Abstract
A variety of surgical options exists for penile reconstruction. The key to success of therapy is holistic management of the patient, with attention to the psychological aspects of treatment. In this article, we review reconstructive modalities for various types of penile defects inclusive of partial and total defects as well as the buried penis, and also describe recent basic science advances, which may promise new options for penile reconstruction.
Keywords: Dyspareunia, penile reconstruction, osteocutaneous flaps, radial forearm flap, penile cancer, priapism, micropenis, phallus, penis
Penile reconstruction presents a complex series of issues. Often, management consists of not only surgical reconstruction, but also psychological rehabilitation. Psychological needs vary greatly, ranging from patients seeking gender reassignment surgery to those presenting after traumatic penile amputation. The loss of the penis negatively affects many different aspects of life, such as one's psychological well-being and relationship with spouse or partner.
In all cases, the goals of surgery are to create or restore a functional and aesthetic phallus. This entails the ability to void standing from the tip of the phallus and the ability to achieve sexual function, with a sensate penis of sufficient bulk to allow penetration for sexual intercourse. Of importance is also the appearance of the reconstructed phallus, which should resemble a normal penis in all aspects from glans to shaft.
The extent of the defect dictates the means of reconstruction. A surgical defect may range from one involving a single tissue or structure, such as skin or urethra, to a total penectomy defect, which would require microsurgical reconstruction. The buried penis is another interesting problem, which entails different surgical requirements. In this article, we review reconstructive modalities for various types of penile defects, and describe recent basic science advances, which may promise new options for penile reconstruction.
PATIENT EVALUATION
The patient presenting for penile reconstruction may not always be in the right frame of mind. This is particularly true if the loss of the penis was acute and unexpected, resulting for example from domestic violence or trauma. Hence, a thorough psychiatric history and often evaluation by a psychiatrist is essential. Many patients suffer from depression and may even have had suicidal ideation. This does not, however, exclude them from surgical reconstruction, as the deformity is the cause of the patient's psychological distress.
The patient's sexual history and desired goals should be evaluated and reviewed to determine such issues as the premorbid length of the penile shaft, and whether the patient is currently able to have an orgasm. The means by which the patient voids is also important. The presence of a perineal urethrostomy will have bearing on the surgical plan. Of relevance is also whether the patient currently has tactile or protective sensation in the region of the remnant of the penis, and whether nerves such as the pudendal or ilioinguinal nerves are intact. In total penile reconstruction, these nerves may be reapproximated to the neophallus to achieve protective and erogenous sensation. As many of these questions are intimate but essential to the success of surgical reconstruction, a trusting relationship must be cultivated between the physician and patient. Realistic patient expectations are key to postoperative success. An important issue is the length of the penis, which was found to average around 6 inches.1,2 If microsurgical reconstruction is to be performed, it is vital to ensure that the patient stops smoking at least 4 weeks before surgery and also abstains after surgery.
PARTIAL PENILE DEFECTS
If there is partial preservation of the penile shaft, measures to augment the length of the penis such as severing the suspensory ligament or with V-Y plasty of the lower abdominal skin, may be sufficient to achieve a functional phallus.3,4 An illusion of increased penile length is perceived resulting from penile descent and increased convexity of the penile base. This reconstructive option is suitable for defects where a remnant penile length of 2–3 cm is preserved, with the ability to urinate in a standing position. In older patients with multiple comorbidities, penile augmentation may be an adequate option in lieu of complex free flap surgery. Complications may include “scrotalization” where the penis is covered by unsightly scrotal corrugated skin rather than by natural smooth skin, hypertrophic scarring, and a low hanging penis.5
Isolated skin loss may result from penile trauma, burns, or excisional debridement of hidradenitis suppurativa. In these cases, split thickness skin grafting allows good reliable coverage. Increased use of vacuum assisted closure (VAC) dressing (Kinetics Concepts Incorporated, San Antonio, TX) in these difficult areas has resulted in increased skin graft take and improved results. In cases not amenable to immediate split-thickness skin grafting, Integra (Integra Life Sciences Corp., Plainsboro, N.J.), a dermal substitute comprised of bovine collagen, results in a vascularized bed and may allow for skin grafting after removal of its overlying silicone sheeting 3 weeks after initial application. Scrotal skin flaps based on the anterior and posterior scrotal arteries may also be used in cases of skin deficiency,6 and are also used in reconstruction of partial penectomy defects.7
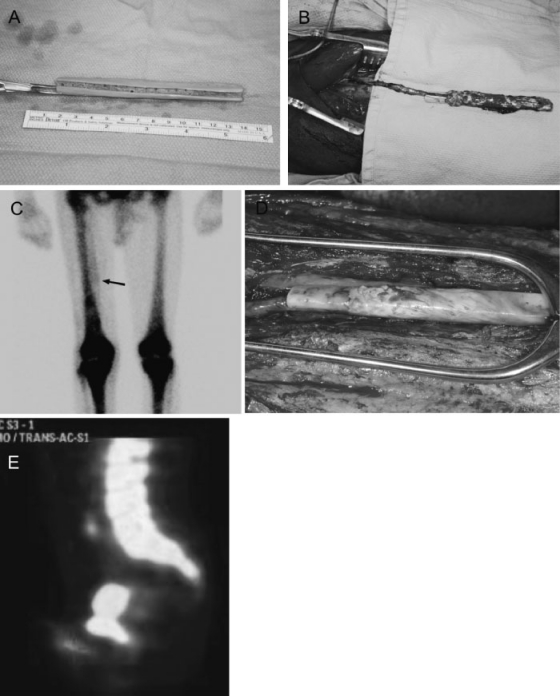
In rare cases, a patient may present with isolated erectile dysfunction not amenable to reconstruction with prostheses. This can result from sickle cell disease. A prefabricated cadaveric bone flap based on the descending branch of the lateral circumflex femoral artery has been used as a pedicled flap and implanted within the corpora cavernosa to restore sexual function.8 This is illustrated in Fig. 1.
Figure 1.
Prefabricated cadaveric fibula flap for penile autoaugmentation. (A) A trough was created on one side of the fibula segment and multiple holes were drilled to permit for vascular ingrowth. (B) The vascular pedicle was ligated distally and inset into the trough, and the construct subsequently wrapped with fascia to maintain the pedicle in good position. (C) Bone scan 6 weeks after first-stage prefabrication showed radionuclide uptake in the region of the implanted cadaveric bone on the medial aspect of the right thigh (arrow). This is absent on the contralateral thigh. (D) At the second-stage surgery, arborizing vessels are noted over the surface of the bone segment, with previously drilled holes along the sidewalls of the bone filled with vascularized tissue. (E) Bone scan 1 month after second-stage surgery demonstrates continued radionuclide uptake in the region of the autoaugmented penis. Sagittal view of the pelvis is seen, with radionuclide uptake in the lumbosacral spine and symphysis pubis noted in the superior part of the image. Radionuclide uptake is seen in the neophallus, projecting out inferior to the symphysis pubis (marked with cross).
URETHRAL RECONSTRUCTION
Urethral defects requiring reconstruction by the plastic surgeon are rare. In cases of carcinoma of the anterior urethra or penile shaft, partial penectomy or total penectomy with a perineal urethrostomy may occur. These patients are often not candidates for reconstruction due to age or comorbidities. In patients with advanced bladder cancer requiring cystectomy with total urethrectomy, urethral reconstruction is not required as urinary diversion will be performed through the medium of an ileal conduit.
Reconstructive techniques are based on the approaches to treat urethral strictures. The penile urethra may be exposed through an inverted T-shaped incision on the ventral surface of the penis or a circumferential incision ~0.5 cm below the glans. The bulbar urethra is best approached through a midline line perineal incision, which provides good access to the posterior urethra. Urinary diversion is key to success of reconstructive procedures. This may be achieved through a urethral catheter, which concurrently stents the reconstruction to prevent strictures, or a suprapubic catheter. Typically, after 10 to 14 days, a cystourethrogram is performed to verify that absence of urinary extravasation. In cases of significant extravasation, the cystourethrogram is repeated after 1 week and urinary diversion maintained.
In small defects less than 2 to 3 cm, urethral ends may be mobilized from the corporal cavernosa and spatulated.9 A primary interrupted anastomosis is then performed. If a tension-free anastomosis cannot be achieved, anastomosis of the dorsal or ventral strip with augmentation onlay of the opposing side may be performed. A buccal mucosal patch or skin flap may be used to as an onlay to fill the defect.10,11 If a free graft is used, care must be taken to ensure the graft lies against a well-vascularized bed. The location of the buccal mucosa graft on ventral, dorsal or lateral aspects of the bulbar urethra has been shown to result in similar success rates.12 In cases of a circumferential urethral defect, a pedicled skin flap based on the prepuce, penile shaft or scrotum may be used for reconstruction.
RECONSTRUCTION OF TOTAL PENILE DEFECTS
Total penile defects requiring reconstruction may result from a variety of mechanisms ranging from trauma to malignancy. Penile amputation has been reported from causes ranging from domestic violence to such bizarre cases as strangulation by a metallic nut13 or self-amputation due to schizophrenia.14 Where the penis is intact and amputated sharply, microsurgical replantation results in the best outcome. More often, recruitment of adjacent or distant tissue is required to effect a functional and aesthetic reconstruction.
Although pedicled flaps such as groin15 or abdominal skin flaps, rectus abdominis and gracilis, have been used historically and recently for penile reconstruction, these lead to suboptimal results with poor aesthetic and functional outcomes. Hence, microsurgical free flap reconstruction has become the method of choice for penile reconstruction. The ideal flap should be one that is sensate and hairless, with sufficient tissue to allow tubularization, as well as with a long pedicle. The radial forearm flap fulfills these requirements, and is by far the most commonly used free flap for penile reconstruction.
Radial Forearm Flap
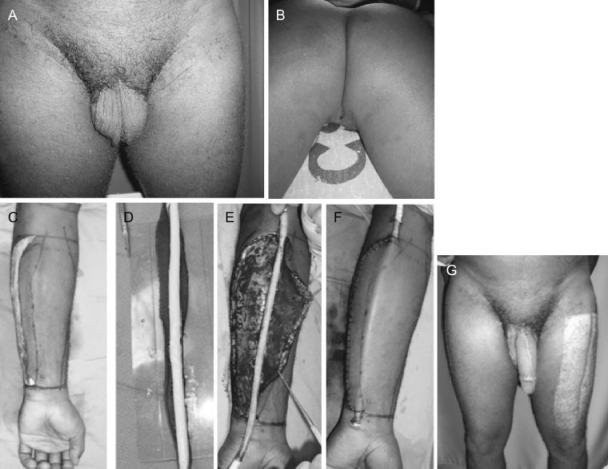
The radial forearm flap was first described by Chang and Hwang in 1984 for total penile reconstruction16 and has been found to be superior to all other techniques.17,18 The radial forearm flap has the advantage of providing thin supple tissue as well as a long pedicle that is easily exposed and dissected. It allows the best recovery of sensation among various flaps used for penile reconstruction. The location of the donor site away from the groin also allows a two-team approach. A typical case is illustrated in Fig. 2.
Figure 2.
A 38-year-old man who underwent total penectomy and bilateral inguinal node dissection for invasive squamous cell carcinoma. (A) Patient is seen 8 months after initial extirpative surgery. (B) Perineal urethrostomy can be seen. (C) Left radial forearm flap was partially raised during the first-stage of surgery. (D,E,F) Prelamination of neo-urethra using a full thickness skin graft which was wrapped circumferentially around a Foley catheter. (G) Postoperative result at 4 months following phalloplasty for total penile reconstruction with radial forearm osteocutaneous flap and palmaris longus tendon graft for glans coronoplasty.
A preoperative Allen test is essential to ensure that vascularity of the hand will not be compromised with harvest of the radial forearm flap. In a typical surgical scenario, two surgical teams operate simultaneously. The urologist performs the resection and also prepares the urethral stump. At the same time, the plastic surgeon raises the flap on the nondominant forearm. Prelamination of the neo-urethra may be performed prior to the definitive surgery, most often with a split-thickness skin graft over a stent.19 Alternatively, if prelamination is not performed and the procedure is performed in a single stage, the ulnar skin can be used to create a “tube-within-a-tube” phallus.18,20
To restore tactile and erogenous sensation, the medial and lateral antebrachial nerves are identified and preserved. These are anastomosed to the ilioinguinal nerve for protective sensation and dorsal penile or dorsal clitoral nerve for erogenous sensation. By nature of the vessels available in the region of the penis, microsurgical reconstruction is most often performed with anastomoses to the femoral artery and great saphenous vein or inferior epigastric vessels.
If bone from the radius is harvested to provide extra rigidity of the neophallus, prophylactic plating may be used to decrease the incidence of subsequent radius fractures.21 Otherwise, penile and testicular implants are placed after 12 months to allow sexual intercourse, following return of protective sensation to the penile tip. The forearm donor site is covered with split-thickness skin grafts or full thickness skin grafts from the groin. Postoperative urinary diversion is essential to protect the urethral anastomosis. Tattooing of the glans can be performed 2 to 3 months latter to improve the aesthetic result.
The incidence of urinary complications such as urethrocutaneous fistula or urinary stricture following penile reconstruction is significant, with a rate of around 41% reported in two studies.18,22 The majority of fistulas can be treated conservatively; however, most strictures can be treated with dilation. Interestingly, the radial forearm flap has also been described for penile reconstruction using nonmicrosurgical technique.23 In this technique, an osteocutaneous radial forearm flap is elevated as a reverse-flow island flap and transferred to the recipient site as a distant flap while maintaining its vascular connection with the forearm. The pedicle is then divided and the reconstruction completed 2 to 3 weeks later.
Free Fibular Flap
The free sensate osteocutaneous fibular flap was described by Sadove et al in 1992 for total phallic reconstruction.24 The advantages of the flap are its intrinsic rigidity, concealed donor site and long vascular pedicle. The increased bone stock available obviates the requirement for a penile implant for intercourse. However, disadvantages include decreased sensibility, increased urethral complications, and a permanently erect penis that may cause distress and social embarrassment. Despite decreased sensibility with the fibular flap, better sexual intercourse has been reported by patients compared with those reconstructed with the radial forearm flap.25 As a result, this flap is preferred by some for penile reconstruction, particularly in patients who refuse the radial forearm donor site. Figure 3 illustrates a use of a free fibular flap for penile reconstruction following penectomy.
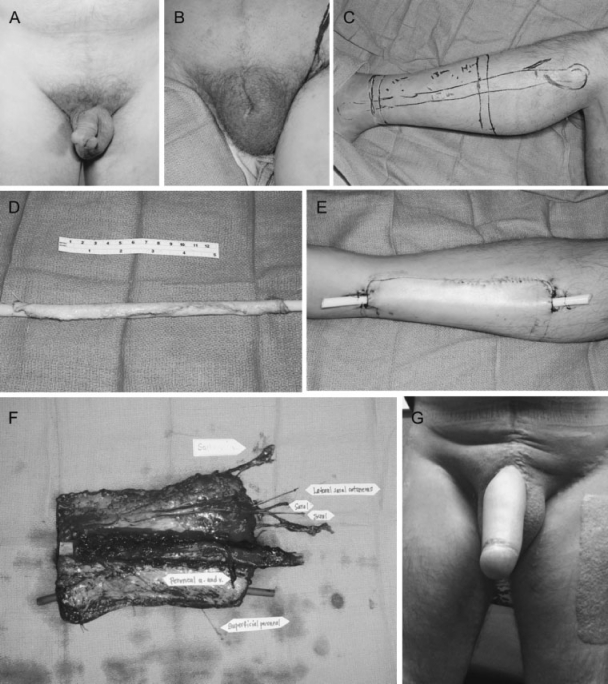
Figure 3.
A 43-year-old man who initially presented with an enlarging and painless mass at the base of the penis and right distal shaft (A). Biopsy revealed squamous cell carcinoma and he subsequently underwent radical penectomy, scrotal urethrostomy, bilateral superficial and deep inguinal lymph node dissection, and bilateral pelvic lymph node dissection. (B) He was tumor-free 8 months after surgery, and underwent the first stage of phalloplasty with plan for reconstruction using a free fibular flap (C). (D, E) Prelamination was performed around a Foley catheter using a full thickness skin graft harvested from the groin. (F) After maturation of the prelaminated neo-urethra, the flap was harvested based on the peroneal artery and vein, including branches of the lateral sural cutaneous nerve. Lateral leg skin was wrapped circumferentially around the fibula to form a neophallus, and periosteum of the proximal fibula sutured to periosteum of the pubic bone. The flap was anastomosed to the interior epigastric artery and great saphenous vein. A primary urethra to neo-urethra anastomosis was performed and the Foley catheter kept in place for 6 weeks. (H) Postoperative result, where patient is able to use the penis for intercourse and able to urinate spontaneously.
Anterolateral Thigh Flap
The pedicled anterolateral thigh (ALT) has experienced increasing popularity in recent years for total phallic reconstruction.26,27,28 Unlike previously used pedicled flaps, the ALT flap provides a superior aesthetic outcome and also allows restoration of sensation through coaptation of the lateral femoral cutaneous nerve to the pudendal or dorsal penile/clitoral nerves. A major advantage over the radial forearm flap is the concealed donor site. An erectile prosthesis can also easily be implanted. Other authors have described using the ALT as a free flap for phalloplasty.29
Other Reconstructive Options
In efforts to improve the donor site scar, the thoracodorsal artery perforator flap has been used for phallic reconstruction.30 The concealed donor site and large reservoir of tissue are obvious advantages of this technique. A pedicled suprapubic abdominal wall flap has also been described in a series of 85 transsexual patients for phalloplasty.31 Although the technique resulted in a good cosmetic result with the ability to achieve sexual intercourse with the aid of a penile implant, a major limitation was the high rate (75%) of urinary complications. Finally, penile transplantation has been described in an isolated case report.32 Although the recipient could urinate standing 10 days after surgery, the transplanted penis was cut off at day 14 due to psychological issues.
MANAGEMENT OF THE BURIED PENIS
The “buried” penis33 is an unusual condition that results in significant psychological and physical symptoms, and is associated with morbid obesity and diabetes mellitus in adults. This has been defined as a penile shaft buried below the surface of the prepubic skin and also to a partial or totally obstructed penis caused by obesity or radical circumcision.34 Increase in suprapubic fat results a moist environment around the penis that may result in chronic infection, skin breakdown, and subsequent scar contracture. This results in a penis that is not obvious on immediate inspection. Other causes may include penoscrotal elephantiasis or chronic genital lymphedema.
Pestana et al33 described a treatment algorithm that may be used in adults with buried penis. Release of scar contracture and removal of adjacent excess abdominal tissue through suction lipectomy, panniculectomy, or both allows exposure of the penis. Tacking sutures from subdermal tissue at the pubis to rectus fascia or pubic periosteum prevent retraction of the penis into the pubis or scrotum and maintains elevation of the suprapubic region.35 Finally, reconstruction of the skin defect is achieved through local tissue rearrangement or skin grafts (split or full thickness).
RECENT DEVELOPMENTS
Advances in tissue engineering promise new options for penile reconstruction. Although research has not been translated beyond animal studies, remarkable progress has been made in recent years. Acellular corporal collagen matrices seeded with autologous cells have been used to replace entire pendular penile corporal bodies in a rabbit model.36 Remarkably, the engineered tissue was similar structurally and functionally to native tissue, and male rabbits were able to successfully impregnate females. Tissue engineered cartilage rods have also been used as a substitute for synthetic penile implants. Autologous chondrocytes seeded on a polymer lattice rod were implanted into corporal spaces of the same rabbits, and explantation after 2 months showed well-formed cartilage structures, with animals able to copulate and impregnate female partners.37 An additional study utilizing human chondrocytes were implanted into subcutaneous spaces of rats for duration of 2 months to produce cartilaginous rods of comparable size and mechanical properties to silicone prostheses.38 Stem cells may also be a novel treatment option. One study has reported differentiating rat muscle-derived stem cells into corporal smooth muscle cells to replace these in situ.39 Another study by Song et al. observed the differentiation of human mesenchymal stem cells into smooth muscle cells or endothelial cells upon transplantation into rat corpus cavernosum.40
CONCLUSION
Penile reconstruction is a complex endeavor that requires close cooperation between the plastic surgeon and urologist. Often, not only the surgical, but also psychological aspects of treatment will determine success or failure of therapy. Regardless of the method of reconstruction, the goals of surgery remain the same. These include creating a functional and aesthetic phallus with the ability to void standing and to achieve sexual function.
References
- Schonfeld W, Beebe G W. Normal growth and variation in the male genitalia from birth to maturity. J Urol. 1942;48:759. [Google Scholar]
- Spyropoulos E, Borousas D, Mavrikos S, Dellis A, Bourounis M, Athanasiadis S. Size of external genital organs and somatometric parameters among physically normal men younger than 40 years old. Urology. 2002;60(3):485–489. discussion 490–491. doi: 10.1016/s0090-4295(02)01869-1. [DOI] [PubMed] [Google Scholar]
- Amukele S A, Lee G W, Stock J A, Hanna M K. 20-year experience with iatrogenic penile injury. J Urol. 2003;170(4 Pt 2):1691–1694. doi: 10.1097/01.ju.0000084147.28987.7f. [DOI] [PubMed] [Google Scholar]
- Ghanem H, Shamloul R, Khodeir F, ElShafie H, Kaddah A, Ismail I. Structured management and counseling for patients with a complaint of a small penis. J Sex Med. 2007;4(5):1322–1327. doi: 10.1111/j.1743-6109.2007.00463.x. [DOI] [PubMed] [Google Scholar]
- Alter G J. Penile enlargement surgery. Tech Urol. 1998;4(2):70–76. [PubMed] [Google Scholar]
- Zhao Y Q, Zhang J, Yu M S, Long D C. Functional restoration of penis with partial defect by scrotal skin flap. J Urol. 2009;182(5):2358–2361. doi: 10.1016/j.juro.2009.07.048. [DOI] [PubMed] [Google Scholar]
- Mazza O N, Cheliz G M. Glanuloplasty with scrotal flap for partial penectomy. J Urol. 2001;166(3):887–889. [PubMed] [Google Scholar]
- Salgado C J, Chim H, Rowe D, Bodner D R. Vascularized cadaveric fibula flap for treatment of erectile dysfunction following failure of penile implants. J Sex Med. 2010;7(10):3504–3509. doi: 10.1111/j.1743-6109.2010.01914.x. [DOI] [PubMed] [Google Scholar]
- Micheli E, Ranieri A, Peracchia G, Lembo A. End-to-end urethroplasty: long-term results. BJU Int. 2002;90(1):68–71. doi: 10.1046/j.1464-410x.2002.02832.x. [DOI] [PubMed] [Google Scholar]
- Guralnick M L, Webster G D. The augmented anastomotic urethroplasty: indications and outcome in 29 patients. J Urol. 2001;165(5):1496–1501. [PubMed] [Google Scholar]
- El-Kassaby A W, El-Zayat T M, Azazy S, Osman T. One-stage repair of long bulbar urethral strictures using augmented Russell dorsal strip anastomosis: outcome of 234 cases. Eur Urol. 2008;53(2):420–424. doi: 10.1016/j.eururo.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Barbagli G, Palminteri E, Guazzoni G, Montorsi F, Turini D, Lazzeri M. Bulbar urethroplasty using buccal mucosa grafts placed on the ventral, dorsal or lateral surface of the urethra: are results affected by the surgical technique? J Urol. 2005;174(3):955–957. discussion 957–958. doi: 10.1097/01.ju.0000169422.46721.d7. [DOI] [PubMed] [Google Scholar]
- Nuhu A, Edino S T, Agbese G O, Kallamu M. Penile gangrene due to strangulation by a metallic nut: a case report. West Afr J Med. 2009;28(5):340–342. doi: 10.4314/wajm.v28i5.55018. [DOI] [PubMed] [Google Scholar]
- Gyan S, Sushma S, Maneesh S, Rajesh S, Misra M. Successful microsurgical penile replantation following self amputation in a schizophrenic patient. Indian J Urol. 2010;26(3):434–437. doi: 10.4103/0970-1591.70589. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Puckett C L, Montie J E. Construction of male genitalia in the transsexual, using a tubed groin flap for the penis and a hydraulic inflation device. Plast Reconstr Surg. 1978;61(4):523–530. doi: 10.1097/00006534-197804000-00005. [DOI] [PubMed] [Google Scholar]
- Chang T S, Hwang W Y. Forearm flap in one-stage reconstruction of the penis. Plast Reconstr Surg. 1984;74(2):251–258. doi: 10.1097/00006534-198408000-00014. [DOI] [PubMed] [Google Scholar]
- Garaffa G, Christopher N A, Ralph D J. Total phallic reconstruction in female-to-male transsexuals. Eur Urol. 2010;57(4):715–722. doi: 10.1016/j.eururo.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Monstrey S, Hoebeke P, Selvaggi G, et al. Penile reconstruction: is the radial forearm flap really the standard technique? Plast Reconstr Surg. 2009;124(2):510–518. doi: 10.1097/PRS.0b013e3181aeeb06. [DOI] [PubMed] [Google Scholar]
- Song C, Wong M, Wong C H, Ong Y S. Modifications of the radial forearm flap phalloplasty for female-to-male gender reassignment. J Reconstr Microsurg. 2011;27(2):115–120. doi: 10.1055/s-0030-1268210. [DOI] [PubMed] [Google Scholar]
- Monstrey S, Hoebeke P, Dhont M, et al. Radial forearm phalloplasty: A review of 91 cases. ANIR-ANHP. 2004;6:193–199. [Google Scholar]
- Waits C A, Toby E B, Girod D A, Tsue T T. Osteocutaneous radial forearm free flap: long-term radiographic evaluation of donor site morbidity after prophylactic plating of radius. J Reconstr Microsurg. 2007;23(7):367–372. doi: 10.1055/s-2007-992342. [DOI] [PubMed] [Google Scholar]
- Hu Z Q, Hyakusoku H, Gao J H, Aoki R, Ogawa R, Yan X. Penis reconstruction using three different operative methods. Br J Plast Surg. 2005;58(4):487–492. doi: 10.1016/j.bjps.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Mutaf M. Nonmicrosurgical use of the radial forearm flap for penile reconstruction. Plast Reconstr Surg. 2001;107(1):80–86. doi: 10.1097/00006534-200101000-00013. [DOI] [PubMed] [Google Scholar]
- Sadove R C, McRoberts J W. Total phallic reconstruction with the free fibula osteocutaneous flap. Plast Reconstr Surg. 1992;89(5):1001. doi: 10.1097/00006534-199205000-00064. [DOI] [PubMed] [Google Scholar]
- Schaff J, Papadopulos N A. A new protocol for complete phalloplasty with free sensate and prelaminated osteofasciocutaneous flaps: experience in 37 patients. Microsurgery. 2009;29(5):413–419. doi: 10.1002/micr.20647. [DOI] [PubMed] [Google Scholar]
- Mutaf M, Isik D, Bulut O, Büyükgüral B. A true one-stage nonmicrosurgical technique for total phallic reconstruction. Ann Plast Surg. 2006;57(1):100–106. doi: 10.1097/01.sap.0000208991.22264.b5. [DOI] [PubMed] [Google Scholar]
- Rubino C, Figus A, Dessy L A, et al. Innervated island pedicled anterolateral thigh flap for neo-phallic reconstruction in female-to-male transsexuals. J Plast Reconstr Aesthet Surg. 2009;62(3):e45–e49. doi: 10.1016/j.bjps.2007.11.056. [DOI] [PubMed] [Google Scholar]
- Lee G K, Lim A F, Bird E T. A novel single-flap technique for total penile reconstruction: the pedicled anterolateral thigh flap. Plast Reconstr Surg. 2009;124(1):163–166. doi: 10.1097/PRS.0b013e3181ab2593. [DOI] [PubMed] [Google Scholar]
- Felici N, Felici A. A new phalloplasty technique: the free anterolateral thigh flap phalloplasty. J Plast Reconstr Aesthet Surg. 2006;59(2):153–157. doi: 10.1016/j.bjps.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Lin C T, Chen L W. Using a free thoracodorsal artery perforator flap for phallic reconstruction—a report of surgical technique. J Plast Reconstr Aesthet Surg. 2009;62(3):402–408. doi: 10.1016/j.bjps.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Bettocchi C, Ralph D J, Pryor J P. Pedicled pubic phalloplasty in females with gender dysphoria. BJU Int. 2005;95(1):120–124. doi: 10.1111/j.1464-410X.2004.05262.x. [DOI] [PubMed] [Google Scholar]
- Hu W, Lu J, Zhang L, et al. A preliminary report of penile transplantation. Eur Urol. 2006;50(4):851–853. doi: 10.1016/j.eururo.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Pestana I A, Greenfield J M, Walsh M, Donatucci C F, Erdmann D. Management of “buried” penis in adulthood: an overview. Plast Reconstr Surg. 2009;124(4):1186–1195. doi: 10.1097/PRS.0b013e3181b5a37f. [DOI] [PubMed] [Google Scholar]
- Ehrilch R M, Alter G J. Buried penis. In: Ehrlich R M, Alter G J, editors. Reconstructive and Plastic Surgery of the External Genitalia: Adult and Pediatric. Vol. 1. Philadelphia: Saunders; 1999. pp. 397–401. [Google Scholar]
- Alter G J. Surgical techniques: surgery to correct hidden penis. J Sex Med. 2006;3(5):939–942. doi: 10.1111/j.1743-6109.2006.00298.x. [DOI] [PubMed] [Google Scholar]
- Chen K L, Eberli D, Yoo J J, Atala A. Bioengineered corporal tissue for structural and functional restoration of the penis. Proc Natl Acad Sci U S A. 2010;107(8):3346–3350. doi: 10.1073/pnas.0909367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo J J, Park H J, Lee I, Atala A. Autologous engineered cartilage rods for penile reconstruction. J Urol. 1999;162(3 Pt 2):1119–1121. doi: 10.1016/S0022-5347(01)68090-X. [DOI] [PubMed] [Google Scholar]
- Kim B S, Yoo J J, Atala A. Engineering of human cartilage rods: potential application for penile prostheses. J Urol. 2002;168(4 Pt 2):1794–1797. doi: 10.1097/01.ju.0000028238.60060.59. [DOI] [PubMed] [Google Scholar]
- Nolazco G, Kovanecz I, Vernet D, et al. Effect of muscle-derived stem cells on the restoration of corpora cavernosa smooth muscle and erectile function in the aged rat. BJU Int. 2008;101(9):1156–1164. doi: 10.1111/j.1464-410X.2008.07507.x. [DOI] [PubMed] [Google Scholar]
- Song Y S, Lee H J, Park I H, Kim W K, Ku J H, Kim S U. Potential differentiation of human mesenchymal stem cell transplanted in rat corpus cavernosum toward endothelial or smooth muscle cells. Int J Impot Res. 2007;19(4):378–385. doi: 10.1038/sj.ijir.3901539. [DOI] [PubMed] [Google Scholar]