Abstract
Although oral bacteria-associated systemic diseases have been reported, association between Streptococcus mutans, pathogen of dental caries, and ulcerative colitis (UC) has not been reported. We investigated the effect of various S. mutans strains on dextran sodium sulfate (DSS)-induced mouse colitis. Administration of TW295, the specific strain of S. mutans, caused aggravation of colitis; the standard strain, MT8148 did not. Localization of TW295 in hepatocytes in liver was observed. Increased expression of interferon-γ in liver was also noted, indicating that the liver is target organ for the specific strain of S. mutans-mediated aggravation of colitis. The detection frequency of the specific strains in UC patients was significantly higher than in healthy subjects. Administration of the specific strains of S. mutans isolated from patients caused aggravation of colitis. Infection with highly-virulent specific types of S. mutans might be a potential risk factor in the aggravation of UC.
Ulcerative colitis (UC) and Crohn’s disease (CD), major inflammatory bowel diseases (IBDs), are chronic, relapsing enteropathies of unknown origin1,2,3,4. Remission and relapses, with symptoms of bloody diarrhea, abdominal pain, and bleeding, characterize these diseases. Recently, the incidence of IBDs has increased globally5. Although the pathogenesis is likely dependent on the interaction between local immune reactions and environmental factors in genetically susceptible individuals, the etiology of IBDs is still unknown1,2,3,4,5. Current theories also suggest that a dysregulated mucosal immune response to unidentified components of normal intestinal microbiota in a genetically susceptible host is at the core of these diseases6,7,8. Therefore, involvement of intestinal bacteria is considered an important factor in the onset of IBD. In fact, administration of antibiotics sometimes shows the amelioration of IBD symptoms in patients9. However, other bacterial associations on access routes into local bowel areas have not been investigated.
Recently, oral bacteria-associated systemic diseases have received increasing attention10,11,12,13,14. Although there are rich microvessels in the dental mucosa, it does not have well-developed systematic and strict immune systems like the intestinal mucosa. Therefore, oral bacteria are considered capable of invading the blood circulation, resulting in bacteremia and other systemic diseases. Previous reports also indicate that bacteremia is associated with such dental procedures as tooth brushing and extraction15,16. Therefore, it should be considered that the mucosa in the dental pocket is an important access route for bacteria invading the blood circulation.
In general, pathogenic oral bacteria are classified into two major groups; one is the pathogen for dental caries, and the other is for periodontitis. Streptococcus mutans (S. mutans), a major pathogen of dental caries, is also known to be a causative agent of infective endocarditis. Recently, we reported the isolation of several specific strains of S. mutans; we confirmed that such strains had different serotypes from that of standard strain, MT814817,18,19. Most of these specific types of S. mutans belong to minor serotypes k or f. Serotype k was isolated from the blood of patients with bacteremia after tooth extraction or infective endocarditis17,18,19. S. mutans is classified into four serotypes (c/e/f/k): in the oral cavity of healthy subjects, serotype c has a prevalence of over 70%; serotype e accounts for approximately 20%; serotype f and k strains amount to less than 5%17,18,19. Thus, S. mutans has been identified as a possible pathogen not only for dental caries but also such systemic diseases as bacteremia and infective endocarditis. However, the association between S. mutans and other systemic diseases has not been elucidated.
Interestingly, we have recently found that the specific strains of S. mutans caused hemorrhagic damages in the murine brain and other tissues20,21. In those studies, we reported that the specific strains of S. mutans that express collagen-binding protein (CBP) and are serotype k (or f) caused hemorrhagic stroke because of the ability to bind the collagen and resistance to phagocytosis21. Therefore, we concluded that CBP-expressing and serotype k (or f) strains of S. mutans are highly virulent in hemorrhagic and inflammatory diseases. In fact, such highly virulent strains of S. mutans specifically caused serious damages on potentially injured or weakened parts. Therefore, it is considered that transient bacteremia of such highly virulent strains of S. mutans may cause the aggravation of ulcerative colitis if subjects have a potential damage or inflammation on colon. In addition, in our preliminary screening study, we found that the detection frequency of the specific strains of S. mutans was comparatively higher in UC patients than in healthy subjects. Thus, we speculate that such specific strains of S. mutans may be involved in the pathogenesis of UC. In the present study, we show that infection by the specific strains of S. mutans is a potential risk factor for the aggravation of ulcerative colitis. Our data presents a concept for new potential risk factors for UC.
Results
Aggravation of mouse colitis by TW295, a specific strain of S. mutans
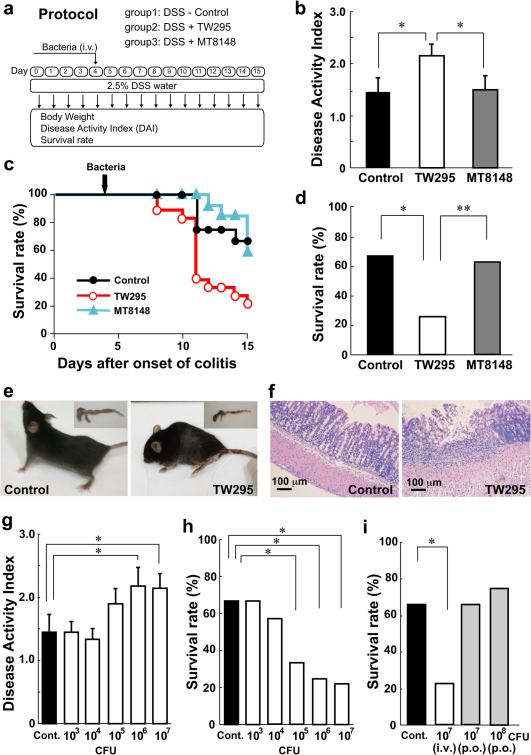
Symptoms of IBD in DSS-induced colitis began 4 days after the mice started drinking water containing DSS. Therefore, we administered the bacteria to the mice on day 4 (Fig. 1a). Intravenous administration of TW295, a serotype k strain of S. mutans, showed an increase in the disease activity index (DAI), which indicates the severity of colitis, in DSS-induced colitis mice compared with controls on day 10 (Fig. 1b). A tendency for increased body weight loss in TW295-infected mice was also observed (Supplementary Fig. S1). Interestingly, a marked decrease in the survival rate of TW295-infected mice was observed compared with controls (Fig. 1c, d). Obvious alterations of the whole animal and the colon in TW295-infected mice were already evident on day 10 (Fig. 1e). Mucosal damage and infiltration of inflammatory cells were microscopically observed in TW295-infected mice (Fig. 1f). In contrast, administration of MT8148, a serotype c standard strain of S. mutans, did not produce any aggravation of the colitis symptoms (Fig. 1b, c, d). These results clearly indicate that only the specific strain of TW295, the serotype k strain, could aggravate the inflammation of DSS-induced mouse colitis.
Figure 1. Aggravation of mouse colitis by TW295, a specific strain of S. mutans.
(a) Intervention study design. Drinking water containing DSS was administered from days 0 to 15. Bacteria were intravenously injected on day 4. (b) Disease activity index (DAI) on DSS-induced colitis mice 6 days after administration of vehicle (Control), TW295, or MT8148. Each column represents the mean ± standard error (SEM) from 12–18 different animals. Statistical significance was determined using Bonferroni’s method after analysis of variance (ANOVA). * p < 0.05. (c) Time-course change of survival rate during the experimental period. Kaplan-Meier analysis was carried out for statistics. TW295; p = 0.0134 versus control, and MT8148; p = 0.9399 versus control, respectively. (d) Survival rate on day 15. Each column represents the mean from 12–18 different animals. ** p < 0.01, * p < 0.05, respectively. (e) Typical appearance of the whole animal and the colon in control and TW295-infected mice on day 10. (f) Typical microscopic images of the colon in control and TW295-infected mice on day 10. Mucosal damage and infiltration of inflammatory cells were observed in TW295-infected mice. (g) & (h) Dose-dependent effects of TW295-mediated aggravation of colitis. (g) DAI on day 10 and (h) survival rate on day 15. A dose-dependent increase in DAI and decrease in survival rate were observed with TW295. Each column represents the mean from 12–18 different animals. Statistical significance was determined using Bonferroni’s method after ANOVA. **p < 0.01, *p < 0.05, respectively. (i) Route-dependent effects of TW295-mediated aggravation of colitis. TW295 was administered intravenously (i.v.) or orally (p.o.). Each column represents the mean from 12–18 different animals (*p < 0.05).
Next, we conducted the dose-dependency investigation of how many bacterial cells were required to cause the TW295-mediated aggravation of colitis. The increase in DAI and decrease in survival rate by TW295 were observed with the administration of 105 bacterial cells (Fig. 1g, h). Since such bacterial cell numbers invading the blood circulation are considered easily achievable with dental procedures15, transient bacteremia may be involved in the aggravation of colitis in clinical situations. We also investigated the route-dependency of TW295-induced aggravation of colitis. Even the oral administration of more than 108 TW295 cells did not produce any aggravation of the colitis symptoms (Fig. 1i). These results clearly indicate that the route the TW295-mediated aggravation of colitis in the intestinal mucosa is through the blood circulation, not from the mucosal lumen side of the gastrointestinal tract.
Target organs of TW295-induced aggravation of colitis
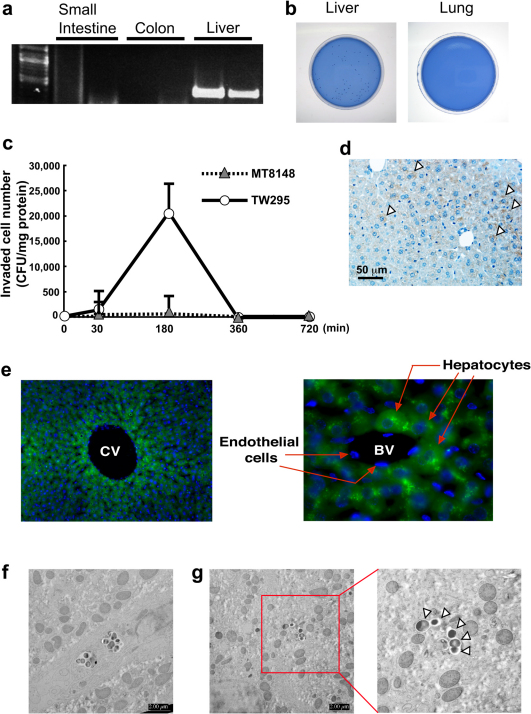
To investigate the target organs causing the TW295-induced aggravation of colitis, we initially tried to detect the invading bacteria in the gastrointestinal tract. However, we could not detect the invading bacteria either in the colon or small intestine (Fig. 2a). Surprisingly, the administered TW295 was specifically localized in the liver. The invaded TW295 was still surviving, which we confirmed by the recovery of living TW295 in liver homogenate (Fig. 2b). In addition, we checked the time-course of TW295 localization in the liver and discovered that the peak of maximum localized time was 180 min after administration (Fig. 2c). In contrast, MT8148, the standard strain of S. mutans, was scarcely localized in the liver. These results indicate that the localization of administered bacteria in the liver is specific for TW295, but not the standard strain of S. mutans.
Figure 2. Investigation of real target organs of TW295-induced aggravation of colitis.
(a) Detection of infected bacteria in organs by PCR. Whole DNA was extracted and the nested PCR method was performed. Each organ represents the results from two different animals. (b) Confirmation of viable bacteria in liver tissue. The tissue specimens were cut into small pieces and squeezed, then inoculated onto mitis salivarais bacitracin (MSB) agar plates. (c) Time-dependent localizations of viable bacteria in liver tissues. Viable cell numbers are expressed as colony forming units (CFU)/mg tissue protein. Each point represents the mean ± SEM from 3–5 different animals. (d) Immunohistochemical stainig of serotype k bacteria in the DSS mouse liver. Arrowheads represent positive staining. (e) Localization of GFP-expressing TW295 in the mouse liver. Administered GFP-expressing TW295 were uptaken by hepatocytes, but not by endothelial cells. Left, low magnification of central vein area; right, high magnification of small blood vessel. The blue areas represent nuclear staining by 4’6-diamino-2-phenylindole (DAPI). CV, central vein; BV, blood vessel. (f) Uptake of TW295 by Kupffer cells using ex vivo electron microscopy. (g) Uptake of TW295 by hepatocytes using ex vivo electron microscopy. Arrowheads represent TW295 uptake by hepatocyte.
To investigate where the invading were localized in the liver, immunohistochemical staining of serotype k strain using anti-serotype k antibody was performed. As shown in Fig. 2d, localization of serotype k strain was observed in hepatocytes. In addition, we prepared green fluorescent protein (GFP)-expressing TW295 and used it to confirm the localization in hepatocytes. We observed uptake of administered GFP-expressing TW295 by hepatocytes, but not endothelial cells (Fig. 2e). Furthermore, we confirmed the uptake of TW295 by hepatocytes and other cells using ex vivo electron microscopic observation. As shown in Fig. 2f, there was uptake of TW295 by Kupffer cells. This was not unexpected since Kupffer cells usually uptake bacteria. Interestingly, it was observed that hepatocytes also uptake TW295 (Fig. 2g). Since the majority of cells in the liver are hepatocytes, these results clearly indicate that the localized TW295 was mainly uptaken by hepatocytes.
Interestingly, increases in plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were observed in TW295-administered mice 24 hours after infection (Supplementary Fig. S2a). However, the increase was transient: the increased ALT and AST levels returned to base levels similar to those of control mice on day 9 (Supplementary Fig. S2b). In addition, visible tissue damage to the liver was not observed in TW295-administered mice (Supplementary Fig. S2c). Since the TW295-administered mice began to die on day 8—10 (Fig. 1c), this indicates that the cause of death in TW295-administered mice was not due to direct liver toxicity. Transient localization of TW295 in the liver may trigger unknown pathways in the aggravation of colitis.
Target molecules involved in localization of TW295 in the liver
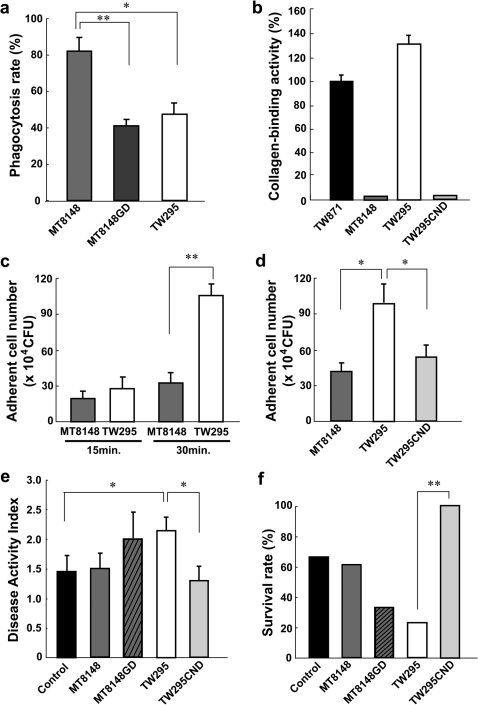
We considered why only TW295, not MT8148, can survive in the blood circulation, reach the liver, and be uptaken by hepatocytes. An important difference between TW295 and MT8148 is that of serotype. Therefore, we focused on the surface glucose side chains that define the serotype of S. mutans. It is believed that the difference in these surface glucose side chains plays a role in the phagocytosis17. The phagocytosis rate of TW295 by polymorphonuclear leukocytes (PMNs) is much lower than that of MT8148, the standard strain (Fig. 3a). To confirm the effect of the glucose side chains, we prepared an isogenic mutant strain of MT8148 with a glucose defection in the side chains (MT8148GD); the mutant strain had similar glucose side chains to those of TW295 (serotype k). As shown in Fig. 3a, the phagocytosis rate of MT8148GD was lower than that of MT8148. In addition, MT8148GD showed the high survivability in blood circulation like TW295 in vivo (Supplementary Fig. S3). These results indicate that glucose side chains of serotype k-type S. mutans are involved in the survivability of TW295 type strains that escape phagocytosis in the blood circulation, and are able to reach the liver.
Figure 3. Target molecules involved in localization of TW295 in the liver.
(a) In vitro phagocytosis rates of various S. mutans. MT8148GD is an isogenic mutant strain of MT8148 with defection of glucose from the side chains; it has similar glucose side chains to TW295 (serotype k). Each column represents the mean ± SEM from 3 independent experiments. Statistical significance was determined using Student’s t-test. MT8148 versus MT8148GD; **p = 0.0071, MT8148 versus TW295; *p = 0.0134, respectively. (b) Collagen-binding activity of various strains of S. mutans. The activity was evaluated under the fixed condition of 2 mg of type I collagen and 1x1010 bacterial cells. The results for each strain are expressed as a percentage based on TW871 as 100%. TW295CND is a mutant strain from TW295 with a defect in the expression of CBP. Each column represents the mean ± SEM from 3 independent experiments. (c) Adhesion of TW295 or MT8148 to Huh-7 hepatocytes. Each column represents the mean ± SEM from 6 independent experiments. Statistical significance was determined using Student’s t-test. **p = 0.00914. (d) Decrease in adherent cells of TW295CND, CBP-defect strain generated from TW295, to Huh-7 hepatocytes. Each column represents the mean ± SEM from 4 independent experiments. Statistical significance was determined using Student’s t-test. MT8148 versus TW295; *p = 0.0157, TW295 versus TW295CND; *p = 0.0328, respectively. (e) DAI of TW295CND-administered mice and other S. mutans strains. Each column represents the mean ± SEM from 12–18 different animals. Statistical significance was determined using Bonferroni’s method after ANOVA. *p < 0.05. (f) Survival rate of TW295-administered mice and other S. mutans strains. Each column represents the mean from 12–18 different animals (**p < 0.01).
Next, we investigated the molecule involved in the adhesion and uptake of TW295 by hepatocytes. Recently, a cell-surface 120-kDa collagen-binding adhesin (CBP) was identified in S. mutans, and its coding gene was cloned and sequenced22. Interestingly, TW295 has this protein, and the collagen-binding activity of TW295 is dramatically higher than that of MT8148 (Fig. 3b). In addition, adhesion of TW295 to a Huh-7 hepatocyte, hepatocarcinoma-cell line, was significantly higher than with MT8148 (Fig. 3c). These results indicate that CBP of TW295 is involved in the adhesion to hepatocytes. Therefore, we generated an isogenic mutant strain of TW295 with a defect in the expression of CBP (TW295CND)23. Like MT8148, TW295CND was completely without the ability to bind collagen (Fig. 3b), and it showed lower adherent ability with Huh-7 hepatocytes (Fig. 3d). We administered the TW295CND strain to the DSS-induced mouse colitis model. A drastic reduction in DAI and marked recovery of survival rate were observed in TW295CND-administered mice compared with TW295-administered animals (Fig. 3e, f). These results clearly indicate that cell-surface CBP is important for adhesion to hepatocytes, resulting in colitis aggravation.
In contrast, MT8148GD showed a tendency, but not significant, to aggravate the colitis (Fig. 3e, f). Although MT8148GD acquired high survivability in blood circulation (Supplementary Fig. S3), the accumulation of MT8148GD in liver was much lower than that of TW295 (Supplementary Fig. S4). These results indicate that both CBP and specific glucose side chain are important for the accumulation in liver and colitis aggravation.
TW295-mediated increase in cytokine production
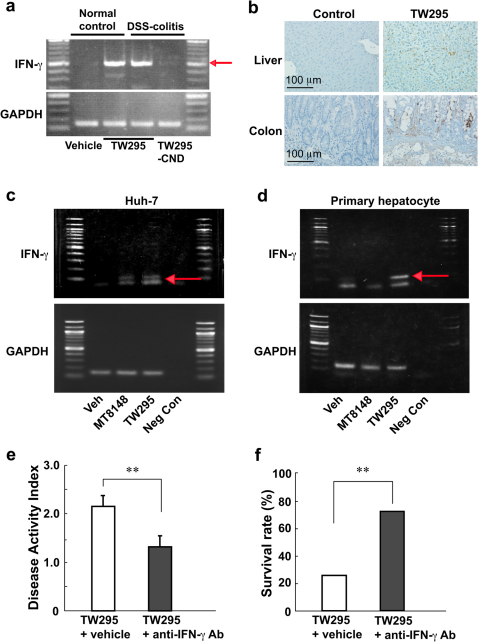
To investigate the upper stream of amplification molecules in the inflammatory response, we examined inflammatory cytokine expression in the TW295-administered mouse liver. As shown in Fig. 4a, administration of TW295 to both DSS-induced colitis mice and normal controls caused increased expressions of IFN-γ in liver tissues. However, no marked alterations were observed in the expression of other inflammatory cytokines, such as TNF-α and IL-6 (Supplementary Fig. S5). In contrast, administration of the CBP-deficient strain, TW295CND, did not produce an increase of IFN-γ in the liver (Fig. 4a). To confirm the results of the polymerase chain reaction (PCR) in tissues, immunohistochemical staining was performed for IFN-γ. As shown in Fig. 4b, increased expression of IFN-γ was observed in the liver and colon. These results indicate that the release of IFN-γ by the liver in TW295 infection may be the first step in the onset of the inflammatory cascade producing various inflammatory-related molecules.
Figure 4. TW295-mediated increase in IFN-γ productions and effect of anti-IFN-γ antibody on TW295-mediated aggravation of colitis.
(a): Increased expression of IFN-γ in liver tissues after TW295 to mice with DSS-induced colitis and normal controls. Arrow indicates the size of IFN-γ. GAPDH is an internal standard for PCR. (b): Immunohistochemical staining of IFN-γ in the liver and colon of mice after the administration of TW295 or vehicle (Control). The brown areas represent positive expression of IFN-γ. (c) & (d): Increased expression of IFN-γ on a hepatocarcinoma cell line, Huh-7 (c) or primary human hepatocytes (d) after the application of bacteria. Neg Con; negative control. GAPDH is an internal standard for PCR. (e): Effect of anti-IFN-γ antibody on DAI in TW295-infected DSS-induced colitis mice at 6 days after infection of TW295. The anti-IFN-γ polyclonal antibody (0.5 mg/mouse) was intraperitoneally administered mice at 4 and 24 hours after the TW295 infection. Each column represents the mean ± SEM from 10–18 different animals. Statistical significance was determined using Student’s t-test. **p = 0.0099. (f): Effect of anti-IFN-γ antibody on survival rate in TW295-infected DSS-induced colitis mice at 11 days after infection of TW295. The anti-IFN-γ polyclonal antibody (0.5 mg/mouse) was intraperitoneally administered mice at 4 and 24 hours after the TW295 infection. Each column represents the mean from 10–18 different animals. Statistical significance was determined using Student’s t-test. **p = 0.0059.
To confirm the results observed in the in vivo colitis model, we performed an in vitro cultured cell experiment. In contrast to MT8148, TW295-applied Huh-7 hepatocarcinoma cell lines showed increased expression of IFN-γ (Fig. 4c). Furthermore, we used human primary hepatocytes to confirm the results of Huh-7 and found that TW295-treated human hepatocytes showed increased expression of IFN-γ (Fig. 4d). These results suggest that release of IFN-γ from TW295-applied hepatocytes is the real trigger of the inflammatory cascade.
In addition, to confirm the further evidence that the TW295-mediated release of IFN-γ from hepatocytes is the real trigger of the inflammatory cascade in vivo model, we administered anti-IFN-γ antibody to TW295-infected colitis mice. The administration of anti-IFN-γ antibody dramatically suppressed TW295-mediated aggravation of colitis in comparison to no antibody administration group in vivo model (Fig. 4e, f). These results clearly prove that the TW295-mediated release of IFN-γ from liver is the real trigger of the inflammatory cascade in oral bacteria-induced aggravation of colitis.
Detection and isolation of TW295-type S. mutans strains from IBD patients
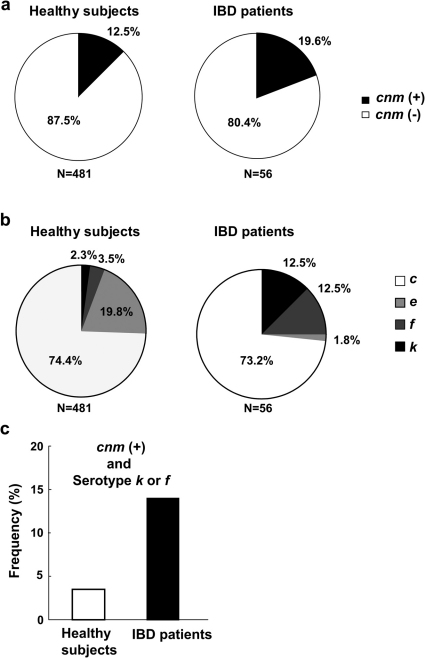
To further support our hypothesis that infection by TW295-type S. mutans is a potential risk factor in colitis aggravation, we compared the frequency of TW295-type S. mutans between IBD patients (Supplementary Tables S1, S2) and non-IBD healthy subjects using oral samples. The characteristics of TW295-type S. mutans are the existence of CBP and specific glucose side chains, both of them are closely related to the effects of TW295-induced colitis aggravation. As shown in Fig. 5a, the detection frequency of the CBP-encoding cnm gene expressing S. mutans was higher in UC patients than in healthy subjects (odds ratio, 1.72). Furthermore, it has been reported that two minor serotype-specific glucose side chains are involved in escape from phagocytosis, serotypes k and f17. Both serotypes are generally very rare (2.3% + 3.5% = 5.8%) in healthy subjects compared with major serotypes, such as serotypes c and e (Fig. 5b, left). In contrast, the detection frequency of both serotypes k and f was significantly higher (12.5% + 12.5% = 25.0%) in UC patients than in healthy subjects (Fig. 5b). We also calculated the frequency of specific strains of S. mutans that have both the cnm gene and a specific serotype (k or f) between UC patients and healthy subjects. As shown in Fig. 5c, the detection frequency of such specific strains of S. mutans in UC patients was dramatically higher than in healthy subjects (14.29% vs. 3.53%, respectively; p = 0.0012; odds ratio, 4.55; 95% confidence interval, 1.87—11.09). These results may indicate that infection of such specific strains of S. mutans that have both the cnm gene and a specific serotype (k or f) may affect aggravation of the UC pathology.
Figure 5. Detection and isolation of TW295-type S. mutans strains from IBD patients.
(a) Detection frequency of CBP-encoding cnm gene expressing S. mutans in IBD patients and healthy subjects. Amomg 98 IBD patients, 56 were S. mutans positive (57.14%). (b) Detection frequency of various serotypes. Serotype k and f are very rare (2.3% + 3.5% = 5.8%) in healthy subjects compared with major serotypes, such as serotypes c and e. In contrast, the detection frequency of serotype k and f was dramatically higher (12.5% + 12.5% = 25.0%) than with serotypes c and e. (c) The frequency of specific strains of S. mutans that have both the cnm gene and a specific serotype (k or f) in IBD patients and healthy subjects. The detection frequency of such specific strains of S. mutans in IBD patients was dramatically higher than in healthy subjects (14.29% vs. 3.53%, respectively; odds ratio, 4.55, 95%; confidence interval, 1.87–11.09; p = 0.0012).
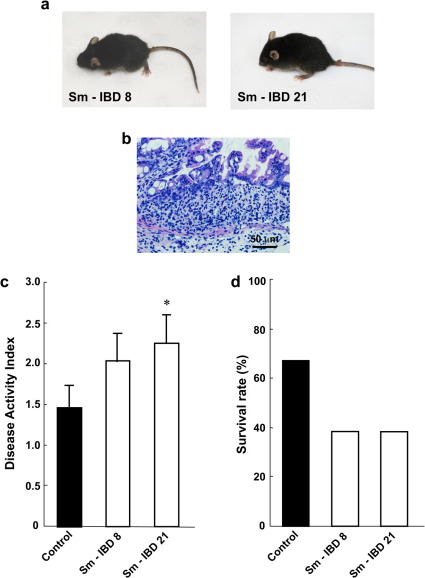
To confirm our observations, we tested the virulent effect of the Cnm-expressing and serotype k or f type of S. mutans strains isolated from IBD patients. We tested two CBP-expressing S. mutans strains from IBD patients (Sm-IBD8, serotype k; and Sm-IBD21, serotype f) in the DSS-colitis model, since they showed the expression of CBP and comparatively strong collagen-binding activity. Aggravation of colitis, evident in the increase in DAI and decrease in survival rates, was observed in mice administered with the two isolated CBP-expressing specific S. mutans strains compared with controls (Fig. 6). These results clearly indicate that infection of specific strains of TW295-type S. mutans is a risk factor in colitis aggravation.
Figure 6. Specific strains of S. mutans isolated from IBD patients aggravate the colitis.
(a) Typical photos of whole mice administered isolated S. mutans. Sm-IBD 8 was isolated from patient 8 and Sm-IBD 21 from patient 21 in Supplementary Table 2. (b) Typical microscopic image of the colon of Sm-IBD8-infected mice on day 10. Mucosal damage and infiltration of inflammatory cells were observed in Sm-IBD 8-infected mice. (c) DAI of isolated S. mutans strains from IBD patients. Each column represents the mean ± SEM from 10–12 different animals. Statistical significance was determined using Bonferroni’s method after ANOVA. *p < 0.05. (d) Survival rate of isolated S. mutans strains from IBD patients. Each column represents the mean from 10–12 different animals.
Discussion
In the present study, we clearly showed that infection of specific strains of S. mutans is one of the risk factors in aggravating inflammation of UC. This is the first report describing the involvement of oral bacteria in UC pathology.
Oral bacteria-related aggravation of colitis was clearly demonstrated in the mouse colitis model infected with serotype k and the CBP-expressing S. mutans strain, TW295. In addition, administered bacteria were detected in the liver, but not in the colon and small intestine, indicating that the interaction of serotype k and CBP-expressing S. mutans with hepatocytes is a crucial event in the development of colitis inflammation. These specific strains exhibited common cell-surface conditions, such as expression of CBP and specific glucose side chains that define the bacterial serotypes. Our hypothesis is that both CBP and specific serotypes of k or f on S. mutans are necessary for colitis aggravation. Such hypothesis is supported by our results that the CBP-defective mutant strain, TW295CND, produced no aggravation of colitis and that the isogenic mutant strain of MT8148 with defection of glucose side chains, MT8148GD, escaped phagocytosis, required high survivability in circulation and showed the tendency of colitis aggravation. These specific characteristics of TW295 enabled it to reach the liver and adhere to hepatocytes, resulting in the invasion into hepatocytes. In fact, we have proposed direct evidence of uptake of oral bacteria by hepatocytes. This is the first report about the uptake of infected oral bacteria by hepatocytes. Since the majority of liver cells are hepatocytes, uptake of TW295 by hepatocytes may be the most important step in causing the aggravation of further inflammatory responses. Although the survivability of the specific strains of S. mutans in the blood circulation may be due to characteristics of glucose side chains, it is unclear what mechanisms and molecules are involved in adherence to and uptake by hepatocytes. At least, our results presented here indicate that CBP-related molecule(s) or mechanism(s) are involved in the uptake of TW295-type oral bacteria by hepatocytes.
Adhesion or invasion into hepatocytes by the specific strains of S. mutans may activate the hepatocytes themselves, because both cultured hepatocytes and liver tissues generated IFN-γ upon bacterial stimulation. In general, IFN-γ is produced by the liver in response to bacterial and viral infection. However, the step of S. mutans-mediated generation of IFN-γ may trigger the inflammatory reaction cascade to produce many other inflammatory molecules that accelerate the further inflammation. These molecules, including IFN-γ, may be initially generated from hepatocytes and Kupffer cells and finally reach the colon to aggravate the inflammation of colitis (Supplementary Fig. S6). In fact, the administration of anti-IFN-γ antibody in vivo dramatically suppressed TW295-mediated aggravation of colitis. These results clearly prove that the TW295-mediated release of IFN-γ from liver is the initial trigger to cause the activation of inflammatory cascade in oral bacteria-induced aggravation of colitis. We, therefore, hypothesize the following mechanistic scheme. After the invasion of specific types of S. mutans, they escape phagocytosis through their specific glucose side chains and survive for a long time in the blood circulation. Immune cells may be unable to recognize the structures of such specific glucose side chains as bacteria. After the journey to liver, the specific types of S. mutans adhere to and invade hepatocytes by expression of CBP. Then, bacteria-stimulated hepatocytes generate IFN-γ following the release of various inflammatory molecules. Such molecules reach the colon resulting in acceleration of the inflammatory response to aggravate colitis (Supplementary Fig. S6). This approach of oral bacteria is from the inside of blood circulation, but not from the mucosa of the lumen side of the gastrointestinal tract, because oral administration of TW295 did not produce colitis aggravation. Our results support this novel concept about oral bacteria-mediated aggravation of colitis.
It is unknown why hepatocytes passively uptake such specific types of S. mutans. One possibility is a final exclusion mechanism for dangerous bacteria as a protection against bacteremia, because such specific strains of bacteria have already broken through the intrinsic defense lines of the immune system. Fundamentally, it may be questioned how so many bacteria can easily invade the blood circulation from the oral mucosa through dental procedures. Previous reports indicated that bacteremia can easily occur through such dental procedures as tooth brushing and extraction14,15. In addition, the aggravation by TW295 was observed with only 104 bacterial cells. Such cell numbers are easily achievable with ordinary dental procedures14,15.
The rate of oral cavity infection by specific types of S. mutans with CBP in healthy subjects was 12.5%. In addition, the detection frequency of specific serotypes of S. mutans (k and f) was 2.3% and 3.5%, respectively. Thus, in normal situations, only a limited number of strains are possible risk factors for aggravation of UC caused by S. mutans-induced bacteremia. In contrast, the detection frequency of specific strains of S. mutans, such as CBP-positive and phagocytosis-resistant strains from UC patients, was extremely higher than in non-UC control subjects (p = 0.0012; odds ratio, 4.55). These results indicate that bacteremia with highly virulent TW295-type S. mutans may be an important risk factor for aggravation of colitis in many clinical cases. In addition, we showed that several S. mutans strains isolated from UC patients caused aggravation of colitis in a mouse DSS model, indicating the potential involvement of highly virulent S. mutans in occurrences of UC. These results clearly suggest that infection by highly virulent S. mutans is, in part, involved in the aggravation or occurrence of UC. It has been reported that administration of antibiotics shows amelioration of IBD symptoms in patients9. This report may partially support our hypothesis. Further large-scale clinical-screening investigations about the relationship between the infection of highly virulent S. mutans and the occurrence of IBD are required.
In conclusion, infection with highly virulent specific types of S. mutans is a potential risk factor for UC. The important identified virulence factors for the aggravation of colitis are the presence of CBP and the lack of surface glucose side chains. This is the first report describing the involvement of oral bacteria in IBD pathology.
Methods
Reagents and antibodies
Dextran sodium sulfate (DSS; molecular weight, 36,000—50,000) was purchased from MP Biomedicals, LLC (Solon, OH, USA). Anti-interferon gamma (IFN-γ) polyclonal antibody was purchased from BioLegend (San Diego, CA, USA) and Santa Cruz Biotechnology (Santa Cruz, CA, USA). All other chemicals were of reagent grade.
Bacterial strains and culture condition
MT8148, the standard strain of S. mutans (serotype c) isolated from the oral cavity of a healthy child, and TW295, a serotype k strain isolated from a subject with bacteremia after tooth extraction, were used in this study17,18,19,24. In addition, two isolated clinical S. mutans strains (Sm-IBD 8 and Sm-IBD 21) from oral samples of IBD patients were used in this study. We also used mutant strains, TW295CND derived from TW295, and MT8148GD derived from MT8148 to investigate the mechanism. Namely, TW295CND is an isogenic mutant strain of TW295 with insertional inactivation with an erythromycin-resistant gene in the middle of cnm encoding collagen-binding protein (CBP)23. MT8148GD is a glucose side chain-defective mutant strain of MT8148 constructed by inactivation of the gene encoding the enzyme for biosynthesis of the glucose side chain by an erythromycin-resistant gene17. Furthermore, we prepared green-fluorescent protein (GFP)-expressing TW295 and used it in this study21. All strains were grown in brain heart infusion (BHI) broth (Difco Laboratories, Detroit, MI, USA) as well as on mitis salivarius agar with bacitracin (MSB, 100 U/ml; Sigma-Aldrich, St.Louis, MO, USA) and 15% sucrose (MSB agar).
DSS-induced mouse colitis model and administration of S. mutans to mice
All mice were treated humanely in accordance with the National Institutes of Health and AERI-BBRI Animal Care and Use Committee guidelines. All animal experiments were approved by the institutional animal care and use committee of Osaka University Graduate School of Dentistry. DSS-induced colitis model was performed according to the method previously described25,26. Specific pathogen free, male C57BL/6J mice were purchased from CLEA Japan (Shizuoka, Japan). Colitis was induced in 7-week-old mice (weighing 20–25 g) with 2.5% (w/v) DSS in the drinking water ad libitum which results in clinical symptoms of UC after 4 days and perforation of the gut and death at approximately 10–15 days25,26.
Bacterial suspensions in phosphate-buffered saline (PBS) were intravenously administered via the jugular vein (103–107 cells/body) on day4 after the onset of DSS-colitis (Fig. 1a). Mice were weighed and visually inspected for diarrhea and rectal bleeding after induction of colitis. Then, an average disease activity index (DAI), including such signs as diarrhea and bleeding was calculated based on data collected by double-blinded observers according to a scoring method, as previously described25,26. Survival rates of the mice were also recorded.
To investigate the role of IFN-γ on TW295-mediated aggravation of colitis, anti-IFN-γ polyclonal antibody (0.5 mg/mouse) was intraperitoneally administered mice at 4 and 24 hours after the TW295 infection. The DAI and survival rate were evaluated.
Histological analysis and immunohistochemical staining
For histological analysis, tissue samples were fixed in 3.7% formaldehyde-PBS, embedded in paraffin, and 3-micrometer sections were stained with hematoxylin-eosin (H&E). Alterations of IFN-γ expression in the tissues were detected using standard immunohistochemical techniques with each antibody. The vectastain ABC kit (Vector Laboratories, Burlingame, CA) was used with the 3, 3’-diaminobenzidine substrate kit (Vector Laboratories), according to the manufacturer’s instructions, as previously described26.
Detection of bacteria in tissue samples
The colon, small intestine, liver, and lung were extirpated from the mice after the injection of 1x107 colony formation units (CFU) of S. mutans TW295 into the jugular vein. Detection of the infecting bacteria in the organs was carried out by PCR as follows. Whole DNA was extracted from the extirpated tissues, and PCR was carried out using S. mutans-specific sets of primers, as described previously17. The nested PCR method was also performed to determine positive reactions.
To confirm that viable strains were in the tissue, tissue specimens were cut into small pieces and squeezed; this was then inoculated onto MSB agar plates and anaerobically incubated at 37°C for 48 hours. These were used as the culture agar plates for S. mutans. The number of bacterial colonies was counted by visual inspection of the agar plates.
Phagocytosis assay
Phagocytosis assay was performed as described previously17. Briefly, the tested organisms were cultured in BHI for 18 hours at 37°C. After the bacterial cells were washed, the cell concentrations were adjusted with PBS to 1.0x108 CFU/ml. Human peripheral blood (500 µl) was collected from a healthy volunteer and incubated with 500 µl (5.0x107 CFU) of the tested bacteria for 10 min at 37°C. Interactions between polymorphonuclear leukocytes (PMNs) and bacteria were observed using a light microscope (magnification, x100; Olympus Optical, Tokyo, Japan) following Giemsa staining (Wako Pure Chemicals, Osaka, Japan). The rate of phagocytosis was expressed as the mean ratio of phagocytosed PMNs per 100 PMNs, with 500 PMNs being examined. Data are expressed as the mean ± standard errors of triplicate experiments.
Collagen-binding assay
The collagen-binding properties of mutants and parent strains were evaluated according to the method described by Waterhouse and Russell27, with some modifications as described previously28. The results for each strain are expressed as a percentage compared with TW871 binding, which was defined as 100%.
Bacterial adhesion assay
Adhesion of bacteria onto hepatic cells was investigated as follows. Approximately 1x105 Huh-7 cells were seeded in parallel wells of 6-well tissue-culture plates. Prior to infection, the wells were washed 3 times with PBS, then antibiotic-free medium was added. Huh-7 cells were infected by the addition of 1x108 CFU of strains TW295 and MT8148. After 15 and 30 minutes of aerobic incubation, the medium was removed and the infected cells were washed 3 times with PBS. To test for adherence, 1.0 ml of sterile distilled water was added and the cells were allowed to burst. Dilutions of the cell lysate infected with S. mutans were plated on MSB agar and cultured at 37°C for 48 hours in an anaerobic condition. The number of adherent bacteria was determined and expressed as the mean ± standard errors of triplicate experiments.
Quantitative RT-PCR for detection of cytokine mRNA
Quantification of mRNA encoding for TNF-α, IL-6, and IFN-γ in the tissues after the administration of S. mutans was conducted using an RT-PCR system. Briefly, tissue specimens were extirpated 24 hours after infection of 1x107 CFU of S. mutans TW295 via the jugular vein. For reverse transcription-PCR (RT-PCR), total RNA was prepared using the SV Total RNA Isokation System (Promega), and total RNA was reversed transcribed by using the High-capacity cDNA Reverse Transcription kit (Applied Biosystems) according to the manufacturer’s instructions. The primer information is described in Supplementary Table S3. Messenger RNA expression of TNF-α, IL-6, and IFN-γ in the tissues was determined. Data were normalized using GAPDH as an internal standard.
In the case of cultured cells, S. mutans TW295 strains (1x108 CFU) were added to Huh-7 cells (1×105 cells/well) in a 6-well culture plate, and the cultures were incubated at 37°C in 5% CO2 for 5 hours. RT-PCR was performed by the method described above. In addition, we used primary hepatocytes isolated from a normal human liver (Lonza Walkersville,Inc., MD) to confirm the results with the Huh-7 cells.
Ethics regarding human subjects
Study protocols in humans were approved by the Ethics Committee of Yokohama City University School of Medicine and Osaka University Graduate School of Dentistry.
All subjects were informed of the protocols and gave their written consent prior to participating in the study. Oral samples were collected from Yokohama City University Hospital and Osaka University Dental Hospital. A summary and detailed information of the IBD patients are presented in Supplementary Tables S1 and S2. Non-IBD subjects who did not have a history of any other disease, such as IBD, heart failure, rheumatoid arthritis, and kidney disease, were defined as control subjects.
Detection of bacteria in the oral samples was performed according to the method described previously17.
Statistical analysis
All results are expressed as means ± standard errors (SEM) or standard deviation (SD). Fisher’s protected least-significant difference test, Student’s t-test, Bonferroni’s method after analysis of variance (ANOVA), regression analysis and Kaplan-Meier analysis were carried out. The results were considered significantly different at p < 0.05.
Author Contributions
KW designed the entire study protocol under the supervision of AN, YK, KU and TO. In vitro studies were performed by KW, AK, KK, RN, and in vivo experiments were carried out by KW, AK, KH, KN, RN prepared and cultured S. mutans. Electron microscopy was performed by YM. Clinical sample collections and suggestions for IBD patients were provided by HT, TH, NM, AN. KN, KW performed the statistical analyses and interpretation of the results. KW wrote the manuscript, which all authors read and approved.
Supplementary Material
Supplementary Information
Acknowledgments
The authors thank Prof. Ichiro Nakagawa, Tokyo Medical and Dental University for preparing GFP-expressing TW295. This study was supported by a Grant-in-Aid for Scientific Research (A) 19209063, (C) 21592357 from the Japan Society for Promotion of Science, as well as a Grant-in-Aid for Young Scientists (A) 21689052 and (B) 21792067 from the Ministry of Education, Culture, Sports, Science and Technology of Japan; it was also supported by the Osaka Medical Research Foundation for Intractable Diseases.
Patients consent: Obtained.
Ethics approval: Study protocols in humans were approved by the Ethics Committee of Yokohama City University School of Medicine and Osaka University Graduate School of Dentistry.
References
- Blumberg R. S. & Strober W. Prospects for research in inflammatory bowel disease. JAMA 285, 643–647 (2001). [DOI] [PubMed] [Google Scholar]
- Shanahan F. Inflammatory bowel disease: immunodiagnostics, immunotherapeutics, and ecotherapeutics. Gastroenterol 120, 622–635 (2001). [DOI] [PubMed] [Google Scholar]
- Wada K., Nakajima A. & Blumberg R. S. PPARgamma and inflammatory bowel disease: A new therapeutic target for ulcerative colitis and Crohn’s disease. Trends Mol Med 7, 329–331 (2001). [DOI] [PubMed] [Google Scholar]
- Kaser A. & Blumberg R. S. Lesson from type I interferons in ulcerative colitis. Gut 60, 430–431 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus E. V. Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 126, 1504–1517 (2004). [DOI] [PubMed] [Google Scholar]
- Nagalingam N. A., Kao J. Y. & Young V. B. Microbial ecology of the murine gut associated with the development of dextran sodium sulfate-induced colitis. Inflamm Bowel Dis 17, 917–926 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nell S., Suerbaum S. & Josenhans C. The impact of the microbiota on the pathogenesis of IBD: Lessons from mouse infection models. Nat Rev Immunol 8, 564–577 (2010). [DOI] [PubMed] [Google Scholar]
- Saleh M. & Trinchieri G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat Rev Immunol 11, 9–20 (2011). [DOI] [PubMed] [Google Scholar]
- Khan K. J. et al. Antibiotic therapy in inflammatory bowel disease: A systematic review and meta-analysis. Am J Gastroenterol 106, 661–673 (2011). [DOI] [PubMed] [Google Scholar]
- Kinane D. F., Riggio M. P., Walker K. F., MacKenzie D. & Shearer B. Bacteremia following periodontal procedures. J Clin Periodontol 32, 708–713 (2005). [DOI] [PubMed] [Google Scholar]
- Friedewald V. E. et al. The Americal Journal of Cardiology and Journal of Periodontology Editors’ consensus: periodontitis and atherosclerotic cardiovascular disease. Am J Cardiol 104, 59–68 (2009). [DOI] [PubMed] [Google Scholar]
- Wada K. & Kamisaki Y. Roles of oral bacteria in cardiovascular diseases–From molecular mechanisms to clinical cases: Involvement of Porphyromonas gingivalis in the development of human aortic aneurysm. J Pharmacol Sci 113, 115–119 (2010). [DOI] [PubMed] [Google Scholar]
- Wada K. & Kamisaki Y. Molecular dissection of Porphyromonas gingivalis–related arteriosclerosis: A novel mechanisms of vascular disease. Periodontol 2000 54, 222–234 (2010). [DOI] [PubMed] [Google Scholar]
- Moreillon P. & Que Y. A. Infective endocarditis. Lancet 363, 139–149 (2004). [DOI] [PubMed] [Google Scholar]
- Sconyer J. R., Crawford J. J. & Moriarty J. D. Relationship of bacteremia to toothbrushing in patients with periodontitis. J Am Dent Assoc 87, 616–622 (1973). [DOI] [PubMed] [Google Scholar]
- Lockhart P. B. et al. Bacteremia associated with toothbrushing and dental extraction. Circulation 117, 3118–3125 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K. et al. Demonstration of Streptococcus mutans with a cell wall polysaccharide specific to a new serotype, k, in the human oral cavity. J Clin Microbiol 42, 198–202 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K. et al. Detection of novel serotype k Streptococcus mutans in infective endocarditis patients. J Med Microbiol 56, 1413–1415 (2007). [DOI] [PubMed] [Google Scholar]
- Ooshima T., Izumitani A., Sobue S., Okahashi N. & Hamada S. Non-cariogenicity of the disaccharide palatinose in experimental dental caries of rats. Infect Immun 39, 43–49 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K., Nakano K., Ooshima T. & Kamisaki Y. Bacteremia by virulent oral bacteria is a potent risk factor for stroke under endothelial cell injury condition. J Pharmacol Sci 112:(Supple 1), 47P (2010). [Google Scholar]
- Nakano K. et al. The collagen-binding protein of Streptococcus mutans is involved in hemorrhagic stroke. Nat Commun 10.1038/ncomms1491 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y. et al. Streptococcus mutans strains harboring collagen-binding adhesin. J Dent Res 83, 534–539 (2004). [DOI] [PubMed] [Google Scholar]
- Nakano K. et al. Molecular characterization of Streptococcus mutans strains containing the cnm gene encoding a collagen-binding adhesin. Arch Oral Biol 55, 34–39 (2010). [DOI] [PubMed] [Google Scholar]
- Nakano K. et al. Protein antigen in serotype k Strepococcus mutans clinical isolates. J Dent Res 87, 964–968 (2008). [DOI] [PubMed] [Google Scholar]
- Saubermann L. J. et al. Peroxisome proliferator-activated receptor gamma agonist ligands stimulate a Th2 cytokine response and prevent acute colitis. Inflamm Bowel Dis 8, 330–339 (2002). [DOI] [PubMed] [Google Scholar]
- Katayama K. et al. A novel PPAR gamma gene therapy to control inflammation associated with inflammatory bowel disease in a murine model. Gastroentarol 124, 1315–1324 (2003). [DOI] [PubMed] [Google Scholar]
- Waterhouse J. C. & Russell R. R. B. Dispensable genes and foreign DNA in Streptococcus mutans. Microbiology 152, 1777–1788 (2006). [DOI] [PubMed] [Google Scholar]
- Nomura R. et al. Molecular and clinical analyses of the gene encoding the collagen-binding adhesin of Streptococcus mutans. J Med Microbiol 58, 469–475 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information