ABSTRACT
Annexin A5 (AnxA5) has a high affinity for phosphatidylserine. The protein is widely used to detect apoptotic cells because phosphatidylserine, a phospholipid that is normally present in the inner leaflets of cytoplasmic membranes, becomes translocated to the outer leaflets during programmed cell death. Here we report the novel observation that AnxA5 binds to Gram-negative bacteria via the lipid A domain of lipopolysaccharide (LPS). Binding of AnxA5 to bacteria was measured quantitatively, confirmed by fluorescence microscopy, and found to be inhibited by antibodies against lipid A. AnxA5 also bound to purified dot-blotted LPS and lipid A. Through ellipsometry, we found that the binding of AnxA5 to purified LPS was calcium dependent and rapid and showed a high affinity—characteristics similar to those of AnxA5 binding to phosphatidylserine. Initial functional studies indicated that AnxA5 can affect LPS activities. AnxA5 inhibited LPS-mediated gelation in the Limulus amebocyte lysate assay. Incubation of LPS with the protein reduced the quantity of tumor necrosis factor alpha (TNF-α) released by cultured monocytes compared to that released upon incubation with LPS alone. Initial in vivo experiments indicated that injection of mice with LPS preincubated with AnxA5 produced serum TNF-α levels lower than those seen after injection of LPS alone. These data demonstrate that AnxA5 binds to LPS and open paths to investigation of the potential biological and therapeutic implications of this interaction.
IMPORTANCE
AnxA5 is highly expressed in cells that have a barrier function—including, among others, vascular endothelium, placental trophoblasts, and epithelial cells lining bile ducts, renal tubules, mammary ducts, and nasal epithelium. The protein has been well characterized for its binding to phospholipid bilayers that contain phosphatidylserine. This report of a previously unrecognized activity of AnxA5 opens the door to investigation of the possibility that this binding may have biological and therapeutic ramifications. In view of the tissue expression of the protein, the present results suggest the possibility that AnxA5 plays a role in modulating the host defense against lipopolysaccharide at these anatomic sites, where cells may interface with microorganisms. These results also raise the intriguing possibility that AnxA5 or analogous proteins or peptides could provide novel approaches to addressing the difficult clinical problem of Gram-negative sepsis.
Introduction
Annexin A5 (AnxA5; a protein that is generally better known by its former name, annexin V) binds to phospholipids in a calcium-dependent manner and forms two-dimensional crystal lattices over the phospholipid bilayers that express phosphatidylserine (1). AnxA5 has become a widely used marker for detecting apoptotic cells because phosphatidylserine, which is normally localized within the internal leaflets of cytoplasmic membranes, is expressed on the cell surface during programmed cell death (2–4).
The biological function of AnxA5 has not been established. The protein is highly expressed by cells that serve a barrier function, including vascular endothelium cells and placental trophoblasts (for a review, see reference 5). A main focus has been on the protein’s anticoagulant properties, which result from its high affinity for anionic phospholipids (6, 7). There is significant evidence that the protein serves an antithrombotic function on vascular endothelial cells and placental trophoblasts since autoantibody-mediated deficiencies are associated with vascular atherothrombosis (8, 9) and with recurrent pregnancy losses (10–12). In addition, AnxA5 has been shown to modulate tissue factor expression (13), to promote endocytosis (14), and to participate in cell protection from engulfment by phagocytosis (15). However, the fact that the protein is highly expressed by cells that have a barrier function but do not play any role in blood coagulation—such as biliary, pancreatic, salivary, and renal ductular epithelial cells (16) and mammary epithelium cells (17)—suggests that it may serve other functions.
Lipopolysaccharide (LPS), a complex lipoglycan that is expressed in the outer membrane of Gram-negative bacteria, is the key molecule responsible for the clinical manifestations of Gram-negative sepsis and septic shock. The lipid A domain, which is mainly responsible for the endotoxin effect of LPS (18), is highly conserved across bacterial species. LPS activates the host defense response through the binding of the lipid A domain to a receptor complex that includes Toll-like receptor 4, CD14, and MD2 (19) on monocytes and other cell types which, in turn, triggers the innate immune response which is characterized by secretion of proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α).
In view of the interesting evidence that suggests a potential role for bacteria in triggering disorders that have an autoimmune component—e.g., the antiphospholipid syndrome (20) and heparin-induced thrombocytopenia with thrombosis (21)—we wondered whether bacteria might also bind AnxA5. To our surprise, we found this to be the case for Gram-negative bacteria but not Gram-positive bacteria. We found that AnxA5 binds to the lipid A portion of LPS and intact LPS. Furthermore, we demonstrated that, similar to the binding of AnxA5 to phospholipid, the binding of AnxA5 to LPS is rapid, shows a high affinity, and is calcium dependent. Finally, in initial studies to evaluate the possibility of functional implications, we showed that AnxA5 binding can also reduce endotoxin effects of LPS.
RESULTS
AnxA5 binding to bacteria.
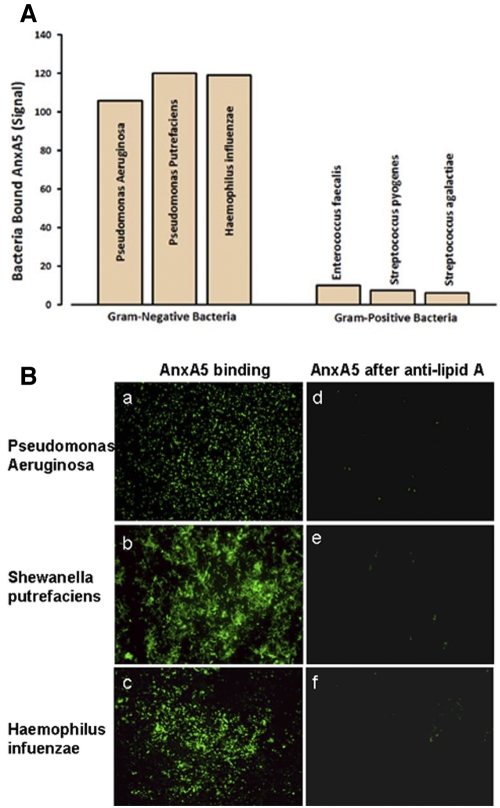
Pure cultures of bacteria were screened for AnxA5 binding activity using a standard fluorescence-tagged AnxA5 protein that is used to detect apoptotic cells. We found that Gram-negative bacteria, including Pseudomonas aeruginosa, Shewanella putrefaciens, and Haemophilus influenzae, bound the protein in the presence of calcium but not in its absence, while there was no appreciable binding to Gram-positive bacteria, including Enterococcus faecalis, Streptococcus pyogenes, and Streptococcus agalactiae (Fig. 1A).
FIG 1 .
AnxA5 binding to bacteria. (A) Measurements of AnxA5 binding to Gram-negative and -positive bacteria. Bacteria were incubated with OG-AnxA5 in HBS containing 5 mM CaCl2. The bound AnxA5 was dissociated from bacteria with EDTA and measured. The Gram-negative bacteria had markedly higher levels of bound AnxA5 than the Gram-positive bacteria (mean fluorescence intensity ± standard deviation, 114.9 ± 7.9 versus 7.7 ± 1.9). (B) Fluorescence microscopic confirmation of OG-AnxA5 binding to Gram-negative bacteria. Bacteria incubated with OG-AnxA5 were stained strongly with Oregon Green (a, c, and e); bacteria that were pretreated with antibody against the lipid A core of LPS prior to incubation with OG-AnxA5 had markedly reduced Oregon Green staining (b, d, and f) (×1,000 magnification under oil immersion).
These quantitative results were confirmed by fluorescence microscopy of the bacteria (Fig. 1B, parts a, b, and c). The apparent specificity of the binding to Gram-negative bacteria suggested that the binding site for AnxA5 might be LPS. If that were the case, we suspected that binding might occur via the highly conserved lipid A domain of LPS because that domain shows some of the structural features of anionic phospholipids (22, 23). Those ideas were supported by our finding that polyclonal antibody against lipid A blocked the binding of AnxA5 to Gram-negative bacteria (Fig. 1B, parts d, e, and f). In addition, in studies described below, we demonstrated that AnxA5 binds directly to purified LPS and to purified lipid A.
AnxA5 binding to LPS.
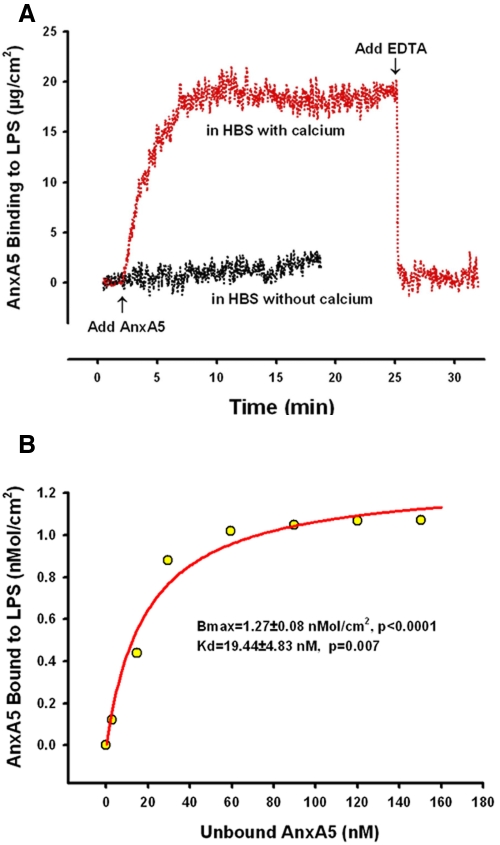
We used ellipsometry, an established precise and quantitative method that has been useful for measuring the adsorption of proteins to phospholipids (6, 24, 25), to monitor the adsorption of AnxA5 to LPS that had been mounted on reflective silicon slides. AnxA5 bound to LPS almost immediately, with maximal binding achieved within 5 min (Fig. 2A). The results of the ellipsometry experiments showed that AnxA5 bound LPS in the presence of ionized calcium but not in its absence (Fig. 2A). In addition, after binding to LPS, AnxA5 was rapidly dissociated when the calcium chelator EDTA was added (Fig. 2A). Binding isotherms confirmed the binding to have a high affinity with an equilibrium constant, KD, for AnxA5-LPS interaction of 19.4 ± 4.8 nM (P = 0.007) (Fig. 2B).
FIG 2 .
Ellipsometry studies of AnxA5 binding to LPS. (A) Ellipsometry tracing of AnxA5 binding to purified LPS. In the presence of calcium, the net binding of the protein to LPS was rapid, reaching a maximum level within 5 min; addition of EDTA immediately dissociated the bound AnxA5 from the LPS. In the absence of calcium, virtually no binding of AnxA5 to LPS was observed. (B) AnxA5 binding isotherm showing the maximum binding densities (Bmax) and KD values for the AnxA5-LPS interaction.
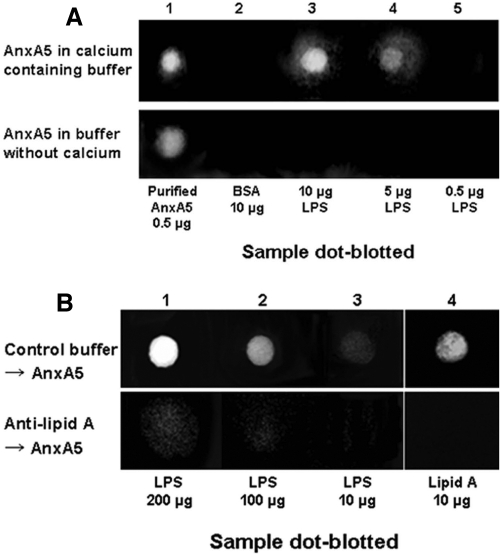
Experiments with purified LPS dot blotted onto membranes confirmed that soluble AnxA5 bound to LPS in the presence of ionized calcium but not in its absence (Fig. 3A). Furthermore, as with intact Gram-negative bacteria, preincubation of purified LPS with antibody against lipid A blocked the binding of AnxA5 (Fig. 3B).
FIG 3 .
Immunoblot studies of AnxA5 binding to extracts of Gram-negative bacteria. (A) Dot-immunoblot study of AnxA5 binding to LPS. Purified LPS, AnxA5 (as positive control), and bovine serum albumin (as a negative control) were applied to immunoblot PVDF membranes; the membranes were incubated with buffer containing AnxA5, followed by antibody against AnxA5. The blots that were exposed to AnxA5 in the presence of 5 mM CaCl2 showed dose-dependent AnxA5 binding to LPS. The blot incubated with added AnxA5 in buffer without calcium showed no staining for bound AnxA5. (B) Dot-immunoblot study of effects of antibody against lipid A on AnxA5 binding. Exposure to anti-lipid A antibody prior to incubation with AnxA5 markedly inhibited the binding of AnxA5.
Effects of AnxA5 on LPS endotoxin activity.
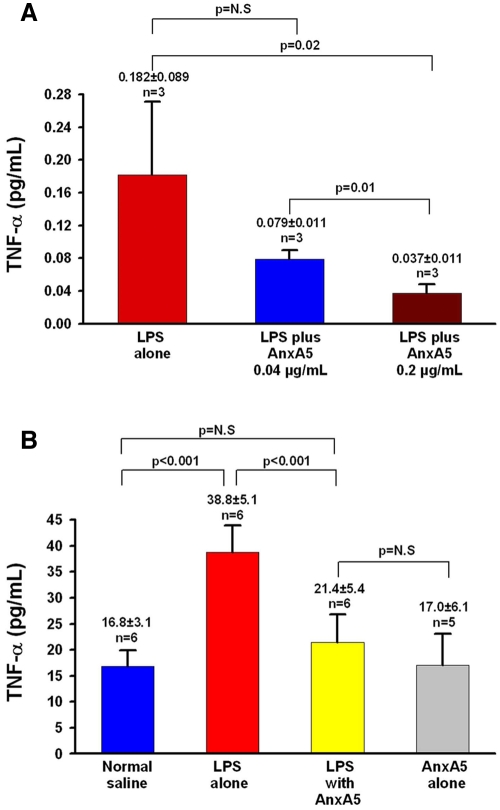
We performed initial proof-of-principle studies to evaluate whether this binding might modulate LPS activity. We investigated the effect of AnxA5 binding with the standard Limulus amebocyte lysate (LAL) assay (26), with measurements of the release of TNF-α (27, 28) from cultured macrophages stimulated with LPS, and with assays of serum TNF-α levels in mice that were intravenously infused with LPS. AnxA5 neutralized LPS activity in the LAL assay; gel formation induced by 0.05 ng/ml LPS was completely inhibited by AnxA5 at 10 ng/ml (Table 1). Similarly, AnxA5 reduced the macrophage response to LPS; exposure of cells to 1 ng/ml LPS in the absence of AnxA5 resulted in an 8-fold-increased level of TNF-α in the culture medium, whereas preincubation of LPS with AnxA5 at 0.04 µg/ml and 0.2 µg/ml significantly reduced this effect in a dose-dependent manner (Fig. 4A).
TABLE 1 .
Effect of AnxA5 on LAL test
| Treatment | Gel formation at AnxA5 concn (µg/ml) of: |
|||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.0001 | 0.001 | 0.01 | 0.1 | 1 | 5 | 10 | |
| AnxA5 + 0.05 ng/ml LPSa in HBS containing 5 mM CaCl2 | + | + | + | − | − | − | − | − |
LPS at 1 ng/ml = 5 EU.
FIG 4 .
Effect of AnxA5 on LPS-induced production of TNF-α in vitro and in vivo. (A) In vitro, mouse macrophages treated with 1 ng/ml LPS and 0.2 µg/ml AnxA5 produced significantly less TNF-α than did cells treated with 1 ng/ml LPS alone and cells treated with a mixture of 1 ng/ml LPS and 0.04 ng/ml AnxA5. (B) In vivo, mice intravenously infused with LPS (1 µg/kg of body weight) with AnxA5 (100 µg/kg of body weight) had significantly lower serum TNF-α levels than mice infused with LPS alone; these levels were not significantly (N.S) different from those of mice infused with control normal saline or with AnxA5 alone.
AnxA5 also reduced the endotoxin effect of LPS in vivo. Mice that were intravenously infused with LPS (1 µg/kg of body weight) that was preincubated with AnxA5 (100 µg/kg of body weight) had significantly lower serum TNF-α concentrations than mice infused with LPS alone (Fig. 4B). The TNF-α concentrations of the mice infused with LPS plus AnxA5 was similar to mice treated with normal saline or with AnxA5 alone (Fig. 4B).
To summarize, we found that AnxA5 binds to Gram-negative bacteria via the lipid A portion of LPS and reverses the molecule’s endotoxin activity in vitro and in vivo. Binding of AnxA5 to Gram-negative bacteria was evidenced by measurements of the binding of fluorescence-tagged AnxA5 to bacteria and by fluorescence microscopic imaging of bacteria. AnxA5 binding was confirmed to occur via LPS through ligand-binding studies on bacterial extracts and with purified LPS. We showed that this binding occurs via the lipid A core of LPS, which is responsible for the endotoxin activity by demonstrating that antibody against lipid A blocks the binding of AnxA5 to bacteria and also by demonstrating that AnxA5 binds to purified lipid A. Through ellipsometric measurements of the binding of AnxA5 to LPS, we found that binding occurs rapidly, demonstrates a high affinity, and exhibits calcium dependence. We then found that the binding of AnxA5 to LPS affects the endotoxin activity of LPS as reflected by the neutralization of gelation in the LAL assay, the reduction of the release of TNF-α by cultured macrophages. Finally, preincubation with AnxA5 also reduced the endotoxin effect of LPS in vivo, as evidenced by the reduction of TNF-α levels in the serum of mice injected with LPS.
DISCUSSION
Despite major advances in the understanding of its pathophysiology, septic shock has had a growing impact on morbidity and mortality and has been relatively resistant to newer therapeutic approaches—for a recent concise review, see reference 29. As cited in that review, hospital mortality rates for septic shock, where data are available from international registries, range from 30% to more than 50% (29). There has been considerable interest in characterizing LPS-binding proteins with an eye toward understanding how these might modulate their biological effects and the additional potential of finding more effective approaches for treating Gram-negative sepsis (for a recent review, see reference 30). A number of serum proteins have been shown to bind to LPS with divergent effects (31). It was previously shown that two other annexins—annexin A1 and annexin A2—can bind to purified lipid A but that those annexins do not bind to LPS itself (32).
The present data showing high-affinity binding of AnxA5 to LPS on Gram-negative bacteria and the reversal of endotoxin activity raise the possibility that the protein plays a role in the host defense against the LPS-induced provocation of the innate (nonadaptive) immune response with its downstream clinical sequelae of Gram-negative endotoxemia by neutralizing LPS in vivo. The fact that, as mentioned above, AnxA5 is highly expressed by cell types that have a barrier function, including vascular endothelium cells (33), placental trophoblasts (34), biliary, pancreatic, salivary, and renal ductular epithelial cells (16), and mammary epithelium cells (17), among others, leads us to speculate that the protein may be concentrated in anatomic locations where it may serve to protect against attack by LPS. Of course, this concept of a biological role for AnxA5 in host defense requires proof by rigorous direct experimentation.
It is also possible that the present findings point to new approaches for addressing Gram-negative sepsis. In view of the disappointments with other candidate LPS inhibitors, we speculate that AnxA5 may be a good prophylactic agent against Gram-negative sepsis or that it may be effective in situations where there may be chronic exposure to LPS, such as ventilator-associated pneumonia.
The high affinity of AnxA5 for LPS and the evidence for its reduction of the glycolipid’s endotoxin activity both in vitro and in vivo raise the interesting potential that supplementation with AnxA5 itself, or treatments with modified forms of the protein or analogous compounds, may provide novel targeted therapies for this difficult-to-treat condition.
MATERIAL AND METHODS
Reagents.
LPS extracted from P. aeruginosa serotype 10, a diphosphoryl form of lipid A prepared from the LPS of a rough strain of E. faecalis, and AnxA5 purified from human placenta were purchased from Sigma-Aldrich. The LPS used for the ellipsometry binding studies and for mouse infusion studies was purified by phenol extraction. To further ensure that AnxA5 was binding to LPS, we performed binding studies with LPS that was taken through a second phenol extraction step as previously described (35). Goat anti-lipid A IgG was purchased from Meridian Life Science, Inc. (Saco, ME). Oregon Green-conjugated AnxA5 (OG-AnxA5) was from Molecular Probes, Inc. (Invitrogen Corp., Carlsbad, CA). Rabbit anti-human AnxA5 IgG was affinity purified as previously described (34). P. aeruginosa (ATCC 27853), S. putrefaciens (ATCC 8071), H. influenzae (ATCC 49776), E. faecalis (ATCC 25922), S. pyogenes (ATCC 19615), and S. agalactiae (ATCC 19615) were purchased from standard clinical laboratory suppliers.
AnxA5 binding to bacteria.
We measured AnxA5 binding using a procedure that we previously described for determining AnxA5 binding to phospholipid suspensions (36), with some modifications. Briefly, approximately 6 × 108 bacteria each of P. aeruginosa, S. putrefaciens, H. influenzae, E. faecalis, S. pyogenes, and S. agalactiae were incubated in duplicate with OG-AnxA5 (1 µg/ml) in HEPES-buffered saline (HBS; 0.01 M HEPES, 0.14 M NaCl, pH 7.5) containing 5 mM CaCl2 (200 µl) for 2 h at room temperature. The bacteria were centrifuged at 15,000 g for 10 min at 25°C, supernatants were removed, and pellets were washed once in 1 ml of the calcium-containing buffer and recentrifuged. The pellets were then resuspended in HBS containing 10 mM EDTA and incubated for 5 min to dissociate bound AnxA5. The supernatants were transferred to a microtiter plate, and AnxA5 was measured with a SpectraMax M5e microplate spectrofluorimeter (Molecular Devices, Sunnyvale, CA) using an excitation wavelength of 485 nm and an emission wavelength of 535 nm.
Fluorescence microscopy studies.
Binding of AnxA5 to Gram-negative bacteria was confirmed by fluorescence microscopy. Approximately 3 × 107 to 5 × 107 bacteria were smeared onto superior adhesive slides (Surgipath), air dried in a biological safety cabinet, rehydrated with HBS, fixed with 5% formalin for 4 min at room temperature, and washed three times in HBS to remove the fixative. The bacteria were then incubated with HBS alone or HBS containing anti-lipid A antibody (50 µg/ml) for 1 h and washed three times in HBS. The bacteria were then incubated with OG-AnxA5 (5 µg/ml) in HBS containing 5 mM CaCl2 for 1 h, washed four times in calcium-containing buffer, and mounted with mounting medium (Vector Laboratories, Inc.). The OG-AnxA5-treated bacteria were observed using an Olympus U-SPT Bx60F-3 fluorescence microscope (Diagnostic Instruments, Inc.), and images were obtained at room temperature with a digital camera (model 2.3.0; Diagnostic Instruments, Inc.) and a computer (Dell Dimension 4500) for storing images as TIFF files. The fluorochrome Oregon Green was imaged by excitation at 488 nm, and the images were viewed at ×1,000 magnification with a 100× objective lens under oil immersion. All of the images were obtained and processed in the same manner; they were all obtained as grayscale images, saved as TIFF files with Adobe Photoshop CS, and converted to RGB. After deletion of blue and red channels, the background was adjusted to black. There was no further processing of the images.
Ellipsometry studies.
Binding of AnxA5 to purified LPS was observed in real time using a thin-film ellipsometer (Rudolph & Sons, Inc., Fairfield, NJ). LPS (1 mg/ml) was applied to a silicon slide in 1.25 mM CaCl2 as previously described (37), and the adsorption of LPS was monitored by ellipsometry. The immobilized LPS layers were flushed with HBS containing 5 mM CaCl2 or HBS alone, AnxA5 (2 µg/ml) was added, and the binding of the protein to the LPS layer was monitored by ellipsometry. After protein adsorption had reached equilibrium, EDTA (final concentration, 10 mM) was added to dissociate LPS-bound AnxA5. Parallel experiments were performed to determine nonspecific AnxA5 binding to background controls in which AnxA5 was applied to a blank silicon slide and protein binding was determined by ellipsometry. Net LPS-bound AnxA5 was then calculated by subtracting the background control from the LPS-bound AnxA5.
To determine the affinity of AnxA5 for LPS, aliquots of AnxA5 were added serially (up to 5 µg/ml) in the calcium-containing buffer after the binding of each prior aliquot had reached equilibrium. Based on the measured free AnxA5 and the total amount of AnxA5 added to the LPS layer, traditional graphs plotting LPS-bound AnxA5 versus free AnxA5 were generated and the Langmuir isotherm was fitted to the binding data to determine the KD and maximum binding capacity using SigmaPlot software (SPSS, Chicago, IL).
Dot-immunoblot studies.
Binding of AnxA5 to purified LPS and lipid A was studied using a dot-immunoblot method as previously described (38), with modification. Briefly, purified LPS was applied to polyvinylidene difluoride (PVDF) membrane and incubated for 1 h in a wet chamber. The blots were washed three times in HBS and incubated with 3% bovine serum albumin for 1 h to block nonspecific binding. The blots were then incubated with AnxA5 (2 µg/ml) for 1 h, washed, and then incubated with rabbit anti-AnxA5 IgG (1 µg/ml), followed by horseradish peroxidase-conjugated goat anti-rabbit antibody (Thermo Scientific, IL); all of the incubations were done in HBS containing 5 mM CaCl2. The blots were then incubated with an enhanced chemiluminescence substrate (Thermo Scientific) for 5 min and scanned using a Kodak Image Station 4000R (Carestream Health, Inc.) to visualize the binding of AnxA5.
Parallel studies were performed to determine whether the binding of AnxA5 to LPS and lipid A might be inhibited by antibody against lipid A in which the blots were incubated with anti-lipid A IgG (50 µg/ml; Sigma) for 1 h prior to incubation with AnxA5. The binding of AnxA5 to LPS and lipid A was then detected as described as above.
LAL test.
The effect of AnxA5 on the endotoxin effect of LPS was investigated using the standard LAL test as previously reported (26), with minor modification. Briefly, after adding various concentrations of LPS in HBS (200 µl) containing 5 mM CaCl2 to single test vials (STVs) of Pyrotell (Associates of Cape Cod, Inc.), it was determined that a positive test was obtained with 0.05 ng/ml LPS. Various concentrations of AnxA5 (0 to 10 µg/ml) were then added to this concentration of LPS and mixed thoroughly, the STVs were immediately placed in a dry block incubator (Thermolyne Dri-bath; Thermo Scientific) at 37°C for 60 ± 2 min, and after the incubation period, gel formation in the STVs was inspected according to the manufacturer’s instructions.
Effects of AnxA5 in vitro on LPS-induced TNF-α production.
Since LPS is known to stimulate macrophages to produce TNF-α (27, 28), we studied whether in vitro the binding of AnxA5 to LPS might affect LPS-induced TNF-α production by a mouse macrophage cell line named RAW 264.7 (American Type Culture Collection). Pilot experiments indicated that treatment of RAW cells with 1 ng/ml LPS increased TNF-α levels 8-fold over those in buffer-treated RAW cells. We therefore used this LPS concentration for the studies described below.
RAW cells were seeded into the wells of a 96-well tissue culture plate (Becton Dickinson Labware) at a density of 1 × 104/well in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum and antibiotics (Gibco), maintained in an incubator (Isotemp; Fisher Scientific) containing 5% carbon dioxide and 95% humidified air at 37°C for 18 h, washed three times, and cultured in serum-free DMEM for 1 h. AnxA5 (0.02 µg/ml and 0.4 µg/ml, respectively) and LPS (1 ng/ml) were added to serum-free DMEM and incubated at 37°C for another 1 h in a rotating incubator (Isotemp, Fisher Scientific). The cells were then treated with AnxA5-LPS-containing, serum-free DMEM (100 µl/well) versus serum-free DMEM that included 1 ng/ml LPS and control serum-free DMEM containing buffer (HBS) without LPS or AnxA5 for 24 h. The conditioned media were collected, and the levels of TNF-α were determined using a mouse TNF-α enzyme-linked immunosorbent assay (ELISA) kit (eBioscience Inc., San Diego, CA) according to the manufacturer’s instructions.
Effects of AnxA5 on LPS-induced TNF-α production in vivo.
We also investigated whether AnxA5 might alter the endotoxin effects of LPS in vivo. All procedures were conducted in accordance with NIH regulations concerning the use and care of experimental animals and approved by the Albert Einstein College of Medicine animal use committee. C57BL/6 mice were housed in the pathogen-free barrier facility at the Albert Einstein College of Medicine. Mixtures of AnxA5 (20 µg/ml) and LPS (0.2 µg/ml) in normal saline were incubated at 37°C for at least 1 h on a rotator prior to injection of the mice. Pilot experiments showed that treatment of mice with 1 µg/kg LPS raised serum TNF-α levels 40-fold above those of normal, saline-treated mice. We therefore used this LPS concentration for the studies described below.
Eighteen C57BL/6 mice 9 to 10 weeks old were randomly divided into 3 groups (n = 6; 3 male and 3 female). LPS (1 µg/kg of body weight) plus AnxA5 (100 µg/kg of body weight), LPS alone (1 µg/kg of body weight), normal saline alone, or AnxA5 alone was injected intravenously at the same volume. After 4 h, the mice were sacrificed and blood was collected by cardiac puncture under anesthesia. The concentration of TNF-α in the serum was determined by ELISA as described above.
Statistical analyses
Statistical analyses were performed using unpaired t tests with GraphPad InStat (GraphPad Software, San Diego, CA).
ACKNOWLEDGMENTS
These studies were supported in part by grant HL-61331 from the National Institutes of Health National Heart Lung and Blood Institute.
We acknowledge the technical support of Andrew V. Nguyen, Department of Biological Sciences, Queensborough Community College of the City University of New York in Bayside, NY.
J.H.R. conceived and designed the study, edited successive drafts of the manuscript, and supervised the project. X.-X.W. performed the experiments, contributed to the writing of the manuscript, composed the figures, and assembled the reference list. E.Y.L. contributed to the design and performed the functional experiments with cultured monocytes and mice and edited successive drafts of the manuscripts. A.G. cultured the bacteria, performed the initial and subsequent binding experiments, and contributed to successive drafts of the manuscript. P.G. helped design and executes the bacterial culture experiments and edited successive drafts of the manuscript. J.C.M. contributed to the experimental design and edited successive drafts of the manuscript.
We have no competing financial interests to declare.
Footnotes
Citation Rand JH, et al. 2012. Annexin A5 binds to lipopolysaccharide and reduces its endotoxin activity. mBio3(2):e00292-11. doi:10.1128/mBio.00292-11.
REFERENCES
- 1. Reviakine I, Bergsma-Schutter W, Brisson A. 1998. Growth of protein 2-D crystals on supported planar lipid bilayers imaged in situ by AFM. J. Struct. Biol. 121:356–361 [DOI] [PubMed] [Google Scholar]
- 2. Bratton DL, et al. 1997. Appearance of phosphatidylserine on apoptotic cells requires calcium-mediated nonspecific flip-flop and is enhanced by loss of the aminophospholipid translocase. J. Biol. Chem. 272:26159–26165 [DOI] [PubMed] [Google Scholar]
- 3. Mirnikjoo B, Balasubramanian K, Schroit AJ. 2009. Suicidal membrane repair regulates phosphatidylserine externalization during apoptosis. J. Biol. Chem. 284:22512–22516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. 1995. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J. Immunol. Methods 184:39–51 [DOI] [PubMed] [Google Scholar]
- 5. Gerke V, Moss SE. 2002. Annexins: from structure to function. Physiol. Rev. 82:331–371 [DOI] [PubMed] [Google Scholar]
- 6. Andree HA, et al. 1990. Binding of vascular anticoagulant alpha (VAC alpha) to planar phospholipid bilayers. J. Biol. Chem. 265:4923–4928 [PubMed] [Google Scholar]
- 7. Tait JF, Gibson D, Fujikawa K. 1989. Phospholipid binding properties of human placental anticoagulant protein-I, a member of the lipocortin family. J. Biol. Chem. 264:7944–7949 [PubMed] [Google Scholar]
- 8. Cederholm A, Frostegård J. 2007. Annexin A5 as a novel player in prevention of atherothrombosis in SLE and in the general population. Ann. N. Y. Acad. Sci. 1108:96–103 [DOI] [PubMed] [Google Scholar]
- 9. Cederholm A, et al. 2005. Decreased binding of annexin V to endothelial cells: a potential mechanism in atherothrombosis of patients with systemic lupus erythematosus. Arterioscler. Thromb. Vasc. Biol. 25:198–203 [DOI] [PubMed] [Google Scholar]
- 10. Hunt BJ, et al. 2011. Resistance to annexin A5 anticoagulant activity in women with histories for obstetric antiphospholipid syndrome. Am. J. Obstet. Gynecol. 205:485.e17–485.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rand JH, et al. 1997. Pregnancy loss in the antiphospholipid-antibody syndrome—a possible thrombogenic mechanism. N. Engl. J. Med. 337:154–160 [DOI] [PubMed] [Google Scholar]
- 12. Wu XX, Guller S, Rand JH. 2011. Hydroxychloroquine reduces binding of antiphospholipid antibodies to syncytiotrophoblasts and restores annexin A5 expression. Am. J. Obstet. Gynecol. 205:576.e7-576.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ravassa S, et al. 2005. Annexin A5 down-regulates surface expression of tissue factor: a novel mechanism of regulating the membrane receptor repertoir. J. Biol. Chem. 280:6028–6035 [DOI] [PubMed] [Google Scholar]
- 14. Kenis H, et al. 2004. Cell surface-expressed phosphatidylserine and annexin A5 open a novel portal of cell entry. J. Biol. Chem. 279:52623–52629 [DOI] [PubMed] [Google Scholar]
- 15. Kenis H, et al. 2006. Annexin A5 inhibits engulfment through internalization of PS-expressing cell membrane patches. Exp. Cell Res. 312:719–726 [DOI] [PubMed] [Google Scholar]
- 16. Katayanagi K, et al. 1999. Generation of monoclonal antibodies to murine bile duct epithelial cells: identification of annexin V as a new marker of small intrahepatic bile ducts. Hepatology 29:1019–1025 [DOI] [PubMed] [Google Scholar]
- 17. Rieanrakwong D, Yonezawa T, Kurusu S, Kawaminami M. 2010. Immunohistochemical localization of annexin A5 in the mammary gland of rats: up-regulation of expression by pup removal. J. Vet. Med. Sci. 72:19–22 [DOI] [PubMed] [Google Scholar]
- 18. Peri F, Piazza M. 2012. Therapeutic targeting of innate immunity with Toll-like receptor 4 (TLR4) antagonists. Biotechnol. Adv. 30:251-260 [DOI] [PubMed] [Google Scholar]
- 19. Takeda K. 2010. The lipid A receptor. Adv. Exp. Med. Biol. 667:53–58 [DOI] [PubMed] [Google Scholar]
- 20. Harel M, Aron-Maor A, Sherer Y, Blank M, Shoenfeld Y. 2005. The infectious etiology of the antiphospholipid syndrome: links between infection and autoimmunity. Immunobiology 210:743–747 [DOI] [PubMed] [Google Scholar]
- 21. Krauel K, et al. 2011. Platelet factor 4 binds to bacteria, inducing antibodies cross-reacting with the major antigen in heparin-induced thrombocytopenia. Blood 117:1370–1378 [DOI] [PubMed] [Google Scholar]
- 22. Rietschel ET, et al. 1987. Lipid A, the endotoxic center of bacterial lipopolysaccharides: relation of chemical structure to biological activity. Prog. Clin. Biol. Res. 231:25–53 [PubMed] [Google Scholar]
- 23. Vishnyakova TG, et al. 2003. Binding and internalization of lipopolysaccharide by Cla-1, a human ortholog of rodent scavenger receptor B1. J. Biol. Chem. 278:22771-22780 [DOI] [PubMed] [Google Scholar]
- 24. Cuypers PA, et al. 1983. The adsorption of prothrombin to phosphatidylserine multilayers quantitated by ellipsometry. J. Biol. Chem. 258:2426–2431 [PubMed] [Google Scholar]
- 25. Rand JH, et al. 1998. Antiphospholipid antibodies accelerate plasma coagulation by inhibiting annexin-V binding to phospholipids: a “lupus procoagulant” phenomenon. Blood 92:1652–1660 [PubMed] [Google Scholar]
- 26. Levin J, Watson SW, Novitsky TJ. 1987. Detection of bacterial endotoxins with the Limulus amebocyte lysate test. Proceedings of an international congress. Woods Hole, Massachusetts, September 8-11, 1985. Prog. Clin. Biol. Res. 231:1–587 [PubMed] [Google Scholar]
- 27. Hawiger J. 2001. Innate immunity and inflammation: a transcriptional paradigm. Immunol. Res. 23:99–109 [DOI] [PubMed] [Google Scholar]
- 28. Valledor AF, Comalada M, Santamaría-Babi LF, Lloberas J, Celada A. 2010. Macrophage proinflammatory activation and deactivation: a question of balance. Adv. Immunol. 108:1–20 [DOI] [PubMed] [Google Scholar]
- 29. Suffredini AF, Munford RS. 2011. Novel therapies for septic shock over the past 4 decades. JAMA 306:194–199 [DOI] [PubMed] [Google Scholar]
- 30. Krasity BC, Troll JV, Weiss JP, McFall-Ngai MJ. 2011. LBP/BPI proteins and their relatives: conservation over evolution and roles in mutualism. Biochem. Soc. Trans. 39:1039–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andrä J, Gutsmann T, Müller M, Schromm AB. 2010. Interactions between lipid A and serum proteins. Adv. Exp. Med. Biol. 667:39–51 [DOI] [PubMed] [Google Scholar]
- 32. Eberhard DA, Vandenberg SR. 1998. Annexins I and II bind to lipid A: a possible role in the inhibition of endotoxins. Biochem. J. 330:67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Flaherty MJ, West S, Heimark RL, Fujikawa K, Tait JF. 1990. Placental anticoagulant protein-I: measurement in extracellular fluids and cells of the hemostatic system. J. Lab. Clin. Med. 115:174–181 [PubMed] [Google Scholar]
- 34. Krikun G, et al. 1994. The expression of the placental anticoagulant protein, annexin V, by villous trophoblasts: immunolocalization and in vitro regulation. Placenta 15:601–612 [DOI] [PubMed] [Google Scholar]
- 35. Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J. Immunol. 165:618–622 [DOI] [PubMed] [Google Scholar]
- 36. Rand JH, et al. 2006. Reduction of circulating annexin A5 levels and resistance to annexin A5 anticoagulant activity in women with recurrent spontaneous pregnancy losses. Am. J. Obstet. Gynecol. 194:182–188 [DOI] [PubMed] [Google Scholar]
- 37. Rand JH, et al. 2003. Human monoclonal antiphospholipid antibodies disrupt the annexin A5 anticoagulant crystal shield on phospholipid bilayers: evidence from atomic force microscopy and functional assay. Am. J. Pathol. 163:1193–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wright SF, Morton JB. 1989. Detection of vesicular-arbuscular mycorrhizal fungus colonization of roots by using a dot-immunoblot assay. Appl. Environ. Microbiol. 55:761–763 [DOI] [PMC free article] [PubMed] [Google Scholar]