually a clear window into the urinoma. Unless the urinoma is grossly infected, a small-bore 8- or 10-Fr catheter will suffice. Output from the drain should be monitored and the catheter removed when the output is consistently below 20 cc per day. If the underlying source of urine leakage is adequately controlled, complete drainage should be accomplished within a few days.
Percutaneous drainage and stenting is successful in sealing off urine leaks in anywhere from 36% to 100% of cases depending on the pathology involved and the series reported.70,88 When percutaneous techniques fail, surgery has usually been the next step to close the leak. There has also been a case reported in which a cyanoacrylate glue was percutaneously injected and used to close a persistent urine leak in a patient who was medically unfit for surgery.89 This technique is interesting and bears further investigation.
References
- McHugh K, Stringer D A, Hebert D, Babiak C A. Simple renal cysts in children: diagnosis and follow-up with US. Radiology. 1991;178(2):383–385. doi: 10.1148/radiology.178.2.1987597. [DOI] [PubMed] [Google Scholar]
- Hartman D S, Weatherby E, III, Laskin W B, Brody J M, Corse W, Baluch J D. Cystic renal cell carcinoma: CT findings simulating a benign hyperdense cyst. AJR Am J Roentgenol. 1992;159(6):1235–1237. doi: 10.2214/ajr.159.6.1442390. [DOI] [PubMed] [Google Scholar]
- Israel G M, Bosniak M A. An update of the Bosniak renal cyst classification system. Urology. 2005;66(3):484–488. doi: 10.1016/j.urology.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Lang E K. Renal cyst puncture studies. Urol Clin North Am. 1987;14(1):91–102. [PubMed] [Google Scholar]
- Mosli H, MacDonald P, Schillinger J. Caliceal diverticula developing into simple renal cyst. J Urol. 1986;136(3):658–661. doi: 10.1016/s0022-5347(17)45007-5. [DOI] [PubMed] [Google Scholar]
- Grantham J J. Pathogenesis of renal cyst expansion: opportunities for therapy. Am J Kidney Dis. 1994;23(2):210–218. doi: 10.1016/s0272-6386(12)80974-7. [DOI] [PubMed] [Google Scholar]
- Patel K, Caro P A, Chatten J. Parapelvic renal cyst causing UPJ obstruction. Investigation by IVP, ultrasound and CT. Pediatr Radiol. 1988;19(1):2–5. doi: 10.1007/BF02388397. [DOI] [PubMed] [Google Scholar]
- Mishal J, Lebovici O, Bregman L, London D, Yoffe B, Sherer Y. Huge renal arteriovenous malformation mimicking simple parapelvic cyst. Clin Imaging. 2000;24(3):166–168. doi: 10.1016/s0899-7071(00)00207-2. [DOI] [PubMed] [Google Scholar]
- Kwon H S, Shin S J, Yun S N, Yang C W, Chang Y S, Bang B K. Renal artery aneurysm manifested as parapelvic cyst on abdominal sonography. Nephron. 1996;74(1):229. doi: 10.1159/000189313. [DOI] [PubMed] [Google Scholar]
- Amis E S, Jr, Cronan J J. The renal sinus: an imaging review and proposed nomenclature for sinus cysts. J Urol. 1988;139(6):1151–1159. doi: 10.1016/s0022-5347(17)42845-x. [DOI] [PubMed] [Google Scholar]
- Whelan T F. Guidelines on the management of renal cyst disease. Can Urol Assoc J. 2010;4(2):98–99. doi: 10.5489/cuaj.10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C R, Stewart P F, Jr, Breckenridge J W. Renal cyst rupture following blunt abdominal trauma: case report. J Trauma. 1995;38(1):28–29. doi: 10.1097/00005373-199501000-00008. [DOI] [PubMed] [Google Scholar]
- Papanicolaou N, Pfister R C, Yoder I C. Spontaneous and traumatic rupture of renal cysts: diagnosis and outcome. Radiology. 1986;160(1):99–103. doi: 10.1148/radiology.160.1.3715054. [DOI] [PubMed] [Google Scholar]
- Davis J M, III, McLaughlin A P. Spontaneous renal hemorrhage due to cyst rupture: CT findings. AJR Am J Roentgenol. 1987;148(4):763–764. doi: 10.2214/ajr.148.4.763. [DOI] [PubMed] [Google Scholar]
- Blakeley C J, Thiagalingham N. Spontaneous retroperitoneal haemorrhage from a renal cyst: an unusual cause of haemorrhagic shock. Emerg Med J. 2003;20(4):388. doi: 10.1136/emj.20.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y Y, Chen J D, How C K, Yen D H. Spontaneous perinephric hemorrhage from a hemorrhagic renal cyst. Intern Med. 2010;49(19):2189–2190. doi: 10.2169/internalmedicine.49.4021. [DOI] [PubMed] [Google Scholar]
- Zerem E, Imamović G, Omerović S. Simple renal cysts and arterial hypertension: does their evacuation decrease the blood pressure? J Hypertens. 2009 October 2009;27(10):2074–2078. doi: 10.1097/HJH.0b013e32832f1458. [DOI] [PubMed] [Google Scholar]
- Pearl M, Klein S. Simple renal cyst and hypertension. Ann Radiol (Paris) 1986;29(3-4):421–423. [PubMed] [Google Scholar]
- de Lichtenberg M H, Nielsen O S. Infected renal cyst simulating acute abdomen. Case report. Acta Chir Scand. 1989;155(2):135. [PubMed] [Google Scholar]
- Ohkawa M, Motoi I, Hirano S, Okasho A, Hisazumi H. Biochemical and pharmacodynamic studies of simple renal cyst fluids in relation to infection. Nephron. 1991;59(1):80–83. doi: 10.1159/000186523. [DOI] [PubMed] [Google Scholar]
- Fortner A, Taylor A, Jr, Alazraki N, Datz F L. Advantage of indium-111 leukocytes over ultrasound in imaging an infected renal cyst. J Nucl Med. 1986;27(7):1147–1149. [PubMed] [Google Scholar]
- Chicoskie C, Chaoui A, Kuligowska E, Dember L M, Tello R. MRI isolation of infected renal cyst in autosomal dominant polycystic kidney disease. Clin Imaging. 2001;25(2):114–117. doi: 10.1016/s0899-7071(01)00244-3. [DOI] [PubMed] [Google Scholar]
- Akinci D, Akhan O, Ozmen M, et al. Long-term results of single-session percutaneous drainage and ethanol sclerotherapy in simple renal cysts. Eur J Radiol. 2005;54(2):298–302. doi: 10.1016/j.ejrad.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Newhouse J H, Pfister R C. Renal cyst puncture. In: Anthasoulis C A, Pfister R C, Greene R E, Roberson G H, editors. Interventional Radiology. Philadelphia: Saunders; 1982. pp. 409–425. [Google Scholar]
- Sandler C M. Renal cyst puncture and percutaneous drainage of perirenal fluid. In: Kadir S, editor. Current practice of interventional radiology. Philadelphia: Decker; 1991. pp. 662–668. [Google Scholar]
- Lang E K. Coexistence of cyst and tumor in the same kidney. Radiology. 1971;101(1):7–16. doi: 10.1148/101.1.7. [DOI] [PubMed] [Google Scholar]
- Lang E K. The differential diagnosis of renal cysts and tumors. Cyst puncture, aspiration, and analysis of cyst content for fat as diagnostic criteria for renal cysts. Radiology. 1966;87(5):883–888. doi: 10.1148/87.5.883. [DOI] [PubMed] [Google Scholar]
- Ljungberg B, Holmberg G, Sjödin J G, Hietala S O, Stenling R. Renal cell carcinoma in a renal cyst: a case report and review of the literature. J Urol. 1990;143(4):797–799. doi: 10.1016/s0022-5347(17)40099-1. [DOI] [PubMed] [Google Scholar]
- Kleist H, Jonsson O, Lundstam S, Nauclér J, Nilson A E, Pettersson S. Quantitative lipid analysis in the differential diagnosis of cystic renal lesions. Br J Urol. 1982;54(5):441–445. doi: 10.1111/j.1464-410x.1982.tb13560.x. [DOI] [PubMed] [Google Scholar]
- Kavoussi L R, Clayman R V, Mikkelsen D J, Meretyk S. Ureteronephroscopic marsupialization of obstructing peripelvic renal cyst. J Urol. 1991;146(2):411–414. doi: 10.1016/s0022-5347(17)37809-6. [DOI] [PubMed] [Google Scholar]
- Lang E K. Renal cyst puncture and aspiration: a survey of complications. AJR Am J Roentgenol. 1977;128(5):723–727. doi: 10.2214/ajr.128.5.723. [DOI] [PubMed] [Google Scholar]
- Zelch J, Lalli A F, Stewart B H, Daughtry J D. Complications of renal cyst exploration versus renal mass aspiration. Urology. 1976;7(3):244–247. doi: 10.1016/0090-4295(76)90450-7. [DOI] [PubMed] [Google Scholar]
- Clayman R V, Surya V, Miller R P, Reinke D B, Fraley E E. Pursuit of the renal mass. Is ultrasound enough? Am J Med. 1984;77(2):218–223. doi: 10.1016/0002-9343(84)90694-6. [DOI] [PubMed] [Google Scholar]
- von Schreeb T, Arner O, Skovsted G, Wikstad N. Renal adenocarcinoma. Is there a risk of spreading tumour cells in diagnostic puncture? Scand J Urol Nephrol. 1967;1(3):270–276. doi: 10.3109/00365596709133549. [DOI] [PubMed] [Google Scholar]
- Jacobsson L, Lindqvist B, Michaelson G, Bjerle P. Fluid turnover in renal cysts. Acta Med Scand. 1977;202(4):327–329. doi: 10.1111/j.0954-6820.1977.tb16837.x. [DOI] [PubMed] [Google Scholar]
- Wahlqvist L, Grumstedt B. Therapeutic effect of percutaneous puncture of simple renal cyst. Follow-up investigation of 50 patients. Acta Chir Scand. 1966;132(4):340–347. [PubMed] [Google Scholar]
- Ozgür S, Cetin S, Ilker Y. Percutaneous renal cyst aspiration and treatment with alcohol. Int Urol Nephrol. 1988;20(5):481–484. doi: 10.1007/BF02550607. [DOI] [PubMed] [Google Scholar]
- Holmberg G, Hietala S O. Treatment of simple renal cysts by percutaneous puncture and instillation of bismuth-phosphate. Scand J Urol Nephrol. 1989;23(3):207–212. doi: 10.3109/00365598909180843. [DOI] [PubMed] [Google Scholar]
- Bean W J. Renal cysts: treatment with alcohol. Radiology. 1981;138(2):329–331. doi: 10.1148/radiology.138.2.7455112. [DOI] [PubMed] [Google Scholar]
- Reiner I, Donnell S, Jones M, Carty H L, Richwood A M. Percutaneous sclerotherapy for simple renal cysts in children. Br J Radiol. 1992;65(771):281–282. doi: 10.1259/0007-1285-65-771-281. [DOI] [PubMed] [Google Scholar]
- Raskin M M, Poole D O, Roen S A, Viamonte M., Jr Percutaneous management of renal cysts: results of a four-year study. Radiology. 1975;115(3):551–553. doi: 10.1148/15.3.551. [DOI] [PubMed] [Google Scholar]
- Pfister R C, Schaffer D. Percutaneous ablation of renal cysts. AJR Am J Roentgenol. 1979;132:1031. [Google Scholar]
- Gelet A, Sanseverino R, Martin X, Leveque J M, Dubernard J M. Percutaneous treatment of benign renal cysts. Eur Urol. 1990;18(4):248–252. doi: 10.1159/000463923. [DOI] [PubMed] [Google Scholar]
- Phelan M, Zajko A, Hrebinko R L. Preliminary results of percutaneous treatment of renal cysts with povidone-iodine sclerosis. Urology. 1999;53(4):816–817. doi: 10.1016/s0090-4295(98)00557-3. [DOI] [PubMed] [Google Scholar]
- Kim S H, Moon M W, Lee H J, Sim J S, Kim S H, Ahn C. Renal cyst ablation with n-butyl cyanoacrylate and iodized oil in symptomatic patients with autosomal dominant polycystic kidney disease: preliminary report. Radiology. 2003;226(2):573–576. doi: 10.1148/radiol.2262011574. [DOI] [PubMed] [Google Scholar]
- Choi Y D, Ham W S, Kim W T, et al. Clinical experience of single-session percutaneous aspiration and OK-432 sclerotherapy for treatment of simple renal cysts: 1-year follow-up. J Endourol. 2009;23(6):1001–1006. doi: 10.1089/end.2008.0137. [DOI] [PubMed] [Google Scholar]
- Demir E, Alan C, Kilciler M, Bedir S. Comparison of ethanol and sodium tetradecyl sulfate in the sclerotherapy of renal cyst. J Endourol. 2007;21(8):903–905. doi: 10.1089/end.2006.0462. [DOI] [PubMed] [Google Scholar]
- Egilmez H, Gok V, Oztoprak I, et al. Comparison of CT-guided sclerotherapy with using 95% ethanol and 20% hypertonic saline for managing simple renal cyst. Korean J Radiol. 2007;8(6):512–519. doi: 10.3348/kjr.2007.8.6.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Sakaguchi H, Anai H, et al. Sclerotherapy for simple cysts with use of ethanolamine oleate: preliminary experience. Cardiovasc Intervent Radiol. 2005;28(6):751–755. doi: 10.1007/s00270-004-0277-0. [DOI] [PubMed] [Google Scholar]
- Yoo K H, Lee S J, Jeon S H. Simple renal cyst sclerotherapy with acetic acid: our 10-year experience. J Endourol. 2008;22(11):2559–2563. doi: 10.1089/end.2008.0110. [DOI] [PubMed] [Google Scholar]
- Xu X X, Du Y, Yang H F, Zhang Q, Li Y, Zee C S. CT-guided sclerotherapy with ethanol concentration monitoring for treatment of renal cysts. AJR Am J Roentgenol. 2011;196(1):W78-82. doi: 10.2214/AJR.10.4671. [DOI] [PubMed] [Google Scholar]
- Fontana D, Porpiglia F, Morra I, Destefanis P. Treatment of simple renal cysts by percutaneous drainage with three repeated alcohol injection. Urology. 1999;53(5):904–907. doi: 10.1016/s0090-4295(98)00634-7. [DOI] [PubMed] [Google Scholar]
- Delakas D, Karyotis I, Loumbakis P, Daskalopoulos G, Charoulakis N, Cranidis A. Long-term results after percutaneous minimally invasive procedure treatment of symptomatic simple renal cysts. Int Urol Nephrol. 2001;32(3):321–326. doi: 10.1023/a:1017566723756. [DOI] [PubMed] [Google Scholar]
- Paananen I, Hellström P, Leinonen S, et al. Treatment of renal cysts with single-session percutaneous drainage and ethanol sclerotherapy: long-term outcome. Urology. 2001;57(1):30–33. doi: 10.1016/s0090-4295(00)00889-x. [DOI] [PubMed] [Google Scholar]
- Zerem E, Imamovíc G, Omerovíc S. Symptomatic simple renal cyst: comparison of continuous negative-pressure catheter drainage and single-session alcohol sclerotherapy. AJR Am J Roentgenol. 2008;190(5):1193–1197. doi: 10.2214/AJR.07.2867. [DOI] [PubMed] [Google Scholar]
- Camacho M F, Bondhus M J, Carrion H M, Lockhart J L, Politano V A. Ureteropelvic junction obstruction resulting from percutaneous cyst puncture and intracystic isophendylate injection: an unusual complications. J Urol. 1980;124(5):713–714. doi: 10.1016/s0022-5347(17)55623-2. [DOI] [PubMed] [Google Scholar]
- Peyromaure M, Debré B, Flam T A. Sclerotherapy of a giant renal cyst with povidone-iodine. J Urol. 2002;168(6):2525. doi: 10.1016/S0022-5347(05)64183-3. [DOI] [PubMed] [Google Scholar]
- Madeb R, Feldman P A, Knopf J, Rub R, Erturk E, Yachia D. Povidone-iodine sclerotherapy is ineffective in the treatment of symptomatic renal cysts. J Endourol. 2006;20(6):402–404. doi: 10.1089/end.2006.20.402. [DOI] [PubMed] [Google Scholar]
- Meng M V, Grossfeld G D, Stoller M L. Renal carcinoma after laparoscopic cyst decortication. J Urol. 2002;167(3):1396. [PubMed] [Google Scholar]
- Nieh P T, Bihrle W., III Laparoscopic marsupialization of massive renal cyst. J Urol. 1993;150(1):171–173. doi: 10.1016/s0022-5347(17)35426-5. [DOI] [PubMed] [Google Scholar]
- Morgan C, Jr, Rader D. Laparoscopic unroofing of a renal cyst. J Urol. 1992;148(6):1835–1836. doi: 10.1016/s0022-5347(17)37043-x. [DOI] [PubMed] [Google Scholar]
- Hulbert J C. Laparoscopic management of renal cystic disease. Semin Urol. 1992;10(4):239–241. [PubMed] [Google Scholar]
- Amar A D, Das S. Surgical management of benign renal cysts causing obstruction of renal pelvis. Urology. 1984;24(5):429–433. doi: 10.1016/0090-4295(84)90315-7. [DOI] [PubMed] [Google Scholar]
- Atug F, Burgess S V, Ruiz-Deya G, Mendes-Torres F, Castle E P, Thomas R. Long-term durability of laparoscopic decortication of symptomatic renal cysts. Urology. 2006;68(2):272–275. doi: 10.1016/j.urology.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Roberts W W, Bluebond-Langner R, Boyle K E, Jarrett T W, Kavoussi L R. Laparoscopic ablation of symptomatic parenchymal and peripelvic renal cysts. Urology. 2001;58(2):165–169. doi: 10.1016/s0090-4295(01)01145-1. [DOI] [PubMed] [Google Scholar]
- Hoenig D M, McDougall E M, Shalhav A L, Elbahnasy A M, Clayman R V. Laparoscopic ablation of peripelvic renal cysts. J Urol. 1997;158(4):1345–1348. [PubMed] [Google Scholar]
- Hulbert J C, Hunter D, Young A T, Castaneda-Zuniga W. Percutaneous intrarenal marsupialization of a perirenal cystic collection—endocystolysis. J Urol. 1988;139(5):1039–1041. doi: 10.1016/s0022-5347(17)42763-7. [DOI] [PubMed] [Google Scholar]
- Kang Y, Noble C, Gupta M. Percutaneous resection of renal cysts. J Endourol. 2001;15(7):735–738. discussion 738–739. doi: 10.1089/08927790152596343. [DOI] [PubMed] [Google Scholar]
- Heikkilä J, Taskinen S, Rintala R. Urinomas associated with posterior urethral valves. J Urol. 2008;180(4):1476–1478. doi: 10.1016/j.juro.2008.06.056. [DOI] [PubMed] [Google Scholar]
- Umbreit E C, Routh J C, Husmann D A. Nonoperative management of nonvascular grade IV blunt renal trauma in children: meta-analysis and systematic review. Urology. 2009;74(3):579–582. doi: 10.1016/j.urology.2009.04.049. [DOI] [PubMed] [Google Scholar]
- Boone T B, Gilling P J, Husmann D A. Ureteropelvic junction disruption following blunt abdominal trauma. J Urol. 1993;150(1):33–36. doi: 10.1016/s0022-5347(17)35390-9. [DOI] [PubMed] [Google Scholar]
- Bennett L N, Voegeli D R, Crummy A B, McDermott J C, Jensen S R, Sollinger H W. Urologic complications following renal transplantation: role of interventional radiologic procedures. Radiology. 1986;160(2):531–536. doi: 10.1148/radiology.160.2.3523596. [DOI] [PubMed] [Google Scholar]
- Alkibay T, Karaoğlan U, Gündoğdu S, Bozkirli I. An unusual complication of extracorporeal shock wave lithotripsy: urinoma due to rupture of the renal pelvis. Int Urol Nephrol. 1992;24(1):11–14. doi: 10.1007/BF02552110. [DOI] [PubMed] [Google Scholar]
- Portela L A, Patel S K, Callahan D H. Pararenal pseudocyst (urinoma) as complication of percutaneous nephrostomy. Urology. 1979;13(5):570–571. doi: 10.1016/0090-4295(79)90478-3. [DOI] [PubMed] [Google Scholar]
- Rajendran L J, Rao M S, Bapna B C, et al. Peripelvic extravasation and formation of perinephric urinoma after cystoscopy. Urology. 1980;16(2):199–201. doi: 10.1016/0090-4295(80)90085-0. [DOI] [PubMed] [Google Scholar]
- Oefelein M G. Delayed presentation of urinoma after radiofrequency ablation-assisted laparoscopic partial nephrectomy. J Endourol. 2006;20(1):27–30. doi: 10.1089/end.2006.20.27. [DOI] [PubMed] [Google Scholar]
- Testa A C, Gaurilcikas A, Licameli A, et al. Sonographic imaging of urinoma. Ultrasound Obstet Gynecol. 2009;33(4):490–491. doi: 10.1002/uog.6349. [DOI] [PubMed] [Google Scholar]
- Titton R L, Gervais D A, Hahn P F, Harisinghani M G, Arellano R S, Mueller P R. Urine leaks and urinomas: diagnosis and imaging-guided intervention. Radiographics. 2003;23(5):1133–1147. doi: 10.1148/rg.235035029. [DOI] [PubMed] [Google Scholar]
- Cullmann H J, Prosinger M. Necrosis of the allograft ureter—evaluation of different examination methods in early diagnosis. Urol Int. 1990;45(3):164–169. doi: 10.1159/000281700. [DOI] [PubMed] [Google Scholar]
- Smith T P, Hunter D W, Letourneau J G, et al. Urine leaks after renal transplantation: value of percutaneous pyelography and drainage for diagnosis and treatment. AJR Am J Roentgenol. 1988;151(3):511–513. doi: 10.2214/ajr.151.3.511. [DOI] [PubMed] [Google Scholar]
- Kinnaert P, Hall M, Janssen F, Vereerstraeten P, Toussaint C, Geertruyden J Van. Ureteral stenosis after kidney transplantation: true incidence and long-term followup after surgical correction. J Urol. 1985;133(1):17–20. doi: 10.1016/s0022-5347(17)48766-0. [DOI] [PubMed] [Google Scholar]
- Maillet P J, Pelle-Francoz D, Leriche A, Leclercq R, Demiaux C. Fistulas of the upper urinary tract: percutaneous management. J Urol. 1987;138(6):1382–1385. doi: 10.1016/s0022-5347(17)43648-2. [DOI] [PubMed] [Google Scholar]
- Anderson H, Alyas F, Edwin P J. Intra-urinoma rendezvous using a transconduit approach to re-establish ureteric integrity. Cardiovasc Intervent Radiol. 2005;28(1):95–97. doi: 10.1007/s00270-004-0051-3. [DOI] [PubMed] [Google Scholar]
- Horikami K, Matsuoka Y, Nagaoki K, et al. Treatment of post-traumatic urinoma by means of selective arterial embolization. J Vasc Interv Radiol. 1997;8(2):221–224. doi: 10.1016/s1051-0443(97)70544-x. [DOI] [PubMed] [Google Scholar]
- Pinto I T, Chimeno P C. Treatment of a urinoma and a post-traumatic pseudoaneurysm using selective arterial embolization. Cardiovasc Intervent Radiol. 1998;21(6):506–508. doi: 10.1007/s002709900313. [DOI] [PubMed] [Google Scholar]
- Spurlock J W, Burke T W, Dunn N P, Heller P B, Collins H S, Park R C. Calyceal rupture with perirenal urinoma in a patient with cervical carcinoma. Obstet Gynecol. 1987;70(3 Pt 2):511–513. [PubMed] [Google Scholar]
- Lang E K, Glorioso L., III Management of urinomas by percutaneous drainage procedures. Radiol Clin North Am. 1986;24(4):551–559. [PubMed] [Google Scholar]
- Kobayashi K, Censullo M L, Rossman L L, Kyriakides P N, Kahan B D, Cohen A M. Interventional radiologic management of renal transplant dysfunction: indications, limitations, and technical considerations. Radiographics. 2007;27(4):1109–1130. doi: 10.1148/rg.274065135. [DOI] [PubMed] [Google Scholar]
- Aning J J, Stott M A, Watkinson A F. Glue ablation of a late-presentation urinary fistula after partial nephrectomy. Br J Radiol. 2009;82(984):e246–e248. doi: 10.1259/bjr/93776392. [DOI] [PMC free article] [PubMed] [Google Scholar]