Abstract
Nephroureteral stents including antegrade, retrograde, or internal (double-J) stents are routinely placed by interventional radiologists. The purpose of this review is to provide a detailed and comprehensive description of indications, contraindications, technique, and various technical challenges of these procedures. Also pre- and postprocedure management of patients will be discussed including routine follow-up and dealing with potential complications.
Keywords: Antegrade, retrograde, nephroureteral stent, double J stent
RETROGRADE (TRANSILEAL CONDUIT) NEPHROURETERAL STENT PLACEMENT
A retrograde nephroureteral stent (RNUS) is a catheter placed in patients who have undergone surgical treatment, such as cystectomy with ileal conduit formation in which it exits from the conduit and extends retrograde to the renal pelvis. It is important to understand the anatomy in patients with noncontinent urinary diversion for urinary bladder cancer or other conditions of dysfunctional or nonfunctioning urinary bladders. An ileal conduit is created by anastomosing the ureters to a loop of bowel that is then secured to the anterior abdominal wall to allow for ostomy bag drainage.1 Up to 15% of patients develop complications in the form of stricture at the ureteroenteric junction causing obstruction. The importance of this is the eventual outcome of hydronephrosis and kidney loss in neglected patients.1,2,3 These situations can be treated by percutaneous techniques that have proved to be safer and less invasive than open surgical repair. Treatment success of ureteric strictures and subsequent hydronephrosis by percutaneous methods can reach up to 100%. In addition, placement of retrograde transileal conduit nephroureteral stents can be successful in 90 to 95% of cases.1,2,3
The RNUS insertion is a common procedure done in patients with urinary diversion to treat a variety of clinical situations such as ureteric strictures, ureteroenteric anastomotic strictures, and ureteric leaks and fistulas.4 The main contraindications faced are similar to those for the placement of a percutaneous nephrostomy (PCN) and obtaining access to the kidney; otherwise, if the patient already has percutaneous access to the kidney then usually no significant issues are faced except in situations where there is significant urinary infection or hematuria.
Special considerations should be taken into account if initial access to the kidney will be obtained and the technique and approach to the patient being considered for percutaneous nephrostomy is described in elsewhere in this issue. If the patient already has a PCN and this will be changed to a RNUS, then again the main concern will be to treat any significant urinary infection before attempting the exchange to avoid periprocedural urosepsis. In addition, it is worthwhile to delay the exchange until the urine has been cleared of blood so that the newly placed catheter does not become clogged with small clots. Patients should be worked up for sedation because usually these types of procedures carry significant discomfort and pain that does require significant amounts of sedation.
Placement of a RNUS can be performed by two main methods. The first method is placement of the stent from an antegrade approach by obtaining a direct access to the pelvicaliceal system. This is followed by placement a nephroureteral stent, once successfully negotiating the ureteral segment and entering the conduit. Usually, this is the preferred approach when initial RNUS placement is required. This could be performed as both a single or multistage procedure. The second method is to place the RNUS through the ileal conduit in a retrograde fashion without obtaining access to the pelvicalyceal system.
Antegrade Placement of a Retrograde Nephroureteral Stent
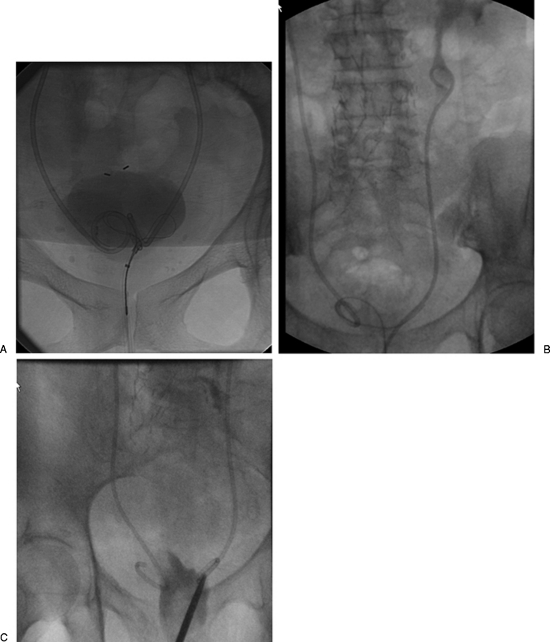
If the patient has a PCN, this can be converted to a RNUS. However, if the patient does not have a PCN, then antegrade access should be performed first. The patient can be placed initially in a lateral oblique position or can be rotated from a prone position after obtaining initial access to the pelvicaliceal system. The lateral oblique position allows simultaneous access to the flank and conduit stoma, which is typically created in the lower abdominal quadrant. The renal collecting system is accessed in a standard manner and contrast is injected providing visualization of the urinary tract to the ileal conduit. The injected contrast should not be dense thus obscuring the catheter, which can make manipulation difficult. A guidewire is advanced through the collecting system and manipulated using a catheter down the ureter and out the stoma (usually a multipurpose catheter-MPA can be used). A wire with a floppy end such as a Bentson or an angled-tip hydrophilic wire can be used. Once the wire is out through the stoma providing through and through access (“body floss” technique) tension should be maintained on both ends of the wire and a retrograde catheter can be advanced through the stoma to form the loop within the renal pelvis. The wire can be removed once the catheter is adequately positioned (Fig. 1). If there is a need to leave behind a PCN then the wire can be pulled back through the PCN skin puncture site and used to place a PCN. Adequate positioning of the retrograde stent is confirmed by injection of the stent with contrast.
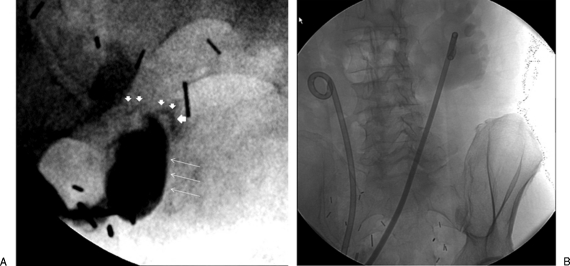
Figure 1.
Patient in prone position with ileal conduit has a right retrograde nephroureteral stent (RNUS) and a left percutaneous internal/external nephroureteral (PCNU) that is to be exchanged for a RNUS. (A) Shows right RNUS and left PCNU. (B) The patient is in lateral oblique position, the left PCNU has been removed over a wire and contrast injection shows left hydronephrosis and a stricture (arrows) in the lower end of the left ureter. (C) Final image shows the final RNUS with a loop formed within the renal pelvis (white arrow).
In patients who do not have a PCN all steps of the above-described method can be performed in one treatment session. However, if there is evidence of infection or significant hematuria then it is preferable to first attempt placement of a PCN, treat with antibiotics until infection or hematuria clears, then bring the patient back at a later date to exchange the PCN to a retrograde stent.
In this group of patients (with no PCN) who have severe obstruction and a tortuous ureter, it can be difficult to place the retrograde stent in one session. Usually these patients need a PCN to be placed first and then allow the collecting system and ureter to decompress for a few days or weeks and reattempt insertion of the retrograde stent later. Usually when the system decompresses, there is less tortuosity in the ureter and also helps for the edema at the site of the ureteric injury or stricture to resolve, which will greatly facilitate guidewire traversal.2,3
Retrograde Placement of a Retrograde Nephroureteral Stent
The second retrograde method is a one-step approach, which includes direct placement of the stent through the ileal conduit. The ileal conduit is opacified by contrast that is allowed to reflux through the ureteroenteric junction leading to visualization of the ureter (Fig. 2). Success rates have been reported to range from 14% to 86% of patients.2,4,5 This can be performed either by inserting a Foley catheter or an angle tip catheter, such as a Kumpe, into the ileal loop. A glidewire is introduced through the catheter to access the ureteroenteric anastomosis and advanced up the ureter. The catheter is advanced into the renal pelvis and exchanged for a stiff guidewire. A nephroureteral catheter can then be advanced over the wire and its loop is formed within the renal pelvis. The distal end of the catheter can be cut to an appropriate length and left within the stoma bag for drainage.
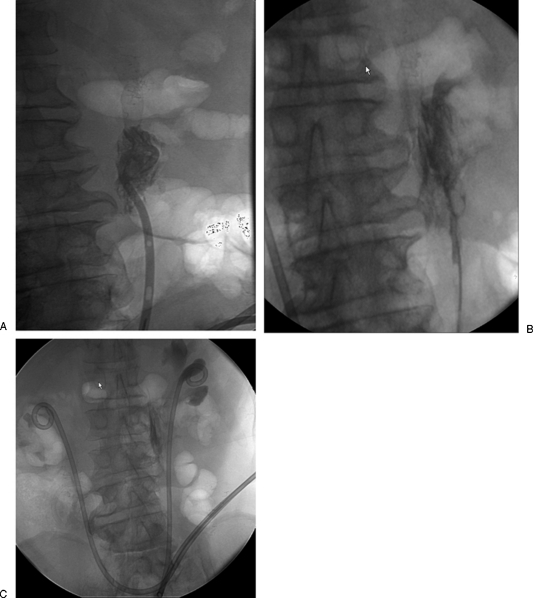
Figure 2.
Patient with ileal conduit who had both retrograde nephroureteral stents (RNUS) accidently removed. (A) Contrast was injected into the ileal conduit (long arrows) which allowed visualization of the lower ends of the ureters (small white arrows) that appear as thin streaks of contrast consistent with lower ureteric strictures. (B) Final image after replacement of RNUSs bilaterally.
This technique is very well tolerated by patients and is relatively safe because it does not include risks of bleeding and renal injury that can complicate initial PCN placement. However, in cases where primary retrograde access is required, the stricture can be severe enough to make this approach not feasible. Also, in patients who need stent placement to treat urine leak or ureteral injury, RNUS placement can be challenging because tissues can be friable hindering access to the ureteroenteric anastomosis. In addition, one can face a situation in which the guidewire will exit from the site of leakage (either at the ureteroenteric anastomosis or in the ureter proper) and making it near impossible to reestablish continuity to the ureter at a more cephalad level. In these situations, one will need to resort back to the first antegrade approach that was described above. The retrograde approach is more preferable and successful in patients who have had a stent that became dislodged.4
General Technical Considerations
Placing bilateral ureteral stents should be considered in patients with fistulous communication between the ileal conduit and surrounding bowel or urinary leak (because sometimes it is very difficult to precisely identify point of leakage), and obviously in cases with bilateral ureteric strictures.4 Occasionally, passing the wire from above and trying to exit the stoma can be very challenging and might require inserting a snare through the stoma to retrieve the wire from the ileal conduit.4
Some centers use a pigtail configuration catheter with a locking (retention) suture. However at our institution we do not use a catheter with a locking suture because encrustations within the catheter can inhibit the release of the locking suture after cutting the catheter, thus causing the exchange of the catheter to be challenging and painful for the patient. In our practice, we find it more convenient to use catheters without a locking suture that can remain secure without significant risk of migration or dislodgement. We typically use catheters with locking sutures only for patients who repeatedly displace their catheters for the theoretical improved stability of the catheter in the presence of a locking suture.
Internal (Double-J) versus Retrograde (RNUS) Stents in Patients with Ileal Conduits
Some authors have described dilatation of ureteric strictures using balloons followed by placement of internal (double-J) stents with the distal loop formed within the ileal conduit. The success rate reported was up to 90% with no major complications on follow-up. None of the patients reported major discomfort on follow-up. The stents were exchanged at 3- to 6-month intervals.1
Others have found the pigtail configuration (RNUS) to be superior to the double-J stents for several reasons. The pigtail nephroureteral stent provides drainage directly into the stoma bag, thus minimizing urine stasis within the ileal conduit, which in turn decreases ureteric reflux and possible ascending infection. Direct contact between the end of the stent and mucus in the ileal conduit is also reduced thus minimizing mucus-related tube malfunction. Additionally, pigtail nephroureteral stents' risk of occlusion is less because 10-French (Fr) or even up to 16-Fr catheters can be used as compared with double-J stents which are sized at 8 Fr. Finally, the exchange of pigtail catheters is technically easier than exchanging double-J stents, as the external presenting part in the stoma bag is readily accessible.3
POSTPROCEDURE
After stent placement, the patient should be closely monitored per hospital protocol. Some authors advise bed rest for 4 hours until any hematuria clears. Antibiotics should be continued, especially if the urinary tract appears to be grossly infected during the procedure. An appropriate antibiotic regimen should then be continued if cultures are positive according to the results and antibiotic sensitivity. One should be ready to replace fluids, adjusted according to urinary output, especially immediately after the procedure. This is important when the procedure is performed to relieve obstruction, in which profuse postobstructive diuresis is expected. Blood-tinged urine may be expected in the first 48 hours. Gross hematuria can be related to initial PCN placement.
Retrograde Ileal Conduit Stent Exchange
The stents should be exchanged at regular intervals to avoid occlusion of the catheter with encrustations. Usually an exchange can be performed at an interval of every 6 to 8 weeks and then can be increased up to 12 weeks if the patient does not develop significant catheter occlusion. Some patients will need an exchange at a shorter time interval. Some authors reported a duration of catheter exchange up to 16 weeks.3 Exchange can be performed as an outpatient procedure that may require sedation depending if the patient has more discomfort than can be managed by local anesthetic. This can be determined during the first exchange, which will indicate how well the patient will tolerate the procedure.
TECHNIQUE
The patient is placed supine on the table. The ileal conduit is cleaned using povidone iodine, and the patient is covered with a sterile drape leaving the stoma exposed. The exchange needs to be done over a stiff wire such as an Amplatz. The catheter is injected with dilute contrast to visualize the renal pelvis and the wire is advanced under fluoroscopic guidance. One should avoid pushing the wire far without fluoroscopic guidance to avoid perforation of the renal pelvis (Fig. 3). The catheter is then exchanged over the wire for a new one. The catheter can be cut and shortened to an appropriate length to fit in the patient's ostomy bag.3
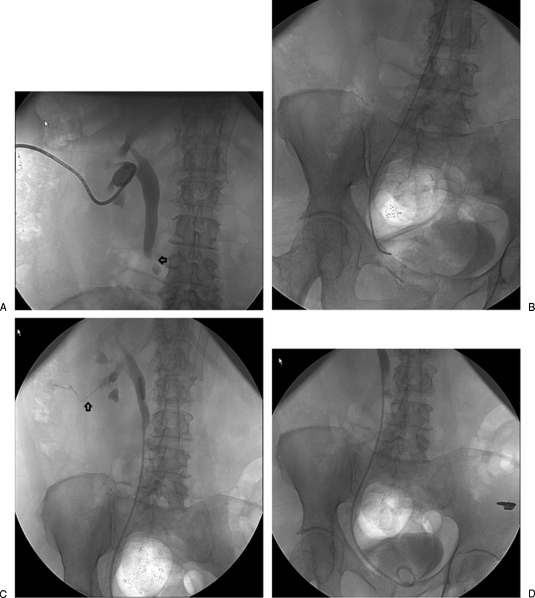
Figure 3.
Patient with ileal conduit and bilateral retrograde nephroureteral stent (RNUS) presenting for routine stent change. (A) Images after placement of the new stent reveal that the loop is retroperitoneal, most likely due to perforation by the catheter during catheter exchange. (B) The stent was removed over a wire and used in combination with an MPA catheter to reaccess the ureter. (C) Final image shows the new catheter with the loop within the pelvicaliceal system. There were no adverse clinical consequences after this exchange.
Sometimes, the catheter can be encrusted and it is not possible to pass a stiff wire through the catheter. In such a case, a hydrophilic wire can be used to exchange the catheter. It is passed up the catheter that is then removed. The wire can then be used to advance the new catheter or can be exchanged using a 5-Fr catheter for a stiffer wire. Then the new ureteral catheter can be advanced over the stiff wire. If the catheter is totally occluded thus not allowing passage of any wire, a modification may help. A suture (0-Prolene) can be placed on the exiting end of the catheter and then a peel-away sheath can be advanced over both the suture and the catheter to the level of the loop. The encrusted stent can then be pulled out, and contrast injected through the peel-away sheath to opacify the renal collecting system. A wire can then be advanced through the peel-away sheath, which is then exchanged over the wire for a new RNUS.6 Caution should be taken when advancing either the wire through the catheter or even when advancing the new catheter over the exchange wire because the caudal aspect of the stents can have a suboptimal curvature, with the initial direction inferior and then superior toward the kidney. This can cause the catheter or the wire to buckle into the ileal conduit, resulting in lost access to the ureter. This can be avoided by passing the wire under continuous fluoroscopic guidance. If the catheter starts to buckle, then a wire that is less stiff or a hydrophilic wire can be used for the exchange. Sometimes, it can be very difficult to advance the retrograde catheter or even impossible due to a severe ureteric stricture. In such cases, balloon dilatation can be performed to allow placement of the catheter.2,3
NEPHROURETERAL STENT PLACMENT
This type of catheter provides drainage of the urinary system from the kidney to the urinary bladder. One of two catheter types can be used, which provides external/internal drainage—the antegrade percutaneous internal/external nephroureteral stent—or total internal drainage—the double-J stent.
ANTEGRADE PERCUTANEOUS INTERNAL/EXTERNAL NEPHROURETERAL STENTS
An antegrade percutaneous internal/external nephroureteral (PCNU) stent is placed percutaneously, establishing antegrade access to the kidney, ureter, and urinary bladder. A segment of the stent remains outside the patient from the flank, which can be capped or connected to gravity drainage. The internal/external catheter provides continuity between the kidney, ureter, and urinary bladder, which is useful in patients with ureteric obstruction, injury, fistulas or those undergoing ureteral surgery. This catheter is also used if there is an obstruction after extracorporeal shock wave lithotripsy (ESWL). It can be placed in both native and transplant kidneys. PCNU stents have side holes, some at the level of the renal pelvis to allow urine to drain distally and exit from side holes in the distal end of the stent. PCNU stents can provide internal drainage by capping the stents after confirming that the patient has a well-functioning urinary bladder. An advantage of maintaining the external portion of the stent is that it facilitates future exchanges and makes it easy to irrigate and flush the catheter to maintain patency. It also provides access to the urinary system for future interventions such as stent placement for a short period after stone removal or for ureteral dilatation. They are also indicated in patients in whom internal double-J stents exchange using cystoscopy is challenging, such as in cases of bladder disease or distortion, and neurologic or orthopedic disease.6
DOUBLE-J STENTS
Double-J stents are internal stents used to bypass an obstructed segment of the ureter and provide internal drainage. They have an advantage over the antegrade PCNU in that there is no external component that is cumbersome and can cause patient discomfort, and they have no risk of skin infection, catheter displacement, and lifestyle limitation.6 Another major advantage of a double-J stent is that it restores the normal route for urinary drainage through the urinary bladder and voiding. Usually these stents are placed in retrograde fashion by urologists utilizing cystoscopy; however, frequently percutaneous placement of double-J stents is required due to an inability to pass a ureteric stricture from the bladder.6,7
INDICATIONS
There are many common indications for PCNU and double-J stents with the latter being placed when an external component is not needed as detailed above. The internal ureteral stents are mostly reserved for patients with a native functioning urinary bladder. Urinary bladder function has to be considered with both devices when planning to reestablish drainage into the bladder.
The indications for stent placement are as follows:
For relief of an obstruction whether benign (iatrogenic, inflammatory) or malignant.
In conjunction with stone therapy in situations where ESWL or intraluminal lithotripsy will be performed or if there is an obstruction.
In certain operative situations where ureteral interventions are to be performed that will require perioperative stent placement as part of management, such as in cases requiring alignment of drainage elements, maintenance of luminal caliber, or for identification of ureters. A PCNU is usually used in such a situation.
To maintain ureter patency during healing or in cases of renal transplant with ureteric obstruction, injury, or leak.8 This helps to prevent the formation of a tight ureteral stricture during the healing phase.9 A PCNU is preferred in this case because of the ability to place larger caliber stents as opposed to double-J stents that cannot be upsized to the same extent.
In cases of ureteral injuries or leak (e.g., fistulas) in nontransplant kidneys, when the goal is to decompress the upper urinary tract and to restore the continuity of the ureter.
CONTRAINDICATIONS
Patients with urinary bladder outlet obstruction, bladder fistulas, or spastic/noncompliant bladder are not candidates for internal drainage. Uncorrectable coagulopathy is a contraindication.8 A neurogenic bladder is also a situation in which one would avoid stents because of the chronic high pressure in the bladder with a propensity for subsequent reflux.7
PREPROCEDURE: PATIENT SELECTION AND PREPARATION
Patients usually require sedation for insertion of ureteral stents. Special considerations should be taken into account if initial access to the kidney is to be obtained. In patients with a previously placed PCN, a change to a PCNU or a double-J stent can be performed after treating urinary infection if present and ensuring that the urine is clear from blood. Patients should be evaluated for any contraindications such as urinary bladder outlet obstruction, fistulas, or incontinence.
TECHNIQUE
PERCUTANEOUS INTERNAL/EXTERNAL NEPHROURETERAL STENT
An antegrade study (nephrostogram) should be performed of the pelvicaliceal system and the ureter with dilute contrast (Fig. 4) to study the morphology and anatomy of the lesion.9 The placement of a PCNU can be performed in one or two steps, depending on the degree of the obstruction and if the system is infected.6 Upon obtaining initial access to the kidney, it is worth while keeping in mind the need for future exchange to an antegrade nephroureteral stent because it is prudent to access the collecting system through an upper pole or interpolar calyx because the favorable angles help to direct the pushing forces toward the ureteropelvic junction and down the ureter.7 If the obstruction is chronic and severe, marked tortuosity of the ureter can occur making placement of the PCNU in one step difficult. Even if a wire was advanced down the tortuous ureter it can be near impossible to advance the catheter over the wire. In this case, it is better to place a PCN in the initial step and decompress the system for a few days or even weeks and then attempt exchanging the PCN later for a PCNU. There are several causes that can result in a very tortuous course to the ureter such as severe obstruction, massive ascites, or a pelvic mass displacing the ureter. Even after decompressing the system it can be challenging to advance the catheter down the ureter, which can be dealt with by advancing the wire into the bladder and then through the urethra possibly by snaring it out to get a through-and-through access. Gentle tension on the wire can straighten it out and make it easy to advance the catheter into the urinary bladder.6
Figure 4.
Patient has a left percutaneous internal/external nephroureteral (PCNU) stent that is to be changed to a double-J stent. (A) A contrast study shows ureteropelvic junction obstruction. (B) A catheter has been successfully manipulated down the ureter into the urinary bladder. The catheter position is confirmed by contrast injection. (C) Final image after a double-J stent insertion showing a proximal loop formed within the renal pelvis. The small arrow indicates a tractogram that clearly identifies a well-formed tract between the kidney and the skin. (D) The distal loop is adequately formed within the urinary bladder.
Converting a Nephrostomy Tube to a Nephroureteral Stent
The first steps are common between the placement of a PCNU and a double-J stent. The PCN is removed over a floppy wire such as a Bentson wire that is coiled within the renal pelvis. Then a catheter is advanced over the wire and used to direct the wire down the ureter and into the urinary bladder. If this is not possible, sometimes converting to a hydrophilic wire can facilitate this. Caution should be taken while using low surface friction wires that can easily perforate the ureter. After having the wire in the urinary bladder, it can be exchanged for a stiffer wire such as an Amplatz wire over which the catheter can be advanced. It is important that once the initial catheter is in the bladder, to inject a small amount of contrast to confirm position within the urinary bladder to verify that the wire did not dissect extraluminal (Fig. 5). Before removing or exchanging the floppy wire for the stiff one, it can be used to measure the distance between the renal pelvis and the urinary bladder where the distal loop to be located. This is done by placing the tip of the Bentson wire where the distal loop is to be located within the urinary bladder and bending the wire at the skin entry site. Then the wire is withdrawn up to where the proximal-most side is within the renal pelvis and another bend in the wire is made. The distance between the two bends can be measured and used to guide the distance between the proximal and (renal pelvis) and distal (bladder) sideholes.7 Regarding placement of a PCNU, there are two types of catheter that are mostly used at our institution. One of them has an additional proximal-looking loop that is formed within the renal pelvis. These catheters come in different lengths; the distance from the renal pelvis to the bladder determines which one to use. Another type of catheter that is used is a straight catheter, which requires additional side holes that must be cut into the tubing. The proximal-most sidehole is placed based on the distance from the renal pelvis to the bladder.
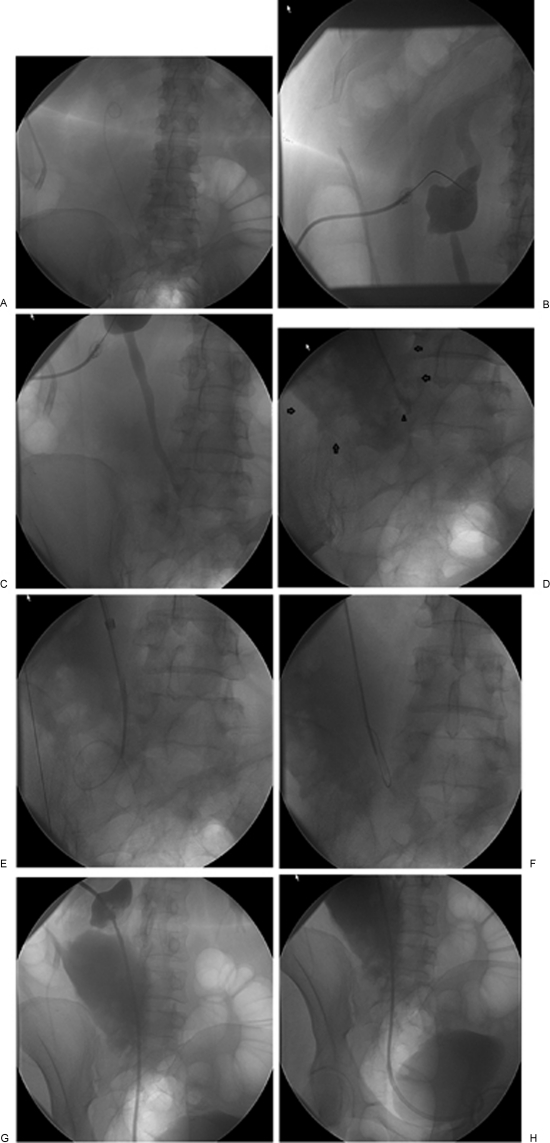
Figure 5.
A 55-year-old woman after surgery for excision of a small tumor over the left ureter with subsequent complete ureteral transection and continuous urinary leak into the peritoneum. The patient has a double-J stent on the left side. (A) Snaring of the double-J stent through the urinary bladder was performed, which revealed a transection of the double-J stent. (B) The cephalad end of the double-J stent was used as a target for accessing the pelvocaliceal system. (C,D) Complete transection of the ureter (arrowhead) with a large urinoma (arrows). (E) Multiple attempts to cross the lesion were unsuccessful and the wire repeatedly entered the urinoma. (F) A hydrophilic wire was used to make a loop and was advanced while maintaining the loop into the distal end of the ureter and finally into the bladder. (G,H) Final images shows the percutaneous internal/external nephroureteral (PCNU) stent in good position (no side holes were created at the level of the ureteric injury).
After advancing your catheter over the stiff wire, its position should be accurately confirmed by contrast injection such that the proximal-most side hole is within the renal pelvis. In cases with ureteral leak, adding side holes at the level of the leak should be avoided. In some cases where crossing ureteric lesions can be very challenging and difficult, filling the urinary bladder with saline can facilitate guidewire passage because it allows for better wire access in the bladder and may change anatomic relationships with the ureter.7
In cases with highly tortuous ureters, maneuvering the catheter down the ureter into the urinary bladder may require advanced skills. The tip of the catheter should be manipulated to the first curve and a floppy tipped wire such as a Bentson wire advanced, which buckles off the wall of the ureter and redirects the tip of the catheter downwards. This can be repeated curve by curve. In addition, the patient can perform a deep expiration that may help to straighten out tortuosity of the ureter.9
Crossing an obstruction can also be very challenging. One of the described techniques is to advance the catheter just proximal to the occlusion and then rotate the tip of the catheter while repeatedly probing the site of obstruction with the wire tip until it is engaged, then manipulate the wire across the occlusion.9 This is encountered in cases with a fistula, where crossing it results in the repeated exit of the wire through the fistula. If much time is spent trying to cross such lesions, a difficult case can become even more challenging due to worsening of ureteral edema. In such cases, it would be wise to abort the procedure and reattempt it after a few days.7
One technical challenge involves the dehiscence of the ureter. It might be possible, but difficult to advance your wire into the distal ureteral segment. If there is a urinoma or a hematoma, sometimes it is worthwhile to perform percutaneous aspiration of the fluid; this will decrease the splaying and separation of the ureter and facilitate passage of the wire. It may be impossible to advance the wire and reenter into the distal ureteral segment if there is complete dehiscence of the ureter. There are techniques to solve this problem. One way is to form a loop using a guidewire and then advancing the wire maintaining the loop so that it avoids further dissection and reenters the distal portion of the ureter beyond the lesion (Fig. 6). When the ureter is completely severed distally, the distance between the distal end of the ureter and the urinary bladder is short, and there are no interposed structures. In this situation, a renal curve catheter is advanced into the distal end of the ureter. Next, the urinary bladder is maximally distended with dilute contrast. By this, you are able to approximate the ureter and the urinary bladder. Next, the stiff end of a wire is advanced under fluoroscopic guidance into the urinary bladder. This access is then used to place the catheter. The uroepithelium readily bridges the defect and scar tissue replaces the absent muscular wall of the ureter.9,10
Figure 6.
Multiple images from different patients showing the steps of a double-J stent change. (A) An MPA-guiding catheter used to distend the bladder with dilute contrast and guide the snare to grab the distal end of the stent. (B) The distal end was snared and pulled out through the urethra. Notice the cephalad end of the double-J stent is maintained high within the ureter to avoid losing access to the ureter. The stent was injected with contrast to visualize the pelvicaliceal system to help in the proper positioning of the new stent. (C) Another patient where a McGill forceps was used to grab the stent.
INTERNAL (DOUBLE-J) STENTS
It is generally recommended to wait about one week between placement of the PCN and placement of an internal stent to decrease the risk of clot or debris occluding the lumen of the stent,9 although clinical judgment should be used and stents may be placed sooner even in the same setting of obtaining renal access in appropriate patients. In a patient with an antegrade PCNU, the catheter should be capped and allowed to drain internally for a few days; this will help to determine the patient's ability to tolerate internal drainage and his or her voiding ability. If the patient tolerates the capping trial, then it is possible to attempt converting the antegrade PCNU to a double-J stent.6
The first steps of placing a double-J stent are very similar to that of a PCNU. As discussed above, a stiff wire is placed through the ureter into the urinary bladder after obtaining the appropriate measurements (Fig. 4).7 Some operators then recommend placing a peel-away sheath over the guidewire into the ureter to facilitate advancing the double-J stent by decreasing any resistance encountered. Others dilate ureter strictures with 5-mm or 7-mm balloons if needed before placement of a double-J stent.7
Once a stiff wire is advanced to the urinary bladder, the double-J stent is mounted on the wire and advanced through the peel-away sheath. Make sure to untangle any loops within the proximal suture to avoid any difficulty in the final removal of the suture. Advance the double-J stent such that the distal tip is beyond the ureteric orifice of the bladder and retract the wire and the stiffener to form the distal loop within the bladder after removing the peel-away sheath. The proximal loop should form within the renal pelvis when the wire and the stiffener are removed proximally. You can reposition the stent at this point by applying gentle traction on the suture and pulling the stent up. Then cut one limb of the suture close to the skin and pull on the other end to remove it under fluoroscopic guidance. Sometimes the suture can be cut and removed before removing the wire and stiffener to avoid proximal migration of the stent during suture removal. The pusher can be used to place a floppy Bentson wire, which is subsequently used to place a PCN at the end of the procedure. An alternative is to initially place an additional safety wire when the PCN is removed at the beginning of the procedure using a sheath. This safety wire can be used to place a PCN at the end of the procedure if the pusher is displaced outside of the kidney. The PCN can be capped if the urine is clear and not bloody. The PCN can then be removed after 24 hours (when the patient tolerates the capping trial) under fluoroscopic guidance to avoid displacement of the double-J stent. An antegrade contrast study should be performed to confirm patency of the stent, and the PCN can be cut to unlock the locking suture and removed.7 If the PCN is entangled with the double-J stent it can be removed over a wire. If the antegrade study does not fill the urinary bladder, ask the patient to void completely and repeat the study to decrease any high pressure that could be in the urinary bladder.11
Patel et al evaluated 41 selected patients in whom double-J stents were placed (with initial renal access) without leaving a PCN behind and they had an 88% technical success rate and an 83% clinical success rate. They excluded patients with infection, coagulopathy, or emergency cases. In patients who underwent two-stage stent placement with leaving a PCN behind, they had a technical success rate of 100% and a clinical success rate of 98%. The difference in the major complication rate between the groups was not significant.12
It is very important to measure the double-J stent accurately to avoid irritation of the urinary tract and particularly to avoid the trigone of the urinary bladder.6,7,8 In a recently published study, a significant association was found between the length of the stent and crossing the midline of the distal end of the stent with stent-related symptoms such as urge incontinence. The authors suggest that selection of the proper length of double-J stents is the most important factor in minimizing stent-related symptoms.13
POSTPROCEDURE
Postprocedure management is similar to that discussed with retrograde stent placement, in addition to advising the patient of expected symptoms of bladder irritation and urinary frequency for a few days. Instructions should be given to the patient to uncap and reconnect the PCN to gravity drainage if he or she develops flank pain, leakage of urine around his or her PCN, significant hematuria, or fevers. The PCN can be removed after 24 hours if the patient remains asymptomatic and his or her creatinine remains stable.
COMPLICATIONS
Perforation of the renal collecting system or ureter can be generally avoided by careful and gentle handling of the guidewires and catheters, but if it does happen, it is usually of no consequence as long as access is maintained and the system is adequately drained at the end of the procedure.7 Up to 80 to 90% of patients develop irritative bladder symptoms; patients should be warned about them and advised as to what to expect. Some develop intolerable symptoms that may require early stent removal. Symptoms can vary from incontinence, hematuria, and pain (that can be in the suprapubic or loin region).13,14,15,16 Loin pain during voiding is usually related to vesicorenal reflux. The nature of the stents that act as a foreign body and subsequent epithelial irritation of the renal collecting system, ureter, and bladder may cause symptoms. Infection and pyuria can also result due to chronic irritation.15,16 Reflux from stents can cause ascending urinary tract infections; in addition, the stents can act as a nidus for bacterial colonization. This can be avoided by delaying stent insertion in patients with infection until the infection is treated and cleared.7
Hematuria is usually mild and caused by urothelial irritation. If more severe hematuria develops then cystoscopy may help identify causes such as erosion of the stent into a tumor.7 Rarely, massive hematuria can develop caused by ureteroarterial fistulas. This happens between the ureter and the common or internal iliac arteries. This is a serious condition and can lead to drastic outcomes; mortality can be up to 23%. Treatment options include stent grafting, embolization, and surgical repair.8,17,18,19
Delay in or neglecting to change a double-J stent can cause urinary crystalline components to deposit on both the inner and outer surfaces of the stent, which could lead to stent encrustation, malfunction, and possible retention. This could also make a stent exchange very difficult; a stent fracture can complicate removal.7,8,20 If a double-J stent that is too short is used and the distal loop is not adequately formed within the urinary bladder, the stent can in time retract into the ureter (“fish reeling”) and make future exchange or retrieval through the urinary bladder very difficult or impossible.8
EXCHANGE: TIMING, SEDATION, TECHNIQUE
TIMING
The stents should be exchanged at regular intervals to avoid occlusion of the catheter with encrustations. Usually PCNU exchange can be performed at an interval of every 6 to 8 weeks and then can be increased up to 12 weeks if the patient does not develop significant encrustations on the catheter. As regards to double-J stents, they can be exchanged every 4 to 6 months.21 Some patients will need an exchange at a shorter time interval. Double-J stents especially in males are mostly exchanged using cystoscopy; however, select female patients can have exchanges done using fluoroscopy alone, which we routinely do in our practice.
SEDATION
A PCNU exchange can be performed as an outpatient procedure that may require sedation if the patient has too much discomfort during the exchange. This can be determined during the first exchange, which will show how well the patient tolerates the procedure. As for double-J stents exchange, almost all patients needs sedation for catheter exchange and some patients require general anesthesia due to severe discomfort and pain during the exchange.21
TECHNIQUE
PCNU: The patient is placed prone on the table; the skin is prepped and draped in a sterile fashion. An exchange should be done over a stiff wire such as an Amplatz. The catheter is injected with dilute contrast to visualize the renal pelvis and bladder. The wire is advanced under fluoroscopic guidance and coiled within the bladder. The catheter is then exchanged over the wire for a new one. If the old catheter had additional side holes then similar holes should be created in the new stent. The catheter is then sutured in place with nonresorbable suture such as Prolene.
Double-J stent: The patient is placed supine on the table; the skin is cleaned using povidone iodine. The patient is covered with a sterile drape leaving the external genitalia exposed. Urethral analgesia is performed using lidocaine gel injected into the urethra. The gel is also applied to the catheters advanced into the bladder. An angiographic sheath can be inserted into the bladder and filled with saline and dilute contrast to facilitate the exchange. This stretches the bladder mucosal folds and prevents it from covering the stents. A snare is advanced into the bladder. A guiding sheath can be used to direct the snare in the urinary bladder to help grab the end of the stent (Fig. 6). Once the stent is snared, it is pulled out from the urethra. One should be very cautious to keep the cephalad loop of the stent within the ureter and at least above its midlevel. Contrast is injected into the stent to visualize the ureter and the pelvicaliceal system to facilitate the positioning of the new stent. An exchange can be done over a stiff wire such as an Amplatz. If it is not possible to grab the stent using a snare, a foreign-body retrieval forceps (Fig. 6) can be used.21
References
- Pappas P, Stravodimos K G, Kapetanakis T, et al. Ureterointestinal strictures following Bricker ileal conduit: management via a percutaneous approach. Int Urol Nephrol. 2008;40(3):621–627. doi: 10.1007/s11255-008-9349-4. [DOI] [PubMed] [Google Scholar]
- Alago W, Jr, Sofocleous C T, Covey A M, et al. Placement of transileal conduit retrograde nephroureteral stents in patients with ureteral obstruction after cystectomy: technique and outcome. AJR Am J Roentgenol. 2008;191(5):1536–1539. doi: 10.2214/AJR.08.1003. [DOI] [PubMed] [Google Scholar]
- Tal R, Bachar G N, Baniel J, Belenky A. External-internal nephro-uretero-ileal stents in patients with an ileal conduit: long-term results. Urology. 2004;63(3):438–441. doi: 10.1016/j.urology.2003.09.062. [DOI] [PubMed] [Google Scholar]
- Zaleski G X, Funaki B, Newmark G. Placement of retrograde nephroureteral stents through ileal conduits. AJR Am J Roentgenol. 1998;170(5):1275–1278. doi: 10.2214/ajr.170.5.9574600. [DOI] [PubMed] [Google Scholar]
- Drake M J, Cowan N C. Fluoroscopy guided retrograde ureteral stent insertion in patients with a ureteroileal urinary conduit: method and results. J Urol. 2002;167(5):2049–2051. [PubMed] [Google Scholar]
- Adamo R, Saad WEA, Brown D B. Management of nephrostomy drains and ureteral stents. Tech Vasc Interv Radiol. 2009;12(3):193–204. doi: 10.1053/j.tvir.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Hausegger K A, Portugaller H R. Percutaneous nephrostomy and antegrade ureteral stenting: technique-indications-complications. Eur Radiol. 2006;16(9):2016–2030. doi: 10.1007/s00330-005-0136-7. [DOI] [PubMed] [Google Scholar]
- Dyer R B, Chen M Y, Zagoria R J, Regan J D, Hood C G, Kavanagh P V. Complications of ureteral stent placement. Radiographics. 2002;22(5):1005–1022. doi: 10.1148/radiographics.22.5.g02se081005. [DOI] [PubMed] [Google Scholar]
- Lang E. Antegrade ureteral stenting for dehiscence, strictures, and fistulae. AJR Am J Roentgenol. 1984;143(4):795–801. doi: 10.2214/ajr.143.4.795. [DOI] [PubMed] [Google Scholar]
- Philiip W, Chao S G, Gordon D H, Glassberg K I. Percutaneous ureteroneocystotomy for treatment of postoperative distal-ureteral stricture. J Endourol. 1987;1(1):55–59. [Google Scholar]
- Rackson M E, Mitty H A, Dan S J, Train J S. Elevated bladder pressure: a cause of apparent ureteral stent failure. AJR Am J Roentgenol. 1988;151(2):335–336. doi: 10.2214/ajr.151.2.335. [DOI] [PubMed] [Google Scholar]
- Patel U, Abubacker M Z. Ureteral stent placement without postprocedural nephrostomy tube: experience in 41 patients. Radiology. 2004;230(2):435–442. doi: 10.1148/radiol.2302030078. [DOI] [PubMed] [Google Scholar]
- Ho CH, Tai HC, Chang HC, et al. Predictive factors for ureteral double-J-stent-related symptoms: a prospective, multivariate analysis. J Formos Med Assoc. 2010;109(11):848–856. doi: 10.1016/S0929-6646(10)60130-1. [DOI] [PubMed] [Google Scholar]
- Lennon G M, Thornhill J A, Sweeney P A, Grainger R, McDermott T E, Butler M R. ‘Firm’ versus ‘soft' double pigtail ureteric stents: a randomised blind comparative trial. Eur Urol. 1995;28(1):1–5. doi: 10.1159/000475010. [DOI] [PubMed] [Google Scholar]
- Joshi H B, Stainthorpe A, MacDonagh R P, Keeley F X, Jr, Timoney A G, Barry M J. Indwelling ureteral stents: evaluation of symptoms, quality of life and utility. J Urol. 2003;169(3):1065–1069. discussion 1069. doi: 10.1097/01.ju.0000048980.33855.90. [DOI] [PubMed] [Google Scholar]
- Joshi H B, Chitale S V, Nagarajan M, et al. A prospective randomized single-blind comparison of ureteral stents composed of firm and soft polymer. J Urol. 2005;174(6):2303–2306. doi: 10.1097/01.ju.0000181815.63998.5f. [DOI] [PubMed] [Google Scholar]
- Gallo F, Gastaldi E, Spirito G, Barile A, Kosir C, Giberti C. A case of iliac-artery-ureteral fistula managed with a combined endoscopic approach. Nat Clin Pract Urol. 2008;5(4):225–228. doi: 10.1038/ncpuro1059. [DOI] [PubMed] [Google Scholar]
- Darcy M. Uretro-arterial fistulas. Tech Vasc Interv Radiol. 2009;12(3):216–221. doi: 10.1053/j.tvir.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Bergqvist D, Pärsson H, Sherif A. Arterio-ureteral fistula—a systematic review. Eur J Vasc Endovasc Surg. 2001;22(3):191–196. doi: 10.1053/ejvs.2001.1432. [DOI] [PubMed] [Google Scholar]
- Rana A M, Sabooh A. Management strategies and results for severely encrusted retained ureteral stents. J Endourol. 2007;21(6):628–632. doi: 10.1089/end.2006.0250. [DOI] [PubMed] [Google Scholar]
- Chang RS, Liang HL, Huang JS, et al. Fluoroscopic guidance of retrograde exchange of ureteral stents in women. AJR Am J Roentgenol. 2008;190(6):1665–1670. doi: 10.2214/AJR.07.3216. [DOI] [PubMed] [Google Scholar]