Abstract
The kidney is the third most common abdominal organ to be injured in trauma, following the spleen and liver, respectively. Several classification systems convey the severity of injury to kidneys, ureter, bladder, and urethra. The most commonly used classification scheme is the American Association for the Surgery of Trauma (AAST) classification of blunt renal injuries, which grades renal injury according the size of laceration and its proximity to the renal hilum. Ureteral injury is graded according to its extent relative to the circumference of the ureter and the extent of associated devascularization. Bladder injury is graded according to its location relative to the peritoneum. Urethral injury is graded according to the extent of damage to surrounding anatomic structures. Although these classification schema may not be always used in common parlance, they do help delineate most important features of urologic tract injury that impact patient management and interventions.
Keywords: Kidney, trauma, embolization, interventional radiology, genitourinary
BACKGROUND
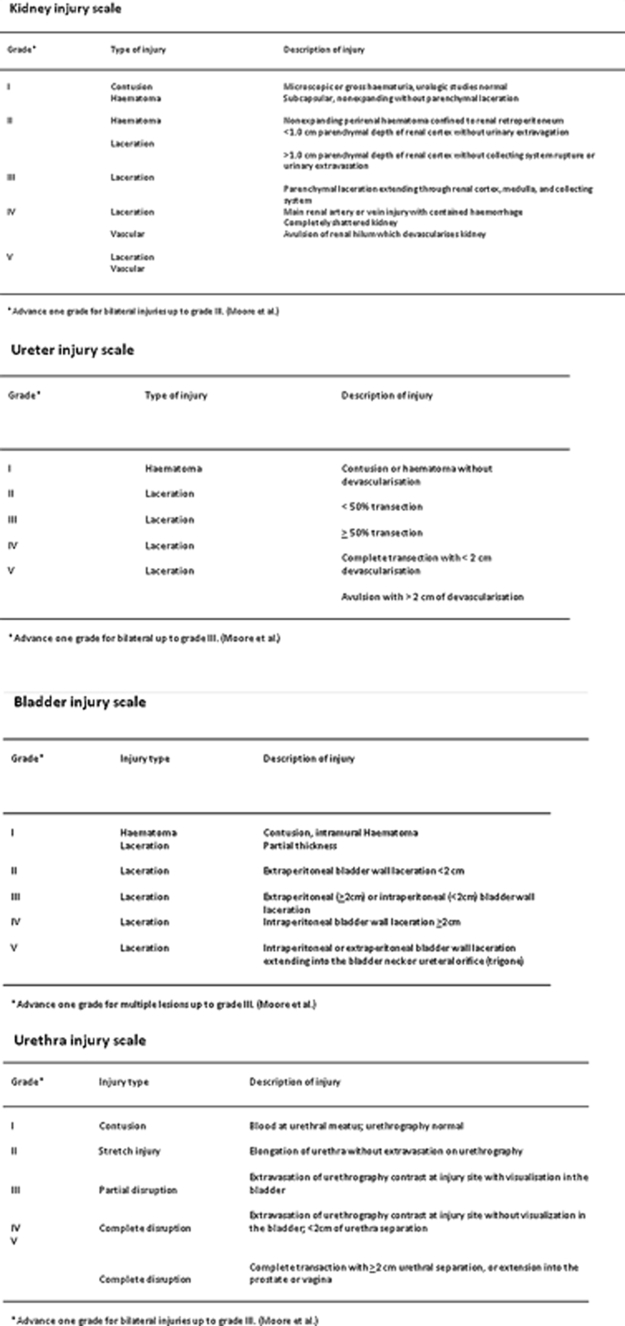
The kidney is the third most common abdominal organ to be injured in trauma, following the spleen and liver, respectively, occurring in ∼10% of cases of blunt and penetrating abdominal trauma.1 Several classification systems convey the severity of injury of kidneys, ureter, bladder, and urethra (Fig. 1). The most commonly used classification scheme is the American Association for the Surgery of Trauma (AAST) classification of blunt renal injuries, which grades renal injury according the size of laceration and its proximity to the renal hilum. Ureteral injury is graded according to its extent relative to the circumference of the ureter and the extent of associated devascularization. Bladder injury is graded according to its location relative to the peritoneum. Urethral injury is graded according to the extent of damage to surrounding anatomic structures. Although these classification schema may not be always used in common parlance, they do help delineate most important features of urologic tract injury that impact patient management and interventions.
Figure 1.
Synopsis of the grading scales for kidney, ureter, urinary bladder, and urethral traumatic injuries.
Traumatic injury to the urologic tract is more prevalent and is associated with higher mortality rates in certain patient populations. The prevalence of renal injury is higher in children than adults due to larger organ-to-body ratio of the pediatric kidneys, greater kidney mobility, and chest wall pliability.2 Although it has long been believed that congenital genitourinary anomalies negatively impacts the severity and morbidity associated with trauma, studies have shown that this belief is unsubstantiated.3 In contrast, complication rates, morbidity, and mortality are all increased in patients with preexisting end-stage renal disease who have sustained trauma.4,5 Therefore, children and patients with end-stage renal disease should be evaluated and treated for urologic tract trauma with a higher degree of scrutiny and caution.
RADIOLOGIC EVALUATION OF UROLOGIC TRAUMA
The radiologic evaluation of the kidneys and urinary tract in the context of trauma is guided primarily by the nature of the traumatic event and the patient's hemodynamic status. Any penetrating trauma in which direct injury to the kidneys and genitourinary tract is suspected should be evaluated by computed tomography (CT), with intravenous contrast whenever possible. In cases of blunt trauma, triphasic CT evaluation is warranted in trauma involving rapid deceleration (as in a motor vehicle collision), suspected multiple organ injuries, and in cases of hemodynamic instability—specifically, in patients who are hypotensive with a systolic blood pressure <90 mm Hg.6 This triphasic CT should include an unenhanced phase to identify internal hemorrhage, a postcontrast corticomedullary phase to identify abnormalities in renal parenchyma, vasculature, and perfusion, and a postcontrast delayed phase obtained 5 to 10 minutes after contrast injection to identify any breech within the urinary collecting system. In circumstances where cross-sectional imaging is warranted but CT is contraindicated, unenhanced magnetic resonance imaging (MRI) should be considered in stable pregnant patients, patients with renal insufficiency, and patients with an iodinated contrast allergy. Additional radiologic examinations should be considered for patients with pelvic fractures or clinical histories of injury to the urinary bladder and distal urethra. Conventional or CT cystography should be considered for patients presenting with pelvic fractures, especially those presenting with gross hematuria, suprapubic pain, and dysuria or inability to void. Retrograde urethrography should be performed for trauma patients presenting with blood at the urethral meatus or vaginal introitus, hematuria, dysuria or inability to void, or swelling in the scrotum, labia, or rectum.
The presence of microhematuria (>5 red blood cells per high-power field) in urinalysis plays a limited role in guiding radiologic evaluation of urologic trauma. Although hematuria may act as a harbinger of injury to the urologic tract, its absence does not exclude it. Hypotensive patients who have sustained blunt trauma should undergo CT evaluation whether or not microhematuria is present, as the degree of microhematuria is neither an absolutely sensitive nor proportionate indicator of urologic injury.6 Hemodynamically stable patients who have sustained blunt trauma pose a diagnostic imaging conundrum: although these patients generally have a low incidence of renal injuries, recent large-population studies have shown that focused assessment with sonography for trauma (FAST) scans have an overall low sensitivity in identifying traumatic injuries in this patient population; therefore, they are not an adequate tool for screening.7 The decision to perform a CT examination on hemodynamically stable patients who have sustained blunt trauma should be guided by patient history, trauma mechanism, physical exam findings, in addition to laboratory values and findings on radiographs.
RADIOLOGIC INTERVENTIONS FOR UROLOGIC TRAUMA
Renal Arterial Injury
Given the increasing alacrity and accuracy of CT angiography in the evaluation of abdominal trauma, the role of conventional angiography, endovascular interventions, and surgical interventions in renal trauma has diminished with time. One recent meta-analysis of the National Trauma Data Bank from 1991 to 2003 reported that 0.05% of all 945,326 blunt trauma admissions had injuries to the renal artery. Of patients who had injuries to the renal artery, 73% did not undergo surgical exploration, 18% underwent immediate nephrectomy, and 9% underwent surgical revascularization.8 There has been a progressive trend to treat traumatic renal arterial injury with conservative management only, diminishing the use of endovascular and surgical interventions.6 However, the delay of warranted endovascular or surgical inventions in patients with renal arterial injury may result in delayed hemorrhage, renal pseudoaneurysm formation, hematuria, hypertension, infection, and urinoma formation.2 Therefore, clinicians should decide from the outset of formulating patient management when intervention is warranted and not delay. Emergent conventional angiography may still be warranted in persistent hypotension associated with hematuria, evaluation with possible treatment of posttraumatic renal artery laceration or occlusion, treatment of arteriovenous fistula or pseudoaneurysm, and for preoperative evaluation.9 Nonemergent conventional angiography should be considered with uncorrectable anemia or delayed renovascular hypertension suspicious for Page kidney (i.e., renovascular hypertension from a compressive subcapsular hematoma).
The management of major renal artery injury in the context of blunt trauma remains a matter of debate. Hemodynamic instability, an expanding or pulsatile renal hematoma, and major renal arterial injury were once considered absolute indications for surgical exploration for potential nephrectomy.10 In the past decade, there has been a progressive trend toward initial conservative treatment in hemodynamically stable patients no matter how severe the renal arterial injury, with an increasing role of interventional radiology to supplant surgical exploration.6,11 There have been recent recommendations for hemodynamically stable patients with AAST grade 3 or higher injuries to undergo conventional angiography to offer possible therapeutic options that are less traumatic than surgical techniques.12 One recent study has reported complete success with preservation of kidney function in a group of 17 patients with grade 4 renal injuries, who were managed with endovascular arterial embolization.13 Similarly, another recent study has reported a 94.4% success rate with angiography, embolization through interventional radiology, and observation alone in patients with grade 4 and 5 renal injuries.14 The use of endovascular therapy in the context of grade 5 renal trauma and hemodynamic instability remains controversial, with conflicting reports of complete success15 or complete failure.9
The two primary endovascular therapies which are offered by interventional radiology are embolization and stenting of injured renal arteries. Superselective arterial embolization can be employed in cases of renal arterial laceration (Fig. 2), arteriovenous fistula, and pseudoaneurysm (Fig. 3) with embolic agents including gelatin sponge or stainless steel coils. Postembolization syndrome has been described in patients who have undergone endovascular embolization, presumably due to renal tissue ischemia invoking hyperpyrexia.16 Additional complications associated with endovascular embolization include ectopic coil placement, iatrogenic dissection, hypertension, and renal abscess.9 With respect to the management of renal arterial dissections, recent studies have supported the use of endovascular stent placement to treat traumatic intimal tears resulting in dissection and luminal stenosis,17,18 with one report advocating the use of directed thrombolytic therapy prior to the deployment of the vascular stent.19
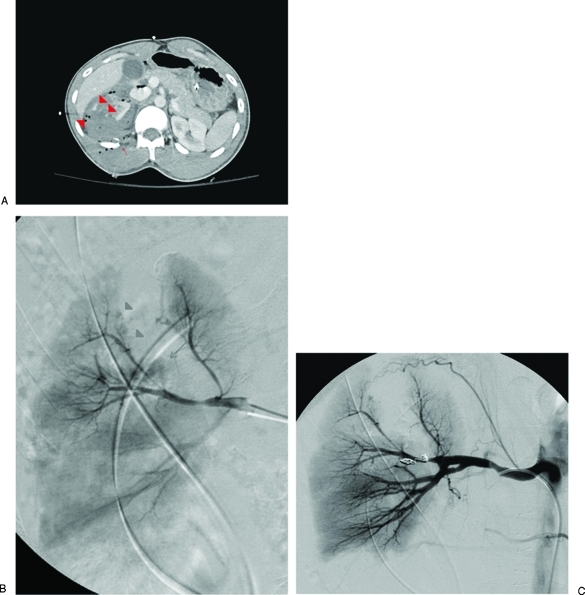
Figure 2.
Embolization of the right posterior segmental artery. (A) Computed tomography (CT) examination of a patient with a penetrating gunshot wound to the right flank shows a grade 4 laceration to the right kidney with multiple foci of active extravasation (arrowheads) as well as a comminuted right posterior 12th rib fracture (arrow). (B) Angiogram demonstrates active extravasation from a superior branch of the right posterior segmental renal artery (arrow), a large wedge-shaped defect corresponding to the renal laceration identified on CT, as well as tiny pseudoaneurysms abutting the margin of the laceration (arrowheads). (C) Successful coil embolization of the right posterior segmental renal artery. Focus of extravasation has largely resolved.
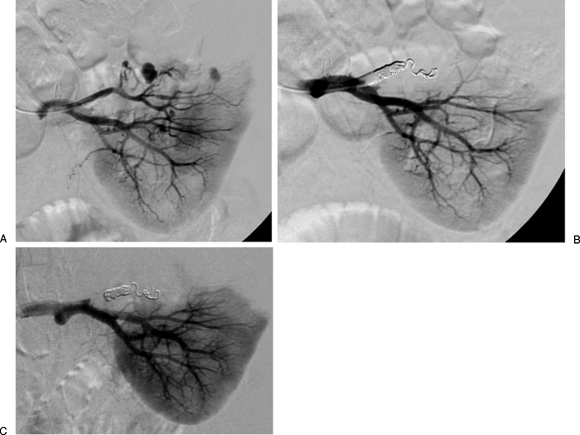
Figure 3.
Embolization of a left superior arterial pseudoaneurysm. (A) A 32-year-old woman underwent a partial left nephrectomy one week prior to the diagnostic angiogram that demonstrates at least three pseudoaneurysms arising from the left superior arterial branch supplying the resection margin. (B) Left superior renal artery was successfully embolized. (C) Follow-up angiogram was performed 2 days after the embolization procedure when the patient presented with dropping hemoglobin and hematocrit levels, though no evidence of extravasation was seen.
Urinary Collecting System Injury
Trauma resulting in urinary leakage, both within the renal parenchyma as well as the ureters, is common and is often iatrogenic in the context of abdominal surgery (Fig. 4). Urinary leakage places a patient at increased risk for the development of urinoma, perinephric abscess, and urinary fistula; such patients should be placed on prophylactic antibiotic therapy. Additionally, undiagnosed ureteral injury can potentially result in ureteral strictures. Imaging evaluation is always warranted when there is clinical suspicion of ureteral injury, as the incidence of hematuria in ureteral injury is only 17%.20 When there is a partial ureteral tear that is less than 50% of the circumference of the ureter, diverting nephrostomy and transureteral stenting are therapeutic options to redirect urinary flow away from the site of injury and hence to promote healing.20 Stents may be left in place for 8 to 12 weeks while the site of injury heals or subsequently undergoes surgical repair.
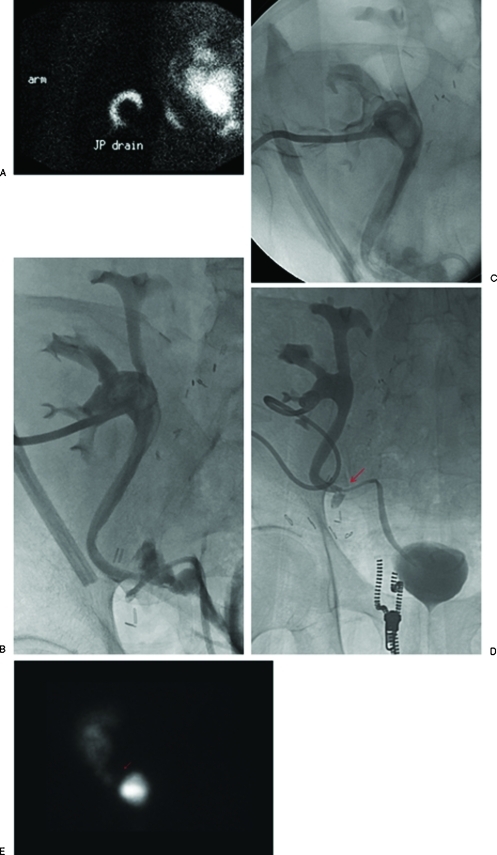
Figure 4.
Percutaneous nephrostomy catheter placement for urinary diversion in a 56-year-old man presenting with abdominal pain and anuria 2 weeks after renal transplantation. (A) Renal scan demonstrated tracer activity in the Jackson-Pruitt drain indicative of a urine leak. (B) Percutaneous nephrostomy catheter was successfully placed for urinary diversion. (C) Antegrade nephrostogram performed 2 days afterward demonstrates a moderate intraperitoneal leak at the ureteral anastomosis of the transplanted kidney. (D) Percutaneous nephrostomy tube was removed ∼3 months later when the leak was shown to be contained on nephrostography (arrow). (E) Follow-up renal scan 20 months after percutaneous nephrostomy catheter removal shows a defect in the region of the contained leak (arrow), but no extravasation of radiotracer to suggest urine leak.
Urinary Bladder and Urethral Injury
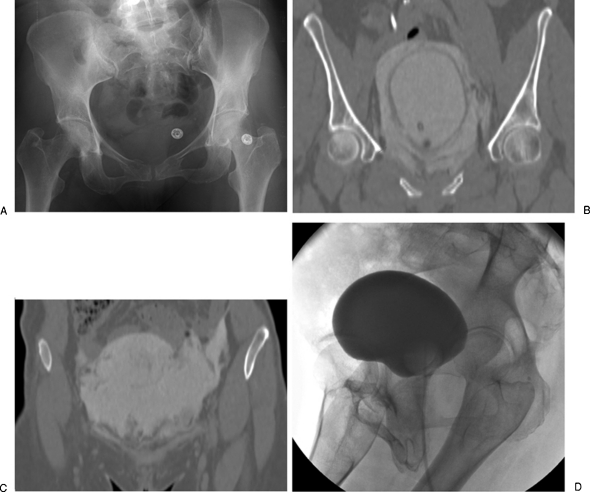
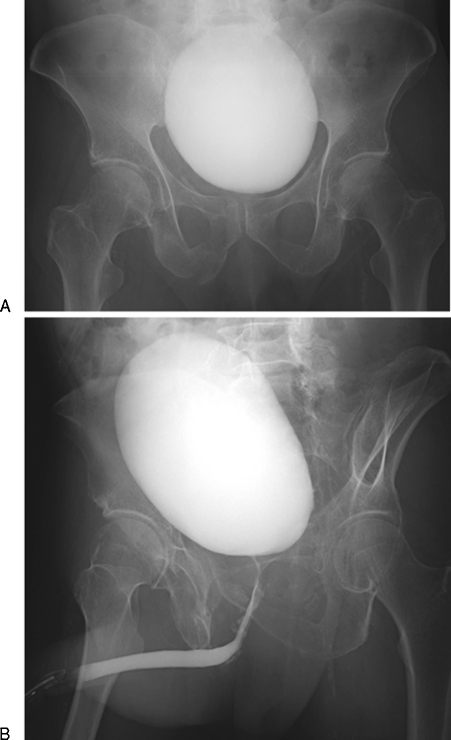
Bladder rupture is commonly seen in the context of pelvic fractures, often presenting with a triad of symptoms including gross hematuria, suprapubic pain and tenderness, and dysuria or inability to void (Fig. 5). Conventional and CT cystography are mandated in these patient presentations, as many bladder ruptures do not become apparent unless intraluminal pressure is applied to the bladder.21 Bladder ruptures are classified according to their location relative to the peritoneum, with extraperitoneal perforations comprising 50 to 71% of all ruptures, intraperitoneal perforations 25 to 43%, and combined perforations comprising 7 to 14%.1 An extraperitoneal bladder rupture with ureteral injury is managed with placement of a suprapubic catheter, and an extraperitoneal bladder rupture without ureteral injury is managed with placement of a Foley catheter. In contrast, an intraperitoneal bladder rupture warrants immediate surgical repair to minimize the risk of sepsis.10
Figure 5.
Urinary bladder rupture in a 52-year-old woman after a motor vehicle collision. (A) Pelvis radiograph demonstrates fractures in the left sacral ala, left ischium, bilateral superior pubic rami, and right inferior pubic ramus. (B) A computed tomography cystogram shows a rupture in the right anterolateral aspect of the urinary bladder wall. (C) Presence of contrast in the right paracolic gutter is indicative of an intraperitoneal bladder rupture. Patient was managed conservatively with placement of a Foley catheter. (D) Follow-up cystogram performed ∼1 month afterwards shows interval resolution of the bladder rupture.
Urethral injuries are also commonly seen in the context of pelvic fractures (Fig. 6). Current guidelines suggest imaging evaluation by retrograde urethrogram in any of the following circumstances: blood at the urethral meatus or vaginal introitus; hematuria; dysuria or inability to void; and penile, labial, or rectal swelling. In a similar vein as ureteral injuries, current practice guidelines suggest immediate surgical repair of urethral injuries, which extend to the bladder neck, rectum, and vagina. Isolated urethral lacerations that do not necessitate surgical repair may be managed by placement of a suprapubic catheter, which diverts urinary flow and promotes healing prior to delayed surgical repair. Delayed urethroplasty may take place up to 3 to 6 months after the time of injury.22
Figure 6.
Urethral injury in a 63-year-old man who was struck by a motor vehicle. (A) Pelvis radiograph demonstrates fractures in bilateral superior pubic rami and right inferior pubic ramus. Fracture of the right sacral ala is obscured by contrast in the urinary bladder. (B) Retrograde urethrogram demonstrates contrast extravasation at the bulbomembranous junction, indicative of a urethral injury. The patient was treated conservatively by placement of a Foley catheter, which was removed after ∼2 months when the urinary leak was shown to have resolved.
PEDIATRIC UROLOGIC TRACT TRAUMA
Trauma is the leading cause of mortality of children older than 1 year, and children are more susceptible than adults to traumatic renal injury due to larger organ-to-body ratio, increased renal mobility, decreased perinephric fat, and increased chest wall mobility.23 Similar to the therapeutic trends seen in adult trauma patients, there has been a progressive movement to increasingly conservative management of blunt renal trauma, no matter how severe. Recent evidence supports the use of conservative management for all grades in hemodynamically stable children with blunt renal injury, with indications for radiologic or surgical interventions including hemodynamic instability, progressive urinoma, or persistent bleeding.24,25,26 If endovascular interventions are warranted, it should be noted that pediatric patients also experience higher rates of complications associated with femoral artery catheterization including hemorrhage, acute thrombosis, dissection, chronic occlusion, as well as arteriovenous fistula and pseudoaneurysm formation. A recent study has shown that risk factors that negatively impacted outcomes after femoral catheterizations include age younger than 3 years, more than three repeated catheterizations, the use of therapeutic interventions, and the use of a 6-French or larger guiding catheter.27 Contrast dosage should be kept <2–5 mL/kg, saline flush should be used conservatively to avoid volume overload, radiation exposure should be kept to an absolute minimum, and ambient room temperature should be kept at ∼27°C (80°F) to curtail vasospasm.
SUMMARY
Familiarity of the evaluation and treatment urologic tract injury is essential in the management of patients who have sustained abdominal trauma. In this review, a systematic approach to the radiologic evaluation of the urologic tract has been delineated, and the treatments offered by interventional radiology have been presented on an anatomic basis from the kidney, ureters, urinary bladder, and urethra. Special considerations regarding pediatric renal trauma and endovascular therapies have also been discussed.
References
- Titton R L, Gervais D A, Boland G W, Mueller P R. Renal trauma: radiologic evaluation and percutaneous treatment of nonvascular injuries. AJR Am J Roentgenol. 2002;178(6):1507–1511. doi: 10.2214/ajr.178.6.1781507. [DOI] [PubMed] [Google Scholar]
- Bixby S D, Callahan M J, Taylor G A. Imaging blunt pediatric blunt abdominal trauma. Semin Roentgenol. 2008;43(1):72–82. doi: 10.1053/j.ro.2007.08.009. [DOI] [PubMed] [Google Scholar]
- McAleer I M, Kaplan G W, LoSasso B E. Congenital urinary tract anomalies in pediatric renal trauma patients. J Urol. 2002;168(4 Pt 2):1808–1810. discussion 1810. doi: 10.1097/01.ju.0000028338.48621.57. [DOI] [PubMed] [Google Scholar]
- Lorelli D R, Kralovich K A, Seguin C. The impact of pre-existing end-stage renal disease on survival in acutely injured trauma patients. Am Surg. 2001;67(7):693–696. [PubMed] [Google Scholar]
- Patel M S, Malinoski D J, Nguyen X M, Hoyt D B. The impact of select chronic diseases on outcomes after trauma: a study from the National Trauma Data Bank. J Am Coll Surg. 2011;212(1):96–104. doi: 10.1016/j.jamcollsurg.2010.09.028. [DOI] [PubMed] [Google Scholar]
- Santucci R A, Fisher M B. The literature increasingly supports expectant (conservative) management of renal trauma—a systematic review. J Trauma. 2005;59(2):493–503. doi: 10.1097/01.ta.0000179956.55078.c0. [DOI] [PubMed] [Google Scholar]
- Natarajan B, Gupta P K, Cemaj S, Sorensen M, Hatzoudis G I, Forse R A. FAST scan: is it worth doing in hemodynamically stable blunt trauma patients? Surgery. 2010;148(4):695–700. discussion 700–701. doi: 10.1016/j.surg.2010.07.032. [DOI] [PubMed] [Google Scholar]
- Sangthong B, Demetriades D, Martin M, et al. Management and hospital outcomes of blunt renal artery injuries: analysis of 517 patients from the National Trauma Data Bank. J Am Coll Surg. 2006;203(5):612–617. doi: 10.1016/j.jamcollsurg.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Breyer B N, McAninch J W, Elliott S P, Master V A. Minimally invasive endovascular techniques to treat acute renal hemorrhage. J Urol. 2008;179(6):2248–2252. discussion 2253. doi: 10.1016/j.juro.2008.01.104. [DOI] [PubMed] [Google Scholar]
- Bent C, Iyngkaran T, Power N, et al. Urological injuries following trauma. Clin Radiol. 2008;63(12):1361–1371. doi: 10.1016/j.crad.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Toutouzas K G, Karaiskakis M, Kaminski A, Velmahos G C. Nonoperative management of blunt renal trauma: a prospective study. Am Surg. 2002;68(12):1097–1103. [PubMed] [Google Scholar]
- Hagiwara A, Sakaki S, Goto H, et al. The role of interventional radiology in the management of blunt renal injury: a practical protocol. J Trauma. 2001;51(3):526–531. doi: 10.1097/00005373-200109000-00017. [DOI] [PubMed] [Google Scholar]
- Morita S, Inokuchi S, Tsuji T, et al. Arterial embolization in patients with grade-4 blunt renal trauma: evaluation of the glomerular filtration rates by dynamic scintigraphy with 99m Technetium-diethylene triamine pentacetic acid. Scand J Trauma Resusc Emerg Med. 2010;18:11. doi: 10.1186/1757-7241-18-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow S J, Thompson K J, Hartman J F, Wright M L. A 10-year review of blunt renal artery injuries at an urban level I trauma centre. Injury. 2009;40(8):844–850. doi: 10.1016/j.injury.2008.11.022. [DOI] [PubMed] [Google Scholar]
- Brewer M E, Jr, Strnad B T, Daley B J, et al. Percutaneous embolization for the management of grade 5 renal trauma in hemodynamically unstable patients: initial experience. J Urol. 2009;181(4):1737–1741. doi: 10.1016/j.juro.2008.11.100. [DOI] [PubMed] [Google Scholar]
- Savastano S, Feltrin G P, Miotto D, Chiesura-Corona M. Renal aneurysm and arteriovenous fistula. Management with transcatheter embolization. Acta Radiol. 1990;31(1):73–76. [PubMed] [Google Scholar]
- Dobrilovic N, Bennett S, Smith C, Edwards J, Luchette F A. Traumatic renal artery dissection identified with dynamic helical computed tomography. J Vasc Surg. 2001;34(3):562–564. doi: 10.1067/mva.2001.116302. [DOI] [PubMed] [Google Scholar]
- Lee J T, White R A. Endovascular management of blunt traumatic renal artery dissection. J Endovasc Ther. 2002;9(3):354–358. doi: 10.1177/152660280200900315. [DOI] [PubMed] [Google Scholar]
- Lupattelli T, Basile A, Iozzelli A, et al. Thrombolytic therapy followed by stenting for renal artery dissection secondary to blunt trauma. Emerg Radiol. 2005;11(3):164–166. doi: 10.1007/s10140-004-0390-z. [DOI] [PubMed] [Google Scholar]
- Best C D, Petrone P, Buscarini M, et al. Traumatic ureteral injuries: a single institution experience validating the American Association for the Surgery of Trauma-Organ Injury Scale grading scale. J Urol. 2005;173(4):1202–1205. doi: 10.1097/01.ju.0000155526.37963.ef. [DOI] [PubMed] [Google Scholar]
- Vaccaro J P, Brody J M. CT cystography in the evaluation of major bladder trauma. Radiographics. 2000;20(5):1373–1381. doi: 10.1148/radiographics.20.5.g00se111373. [DOI] [PubMed] [Google Scholar]
- Martínez-Piñeiro L, Djakovic N, Plas E, et al. European Association of Urology EAU guidelines on urethral trauma. Eur Urol. 2010;57(5):791–803. doi: 10.1016/j.eururo.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Brown S L, Elder J S, Spirnak J P. Are pediatric patients more susceptible to major renal injury from blunt trauma? A comparative study. J Urol. 1998;160(1):138–140. [PubMed] [Google Scholar]
- Margenthaler J A, Weber T R, Keller M S. Blunt renal trauma in children: experience with conservative management at a pediatric trauma center. J Trauma. 2002;52(5):928–932. doi: 10.1097/00005373-200205000-00018. [DOI] [PubMed] [Google Scholar]
- Eassa W, El-Ghar M A, Jednak R, El-Sherbiny M. Nonoperative management of grade 5 renal injury in children: does it have a place? Eur Urol. 2010;57(1):154–161. doi: 10.1016/j.eururo.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Sahai A, Cuthbert F, Niekrash R, Siddiqui M, Gulati M S. Endovascular management of grade V blunt renal trauma with associated splenic injury. Nat Rev Urol. 2009;6(6):335–337. doi: 10.1038/nrurol.2009.79. [DOI] [PubMed] [Google Scholar]
- Lin P H, Dodson T F, Bush R L, et al. Surgical intervention for complications caused by femoral artery catheterization in pediatric patients. J Vasc Surg. 2001;34(6):1071–1078. doi: 10.1067/mva.2001.119043. [DOI] [PubMed] [Google Scholar]