Transarterial chemoembolization (TACE) alone or in combination with thermoablation is commonly used in treating primary and secondary liver malignancies.1,2 It has proven to be safe and effective in treating hepatocellular carcinoma (HCC), neuroendocrine tumors, ocular melanoma, cholangiocarcinoma, and sarcoma. Known complications from hepatic embolization include the self-limiting postembolization syndrome (i.e., pain, nausea, vomiting, fever), liver infarction, liver failure, liver abscess, biloma formation, gastrointestinal bleeding, tumor rupture, septicemia, and pulmonary embolism. Approximately 5% of patients will have serious adverse events after chemoembolization, with the most common being liver abscess or liver infarction.1 This case reviews the incidence, risk factors, diagnosis, and treatment of liver abscess following chemoembolization as well as prophylactic measures to prevent its occurrence.
CASE REPORT
A 77-year-old man presented to our institution with known HCC. Initial magnetic resonance imaging (MRI) revealed the lesion at the junction of segments VII and VIII (Fig. 1). The patient was not a surgical candidate due to his extensive past medical history, which was significant for diabetes, congestive heart failure, coronary artery disease status post four-vessel coronary artery bypass graft, ulcerative colitis, interstitial lung disease from asbestosis, and obstructive sleep apnea. Consequently, he was referred to our interventional radiology service for treatment. For liver malignancies at our institution, we perform TACE followed by microwave ablation (MWA) on the next day. Our prophylactic antibiotic for TACE is 1 g of intravenous (IV) cefoxitin, which the patient received on all occasions.
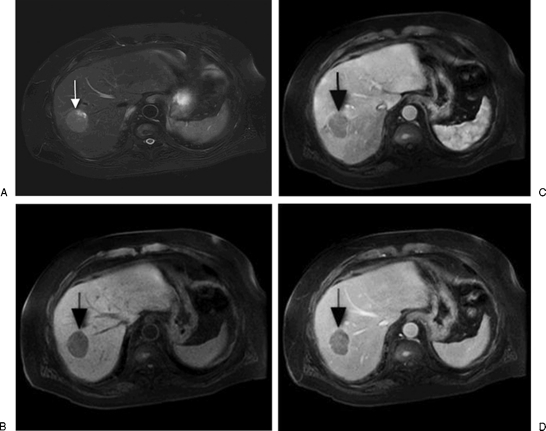
Figure 1.
(A) A T2-weighted, fat-suppression magnetic resonance image shows a slightly lobulated lesion along the junction of segments VII and VIII consistent with known hepatocellular carcinoma (HCC; arrow). (B) Precontrast, (C) early arterial phase, and (D) delayed imaging shows early arterial enhancement of the HCC with washout on the delayed phase. The HCC also demonstrates delayed capsular enhancement.
There were no complications after his initial treatment and he was discharged from the hospital after a few days. An MRI obtained 5 months later revealed recurrence of disease at the treatment site. The patient underwent another round of TACE/MWA shortly after the MRI. Again, the procedures were well tolerated without postprocedural complications. Repeat MRI obtained 4 months later revealed residual/recurrent disease along the peripheral, posterior aspect of the previously ablated region (Fig. 2).
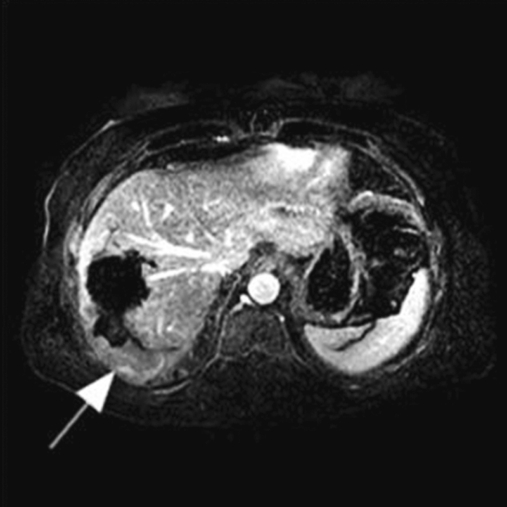
Figure 2.
Subtraction magnetic resonance image shows residual/recurrent disease along the peripheral, posterior aspect of the prior treatment site (arrow).
In addition to recurrent disease, there was a new mass in the pancreatic head suspicious for adenocarcinoma that was causing upstream intrahepatic and extrahepatic biliary duct dilatation as well as pancreatic duct dilatation (Fig. 3).
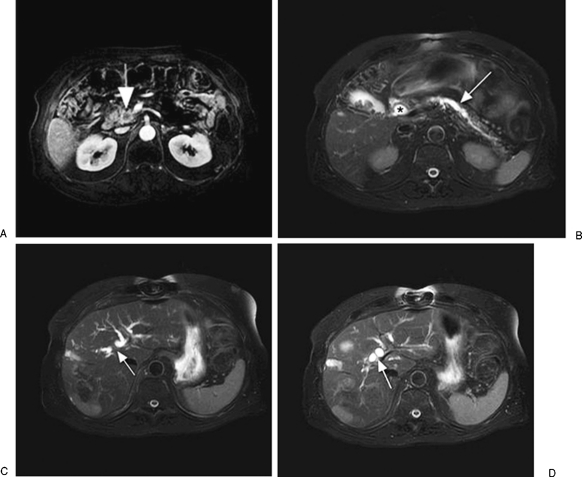
Figure 3.
(A) Postcontrast magnetic resonance image (MRI) shows a heterogeneous pancreatic head mass (arrow). (B) T2-weighted, fat-suppression MRI shows the mass causes common bile duct dilatation (*) as well as pancreatic duct dilatation (arrow). (C,D) A T2-weighted, fat-suppression MRI shows intrahepatic biliary duct dilatation (arrows).
The patient was then referred to the interventional gastrointestinal (GI) service for evaluation with endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP). Core biopsies taken during EUS revealed no evidence of malignancy. A metallic common duct stent and pancreatic duct stent were placed during ERCP (Fig. 4).
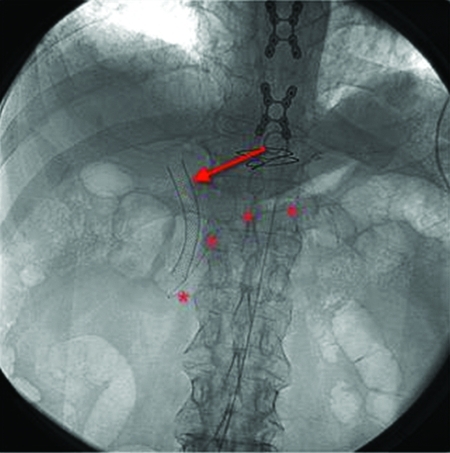
Figure 4.
Spot fluoroscopic image of the abdomen shows a common bile duct stent (arrow) and pancreatic duct stent (*) in place.
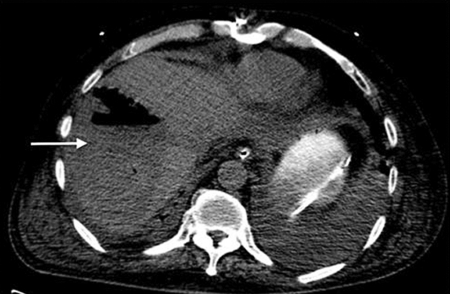
The patient returned a month after the stents were placed for repeat TACE. Chemoembolization on this occasion consisted of 50 mg of epirubicin and two vials of QuadraSpheres with additional embolization performed with 300 to 500 micron Embospheres. The patient returned the following day for MWA of the lesion under computed tomography (CT) guidance. Both procedures were technically successful without immediate complications. However, on postprocedure day 2, the patient developed a fever of 38.5°C, leukocytosis, hypotension unresponsive to intravenous (IV) fluids, and confusion. He was started on broad-spectrum IV antibiotics (ampicillin, cefepime, metronidazole, and micafungin) and subsequently transferred to the intensive care unit where he was intubated. The following day, a CT of the abdomen was obtained, revealing a large right hepatic lobe abscess (Fig. 5).
Figure 5.
Computed tomography image shows a large air-fluid collection in the right lobe of the liver consistent with abscess (arrow).
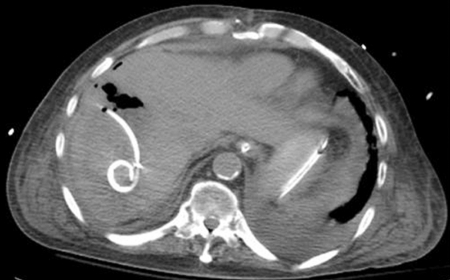
A 10-French percutaneous pigtail drain was placed that same day (Fig. 6) and cultures from the abscess grew Enterococcus, which was also cultured from the patient's blood. The patient was continued on broad-spectrum IV antibiotics, but remained hypotensive despite multiple vasopressors. Given his extensive medical history and poor prognosis, the family reported the patient had earlier expressed that he did not wish to be on prolonged mechanical ventilation. On postprocedure day 5, the family withdrew care and the patient expired.
Figure 6.
Computed tomography image obtained after drain placement shows a pigtail catheter within the fluid collection.
DISCUSSION
Liver abscess following hepatic artery embolization is a rare but serious complication associated with significant morbidity and mortality. The reported rates range from 0.2 to 4.5% of patients who have undergone hepatic artery embolization. The most common predisposing risk factors in these patients were a history of bilioenteric anastomosis or incompetent sphincter of Oddi from stenting or prior sphincterotomy.3,4,5,6,7,8
In a recent retrospective study, Mezhir et al evaluated 971 patients who underwent 2045 hepatic arterial embolization procedures. Hepatic abscess developed in 14 patients after embolization, representing 1.4%. Thirteen of these patients had a history of bilioenteric anastomosis or incompetent sphincter of Oddi. Only one patient had no history of prior biliary procedure. The criteria used for abscess diagnosis were clinical symptoms of infection, imaging findings consistent with abscess, and positive drainage culture results.3 Several authors have also reported a similar increased incidence of liver abscess following transarterial embolization in patients with altered biliary anatomy.4,5,6,7,8
The diagnosis of a postprocedure abscess can be difficult because symptoms can overlap with postembolization syndrome: a constellation of nonspecific signs and symptoms that include nausea, vomiting, fever, abdominal pain, and leukocytosis. This syndrome can occur immediately following solid organ embolization, but is usually well tolerated. Symptoms usually resolve within 1 week and 50% of patients can be discharged one day following embolization.1 Despite the similarities in symptoms, patients with liver abscess generally have a later time course of presentation. Chen et al suggest a liver abscess should be suspected after 1 week if patients continue to have fever, chills, and abdominal pain.9 Several studies also support this delayed presentation. Mezhir et al reported a median time from embolization to abscess diagnosis of 12 days.3 Kim et al reported a median time of 19 days ± 7 days until diagnosis.8 Further confounding the diagnosis of an abscess is the overlap in imaging findings with tumor necrosis, which is expected after embolization. Liquefactive necrosis of embolized tumor can demonstrate an area of low density on CT and can also contain gas. Differentiating these findings from an abscess can be difficult.
Treatment of liver abscess has evolved from surgery to the first-line treatment of percutaneous drainage, which can be performed safely and effectively under image guidance.10 The mainstays of treatment are abscess drainage along with broad-spectrum IV antibiotics.
Prophylactic antibiotics are routinely used prior to embolization procedures although there are no randomized clinical trials to support their use. Venkatesan et al recommends routine prophylaxis, but there is no consensus on which antibiotic agent should be the first choice. The recommendation is for an antibiotic with coverage of skin flora and Gram-negative enteric organisms. The authors also specifically address patients without an intact sphincter of Oddi given their increased risk of abscess formation. Their recommendation in this specific patient population is for coverage of Gram-positive and Gram-negative aerobic and anaerobic organisms with tazobactam/piperacillin being the main consideration in this scenario. The lack of prospective studies also extends to antibiotic use after embolization. Some clinicians recommend continued antibiotic coverage for Gram-negative enteric organisms for 3 to 7 days after embolization.11
Recognizing the increased incidence of liver abscess in patients without an intact sphincter of Oddi, some authors have tried new prophylactic measures including changing the antibiotic regimen and adding a bowel preparation. In a small study by Geschwind et al, seven patients with prior biliary-enteric anastomoses and 1 with prior cholecystectomy with papillotomy were separated into two groups, each containing four patients. Group 1 was administered IV cephalexin for prophylaxis. Group 2 was administered IV tazobactam/piperacillin for a mean duration of 3 days beginning 24 to 36 hours before embolization. A bowel preparation was also initiated the day before embolization, which included oral Fleet Phospho-Soda, oral neomycin, oral erythromycin, oral bisacodyl, and a rectal suppository of bisacodyl. All patients in group 1 developed liver abscess after the procedure. No patients in group 2 developed an abscess.12 Patel et al changed the standard prophylactic antibiotics of a cephalosporin and metronidazole to levofloxacin and metronidazole 2 days prior to embolization and continued for 2 weeks after. Their patients also received a bowel preparation of oral neomycin and erythromycin one day prior. Seven patients were given this regimen, of which two had a history of pancreaticoduodenectomy and five had a history of biliary stent placement. Two of the seven patients after two of 16 procedures developed a liver abscess. This was a trend toward a lower rate of abscess formation in high-risk patients when compared with historical controls.13 The role of a more aggressive antibiotic regimen and bowel preparation are promising in both studies; however, the numbers are too small to draw any definitive conclusions.
CONCLUSION
Liver abscess formation after hepatic artery embolization is rare but associated with significant morbidity and mortality. Several studies have established the significantly increased risk of liver abscess after hepatic artery embolization in patients with bilioenteric anastomosis or incompetent sphincter of Oddi. Current guidelines call for prophylactic antibiotics for hepatic arterial embolization, but special regimens for high-risk patients with altered biliary anatomy remains uncertain. Some studies, however, have shown that aggressive antibiotic regimens and bowel preparation may be helpful.
The overlap of signs and symptoms of postembolization syndrome with abscess formation makes the diagnosis challenging. However, having a high clinical suspicion is important in recognizing this potentially fatal complication. Once diagnosed, early intervention with percutaneous drainage and broad-spectrum IV antibiotics are first-line treatment.
References
- Brown D B, Geschwind J F, Soulen M C, Millward S F, Sacks D. Society of Interventional Radiology Position Statement on Chemoembolization of Hepatic Malignancies. J Vasc Interv Radiol. 2006;17(2 Pt 1):217–223. doi: 10.1097/01.RVI.0000196277.76812.A3. [DOI] [PubMed] [Google Scholar]
- Maluccio M, Covey A M, Gandhi R, et al. Comparison of survival rates after bland arterial embolization and ablation versus surgical resection for treating solitary hepatocellular carcinoma up to 7 cm. J Vasc Interv Radiol. 2005;16(7):955–961. doi: 10.1097/01.RVI.0000161377.33557.20. [DOI] [PubMed] [Google Scholar]
- Mezhir J J, Fong Y, Fleischer D, et al. Pyogenic abscess after hepatic artery embolization: a rare but potentially lethal complication. J Vasc Interv Radiol. 2011;22(2):177–182. doi: 10.1016/j.jvir.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto I, Aso N, Nagaoki K, et al. Complications associated with transcatheter arterial embolization for hepatic tumors. Radiographics. 1998;18(3):605–619. doi: 10.1148/radiographics.18.3.9599386. [DOI] [PubMed] [Google Scholar]
- Song S Y, Chung J W, Han J K, et al. Liver abscess after transcatheter oily chemoembolization for hepatic tumors: incidence, predisposing factors, and clinical outcome. J Vasc Interv Radiol. 2001;12(3):313–320. doi: 10.1016/s1051-0443(07)61910-1. [DOI] [PubMed] [Google Scholar]
- Ong G Y, Changchien C S, Lee C M, et al. Liver abscess complicating transcatheter arterial embolization: a rare but serious complication. A retrospective study after 3878 procedures. Eur J Gastroenterol Hepatol. 2004;16(8):737–742. doi: 10.1097/01.meg.0000108361.41221.8c. [DOI] [PubMed] [Google Scholar]
- de Baère T, Roche A, Amenabar J M, et al. Liver abscess formation after local treatment of liver tumors. Hepatology. 1996;23(6):1436–1440. doi: 10.1002/hep.510230620. [DOI] [PubMed] [Google Scholar]
- Kim W, Clark T W, Baum R A, Soulen M C. Risk factors for liver abscess formation after hepatic chemoembolization. J Vasc Interv Radiol. 2001;12(8):965–968. doi: 10.1016/s1051-0443(07)61577-2. [DOI] [PubMed] [Google Scholar]
- Chen C, Chen P J, Yang P M, et al. Clinical and microbiological features of liver abscess after transarterial embolization for hepatocellular carcinoma. Am J Gastroenterol. 1997;92(12):2257–2259. [PubMed] [Google Scholar]
- Mezhir J J, Fong Y, Jacks L M, et al. Current management of pyogenic liver abscess: surgery is now second-line treatment. J Am Coll Surg. 2010;210(6):975–983. doi: 10.1016/j.jamcollsurg.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Venkatesan A M, Kundu S, Sacks D, et al. Practice guidelines for adult antibiotic prophylaxis during vascular and intervention radiology procedures. J Vasc Interv Radiol. 2010;21:1611–1630. doi: 10.1016/j.jvir.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Geschwind J F, Kaushik S, Ramsey D E, Choti M A, Fishman E K, Kobeiter H. Influence of a new prophylactic antibiotic therapy on the incidence of liver abscesses after chemoembolization treatment of liver tumors. J Vasc Interv Radiol. 2002;13(11):1163–1166. doi: 10.1016/s1051-0443(07)61959-9. [DOI] [PubMed] [Google Scholar]
- Patel S, Tuite C M, Mondschein J I, Soulen M C. Effectiveness of an aggressive antibiotic regimen for chemoembolization in patients with previous biliary intervention. J Vasc Interv Radiol. 2006;17(12):1931–1934. doi: 10.1097/01.RVI.0000244854.79604.C1. [DOI] [PubMed] [Google Scholar]