Abstract
There is substantial evidence that many human cancers are driven by a subpopulation of cells that display stem cell properties. These cancer stem cells (CSCs) may also contribute to metastasis and treatment resistance. Furthermore, just as normal stem cells are regulated by their microenvironment or “niche”, CSCs interact with and in turn are regulated by cells in the tumor microenvironment. These interactions involve inflammatory cytokines including IL-1, IL-6 and IL-8, which in turn activate Stat3/NF-κB pathways in both tumor and stromal cells. Activation of these pathways stimulates further cytokine production generating positive feedback loops which in turn drives CSC self-renewal. These cytokine loops and the pathways they regulate resemble those activated during chronic inflammation and wound healing and may contribute to the known link between inflammation and cancer. Inhibitors of these cytokines and their receptors have been developed as anti-inflammatory agents. By blocking signals from the tumor microenvironment, these agents have the potential to target CSCs. Future clinical trials utilizing these compounds will be needed to determine whether targeting the CSC population has clinical benefit.
Background
Cancer Stem Cells
There is increasing evidence that many human tumors display a hierarchical organization in which a subset of tumor cells with stem cell properties drive tumor growth and metastasis (1, 2). Furthermore, by virtue of their relative resistance to radiation and chemotherapy, these cells may contribute to treatment resistance and relapse following therapy. If this is the case, then more effective treatments will require the effective targeting of this cell population. As is the case with their normal counterparts, CSCs are regulated by intrinsic signals as well as extrinsic signals originating in the tumor microenvironment (3–5). In the case of CSCs, epigenetic as well as genetic changes which occur during carcinogenesis results in disregulation of self-renewal pathways. Stem cell regulatory pathways frequently disregulated in tumors include the Notch, Hedgehog, Wnt, PI-3K, NF-κB and Jak/STAT pathways (6–10). These pathways may be activated via mutation of key regulatory elements. In addition, pathway disregulation may result from altered interactions between these cells and the tumor microenvironment (11). Emerging evidence suggests that evolving tumors, as well as their microenvironment co-evolve during tumor progression (3). Bi-directional paracrine signals coordinately regulate tumorigenic cell populations including cancer stem cells (7, 12–14). Tumorigenic cells, in turn, produce factors which attract and regulate a diverse variety of cell types that constitute the tumor microenvironment (12, 14). Inflammatory cytokines, including IL-1, IL-6 and IL-8 play a pivotal role in mediating the interaction between cancer stem cells and the microenvironment. Interestingly, many of the pathways activated during tumor formation resembled those that mediate normal wound healing. We will review the links between inflammation and cancer stem cells with an emphasis on the cytokine networks and signaling pathways that link these processes. These pathways provide potential targets for the development of novel strategies to target the CSC populations.
Inflammation and Cancer Stem Cells
Considerable clinical evidence exists for links between inflammatory states and cancer development. Epidemiologic studies have demonstrated associations between ulcerative colitis, Hepatitis C and chronic pancreatitis to the development of cancers of the colon, liver and pancreas. Levels of chronic inflammation as assessed by serum C reactive protein or β-amyloid are correlated with risk of breast cancer recurrence after breast cancer recurrence after primary therapy (15). The development of chronic inflammation has been associated with the production of the cytokines IL-1β, IL-6 and IL-8 by a variety of inflammatory cells. Interestingly, genetic polymorphism in genes encoding these cytokines predisposes affected individuals to cancer (16). Furthermore, the Stat3/NF-κB pathway plays a critical role in inducing and maintaining a pro-carcinogenic inflammatory microenvironment at the initiation of malignant transformation and tumor progression (17–19). These inflammatory cytokines including IL-1, IL-6 and IL-8 may influence tumor growth by regulating cancer stem cells populations.
Interleukin 1 (IL-1)
The IL-1 family consists of IL-1α and IL-1β, as well as the antagonist IL-1Ra. These receptors bind IL-1 which is produced in response to infection or tissue injury resulting in activation of NF-κB and downstream targets IL-6 and IL-8. Local production of IL-1 by tumor-associated macrophages promotes angiogenesis, tumor growth and metastasis, whereas blocking IL-1 receptor inhibits these processes in mouse models (20, 21). Elevated levels of IL-1 expression in cancer patients are correlated with advanced metastatic disease (22, 23). Furthermore, gene expression profiling demonstrated higher IL-1 expression in breast cancer stem cells as compared to their more differentiated counterparts (7).
Interleukin 6 (IL-6)
IL-6 is a pleiotropic cytokine secreted by a wide range of cells which plays a crucial role in immunoregulation. Elevated levels of IL-6 have been associated with chronic inflammatory states sepsis, hypertension, obesity, insulin resistance, poor survival among cancer patients, increasing risks for developing malignancies (24, 25). In cancer patients, high levels of IL-6 are associated with poor patient outcome and in pre-clinical models IL-6 has been shown to promote tumorigenesis, angiogenesis and metastasis (26, 27). IL-6 has been shown to be a direct regulator of breast cancer stem cell self-renewal (13), a process mediated by the IL-6 receptor/GP130 complex through activation of Stat3 (Figure 1). In inflammatory cells, IL-6 mediated Stat3 signaling selectively induces a pro-carcinogenic tumorigenic microenvironment (28). Stat3 activation in turn leads to transcriptional activation of NF-κB in inflammatory cells which secrete additional IL-6 and IL-8 acting on tumor cells. Thus, these cytokines generate a positive feedback loop between immune cells and tumor cells which further stimulates the tumor stem cell components accelerating metastasis and therapeutic resistance (Figure 1). Utilizing mouse xenografts, we have recently demonstrated that bone marrow mesenchymal stem cells are recruited to sites of growing breast cancers by gradients if IL-6 (12). Furthermore, Il-6 is a key component of a positive feedback loop involving these bone marrow mesenchymal stem cells and breast cancer stem cells (12). Furthermore, Sethi et al, recently demonstrated that IL-6 meditated Jagged1-Notch1 promotes breast cancer bone metastasis (29). Since Notch is also a stem cell regulator, this suggests that IL-6 may regulate stem cells through multiple pathways. Furthermore, these studies identify IL-6 and its receptor as attractive therapeutic targets.
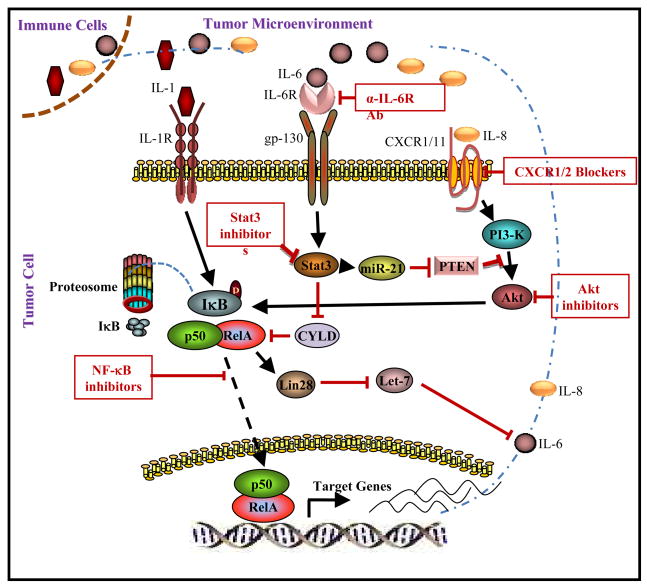
Figure 1.
Regulation of cancer stem cells by inflammatory cytokine networks. Binding of IL-6-IL-6R complex to gp130 and IL-8 to CXCR1/2 activates NF-κB pathway via Stat3 and Akt signaling. IL-6 and IL-8 production by NF-κB generates a positive feedback loop which maintains constitutive pathway activation. Inhibition of pathway components by pharmacologic agents is illustrated.
Interleukin 8 (IL-8)
Interleukin 8 (IL-8) is a proinflammatory cytokine which functions in different biological processes such as neutrophil chemotaxis and angiogenesis. It activates multiple intracellular signaling pathways by binding its receptors, CXCR1 and CXCR2. Within the tumor microenvironment, a diverse variety of cells, including mesenchymal cells, macrophages and immune cells secrete IL-8 (30). Serum IL-8 levels in patients with cancer have been associated with aggressive behavior and poor prognosis (31, 32). Utilizing gene expression profiling we previously identified the IL-8 receptor CXCR1 as highly expressed on breast cancer stem cells (33). Recombinant IL-8 increased breast CSC self-renewal and tumor growth. In contrast, blocking this receptor in mouse xenografts utilizing a small molecule inhibitor repertaxin significantly reduced the breast cancer stem cell population leading to decreased tumorigenicity and metastasis.
NF-κB pathway
The NF-κB family is composed of five related transcription factors p50, p52, RelA (p65), c-Rel and RelB (34, 35). In resting cells, NF-κB proteins are predominantly found in the cytoplasm where they are associated with the IκB family of proteins (Figure 1). Activation of NF-κB by diverse signals results in ubiquitin ligase-dependent degradation of IκB and nuclear translocation of NF-κB protein complexes. A number of cytokines, including IL-6 and IL-8 are regulated by NF-κB. In addition, a positive feedback loop has recently been shown to maintain a chronic inflammatory state in tumor cells. Interestingly, this loop involves the mircroRNA let7, as well as Lin28, a factor involved in embryonic stem cell self-renewal (7). This feedback loop is maintained by IL-6 mediated STAT3 activation which in turn activates NF-κB effecting Lin28 and let7 (Figure 1). The specific role of IL-6 in maintaining this inflammatory loop in breast cancer stem cells has been recently demonstrated (7, 10). NF-κB may also play an important role in normal breast physiology, as well as carcinogenesis. In a HER2-neu model of mammary carcinogenesis, suppression of NF-κB in mammary epithelium reduced the mammary stem cell compartment resulting in a delayed onset of HER2-neu induced tumors (36). NF-κB has also been implicated in the regulation of mouse mammary stem cells during pregnancy. Elevated levels of progesterone during pregnancy induce RANK Ligand (RANKL) in differentiated breast epithelial cells. RANKL in turn stimulates breast stem cells self-renewal via activation of NF-κB in these cells (37, 38). The increased incidence of aggressive breast cancers associated with pregnancy (39) may result from activation of similar pathways in breast cancer stem cells (37, 38).
Clinical Implications
Solid tumors are composed of heterogeneous cell populations which interact in complex networks. As is the case in developing organs, tumor cells interact with and in turn are regulated by these components in the microenvironment. Metastatic tumor cells also recreate complex cellular microenvironments at metastatic sites. Over 120 years ago Paget proposed the “seed and soil” hypothesis of tumor metastasis (40). Reframed in a modern context, the “seeds” are the cancer stem cells and the “soil” is the rich microenvironment composed of diverse cell types which interact with tumor cells via cytokine networks. These networks regulate cancer stem cells and their progeny which form the tumor bulk. Elucidation of these pathways may provide new targets for therapeutic development. Examples include the cytokines IL-6 and IL-8, as well as their receptors IL6R and CXCR1. Blockade of these cytokine pathways reduce breast cancer stem cells in pre-clinical models (33). Clinical trials utilizing IL-6 blocking antibodies have been initiated for the treatment of multiple myeloma with early encouraging results (41). Furthermore, anti-IL-6R antibody, tocilizumab, has been approved for the treatment of arthritis (42) with little clinical toxicity. The small molecule CXCR1 inhibitor, Reperaxin, has been developed to block rejection in renal transplant patients and early clinical trials suggest it is well tolerated. Phase I clinical trials combining this cytokine receptor/inhibitor with chemotherapy are being planned. NF-κB also represents an attractive therapeutic target. Preclinical studies suggest that the NF-κB inhibitor, parthenolide, was able to target leukemic stem cells and early stage clinical trials for the treatment of leukemia utilizing this agent are in progress. Together, these trials will indicate the feasibility of targeting cancer stem cells by blocking interaction of these cells with the tumor microenvironment.
The cancer stem cell model has important implications for clinical trial design. Currently, tumor response rate is determined by tumor size as described by Response Evaluation Criteria In Solid Tumors (RECIST). For many tumors regression does not correlate with increased patient survival (43–45). Since cancer stem cells may constitute only a minor fraction of a tumor, agents which target this population may not produce tumor regression. In fact, stem cell targeting agents would be expected to have more dramatic effects in the adjuvant then advanced tumor settings (46). This suggests that in advanced disease, it will be necessary to combine CSC targeting agents with debulking agents such as chemotherapy or radiation therapy. Time to tumor progression may prove a more useful clinical endpoint then tumor regression in these studies. However, since the non-stem cell fraction of tumors may still retain proliferative capacity, the criteria used to define tumor progression are important so that patients are not removed from treatments prematurely. The evaluation of CSC biomarkers such as CD44, CD133 and aldehyde dehydrogenase-1 in serial biopsies may provide a tool to access the efficacy of CSC targeting agents (47–49). Circulating tumor cells (CTC) may also provide a valuable source of CSC populations for biomarker analysis. These assays will need to be able to capture circulating cancer stem cells which may not express antigens such as Ep-CAM which are currently used. The use of neoadjuvant trial design may prove particularly useful for assessing effects of CSC targeting agents since acquisition of tissue before and after treatment enables assessment of efficacy of CSC targeting. In addition, the effect of these agents on increasing the complete pathologic response rate (CPR) an accepted clinical endpoint, can be readily assessed. Ultimately, randomized trials will be required to determine whether successful targeting of CSCs improves patient outcome.
Acknowledgments
We thank Shawn G. Clouthier for a critical review of this manuscript.
Grant Support
This work was supported in part by NIH grants CA129765 and CA101860 as well as by funds from the AACR (SU2C Dream Team Award), The Breast Cancer Research Foundation and The Taubman Research Institute.
Footnotes
Conflict of interest
Max S. Wicha has financial holdings and is a scientific adviser for OncoMed Pharmaceuticals, is a scientific adviser for Pfizer and has research supported by Dompe Pharmaceuticals.
References
- 1.Korkaya H, Wicha MS. Selective targeting of cancer stem cells: a new concept in cancer therapeutics. BioDrugs. 2007;21:299–310. doi: 10.2165/00063030-200721050-00002. [DOI] [PubMed] [Google Scholar]
- 2.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006;66:1883–90. doi: 10.1158/0008-5472.CAN-05-3153. discussion 95–6. [DOI] [PubMed] [Google Scholar]
- 3.Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009;25:30–8. doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 6.Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7:e1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–71. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:R605–15. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 39:493–506. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma XJ, Dahiya S, Richardson E, Erlander M, Sgroi DC. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res. 2009;11:R7. doi: 10.1186/bcr2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F, et al. Breast Cancer Stem Cells Are Regulated by Mesenchymal Stem Cells through Cytokine Networks. Cancer Res. 2011;71:614–24. doi: 10.1158/0008-5472.CAN-10-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117:3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 15.Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27:3437–44. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michaud DS, Daugherty SE, Berndt SI, Platz EA, Yeager M, Crawford ED, et al. Genetic polymorphisms of interleukin-1B (IL-1B), IL-6, IL-8, and IL-10 and risk of prostate cancer. Cancer Res. 2006;66:4525–30. doi: 10.1158/0008-5472.CAN-05-3987. [DOI] [PubMed] [Google Scholar]
- 17.Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–39. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 18.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 19.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 20.Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, et al. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci U S A. 2003;100:2645–50. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gery I, Waksman BH. Potentiation of the T-lymphocyte response to mitogens. II. The cellular source of potentiating mediator(s) J Exp Med. 1972;136:143–55. doi: 10.1084/jem.136.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elaraj DM, Weinreich DM, Varghese S, Puhlmann M, Hewitt SM, Carroll NM, et al. The role of interleukin 1 in growth and metastasis of human cancer xenografts. Clin Cancer Res. 2006;12:1088–96. doi: 10.1158/1078-0432.CCR-05-1603. [DOI] [PubMed] [Google Scholar]
- 23.Pantschenko AG, Pushkar I, Anderson KH, Wang Y, Miller LJ, Kurtzman SH, et al. The interleukin-1 family of cytokines and receptors in human breast cancer: implications for tumor progression. Int J Oncol. 2003;23:269–84. [PubMed] [Google Scholar]
- 24.Scheller J, Ohnesorge N, Rose-John S. Interleukin-6 trans-signalling in chronic inflammation and cancer. Scand J Immunol. 2006;63:321–9. doi: 10.1111/j.1365-3083.2006.01750.x. [DOI] [PubMed] [Google Scholar]
- 25.Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheller J, Rose-John S. Interleukin-6 and its receptor: from bench to bedside. Med Microbiol Immunol. 2006;195:173–83. doi: 10.1007/s00430-006-0019-9. [DOI] [PubMed] [Google Scholar]
- 27.Fisman EZ, Tenenbaum A. The ubiquitous interleukin-6: a time for reappraisal. Cardiovasc Diabetol. 2010;9:62. doi: 10.1186/1475-2840-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sethi N, Dai X, Winter CG, Kang Y. Tumor-Derived Jagged1 Promotes Osteolytic Bone Metastasis of Breast Cancer by Engaging Notch Signaling in Bone Cells. Cancer Cell. 2011 doi: 10.1016/j.ccr.2010.12.022. [DOI] [PMC free article] [PubMed]
- 30.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 31.Yao C, Lin Y, Chua MS, Ye CS, Bi J, Li W, et al. Interleukin-8 modulates growth and invasiveness of estrogen receptor-negative breast cancer cells. Int J Cancer. 2007;121:1949–57. doi: 10.1002/ijc.22930. [DOI] [PubMed] [Google Scholar]
- 32.Benoy IH, Salgado R, Van Dam P, Geboers K, Van Marck E, Scharpe S, et al. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin Cancer Res. 2004;10:7157–62. doi: 10.1158/1078-0432.CCR-04-0812. [DOI] [PubMed] [Google Scholar]
- 33.Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120:485–97. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffmann A, Natoli G, Ghosh G. Transcriptional regulation via the NF-kappaB signaling module. Oncogene. 2006;25:6706–16. doi: 10.1038/sj.onc.1209933. [DOI] [PubMed] [Google Scholar]
- 35.Moynagh PN. The NF-kappaB pathway. J Cell Sci. 2005;118:4589–92. doi: 10.1242/jcs.02579. [DOI] [PubMed] [Google Scholar]
- 36.Liu M, Sakamaki T, Casimiro MC, Willmarth NE, Quong AA, Ju X, et al. The Canonical NF-{kappa}B Pathway Governs Mammary Tumorigenesis in Transgenic Mice and Tumor Stem Cell Expansion. Cancer Res. 70:10464–73. doi: 10.1158/0008-5472.CAN-10-0732. [DOI] [PMC free article] [PubMed]
- 37.Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, et al. Progesterone induces adult mammary stem cell expansion. Nature. 465:803–7. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- 38.Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, et al. Control of mammary stem cell function by steroid hormone signalling. Nature. 465:798–802. doi: 10.1038/nature09027. [DOI] [PubMed] [Google Scholar]
- 39.Peck JD, Hulka BS, Poole C, Savitz DA, Baird D, Richardson BE. Steroid hormone levels during pregnancy and incidence of maternal breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:361–8. [PubMed] [Google Scholar]
- 40.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 41.Fulciniti M, Hideshima T, Vermot-Desroches C, Pozzi S, Nanjappa P, Shen Z, et al. A high-affinity fully human anti-IL-6 mAb, 1339, for the treatment of multiple myeloma. Clin Cancer Res. 2009;15:7144–52. doi: 10.1158/1078-0432.CCR-09-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohsugi Y, Kishimoto T. The recombinant humanized anti-IL-6 receptor antibody tocilizumab, an innovative drug for the treatment of rheumatoid arthritis. Expert Opin Biol Ther. 2008;8:669–81. doi: 10.1517/14712598.8.5.669. [DOI] [PubMed] [Google Scholar]
- 43.Sevinc A, Turhal NS. Please, desist RECIST criteria in GIST, at least in me. Onkologie. 2008;31:556. doi: 10.1159/000151688. [DOI] [PubMed] [Google Scholar]
- 44.Monetti F, Casanova S, Grasso A, Cafferata MA, Ardizzoni A, Neumaier CE. Inadequacy of the new Response Evaluation Criteria in Solid Tumors (RECIST) in patients with malignant pleural mesothelioma: report of four cases. Lung Cancer. 2004;43:71–4. doi: 10.1016/j.lungcan.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 45.Kimura M, Tominaga T. Outstanding problems with response evaluation criteria in solid tumors (RECIST) in breast cancer. Breast Cancer. 2002;9:153–9. doi: 10.1007/BF02967580. [DOI] [PubMed] [Google Scholar]
- 46.Liu S, Wicha MS. Targeting breast cancer stem cells. J Clin Oncol. 2010;28:4006–12. doi: 10.1200/JCO.2009.27.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–5. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]