Abstract
Objective
To assess long-term developmental outcome in children who received iron-fortified or low-iron formula.
Design
Follow-up at 10 years of randomized controlled trial (1991–1994) of 2 levels of formula iron. Examiners blind to group.
Setting
Urban areas around Santiago, Chile.
Participants
Original study enrolled healthy full-term infants in community clinics; 835 completed the trial. At 10 years, 573 were assessed (57%).
Intervention
Iron-fortified (12.7 mg/l) or low-iron (2.3 mg/l) formula from 6 to 12 months.
Main Outcome Measures
IQ, spatial memory, arithmetic achievement, visual-motor integration, visual perception, and motor functioning. We used covaried regression to compare iron-fortified and low-iron groups and consider hemogobin (HB) prior to randomization and sensitivity analyses to identify 6-month HB at which groups diverged in outcome.
Results
Compared to low-iron, the iron-fortified group scored lower on every 10-year outcome (significant for spatial memory, visual-motor integration; suggestive for IQ, arithmetic, visual perception, motor coordination; 1.4 – 4.6 points lower, effect sizes 0.13 – 0.21). Children with high 6-month HB (> 128 g/l) showed poorer outcome on these measures if they received iron-fortified formula (10.7 – 19.3 points lower; large effect sizes, 0.85 – 1.36); those with low HB (< 105 g/l) showed better outcome (2.6 – 4.5 points higher; small but significant effects, 0.22 – 0.36). High HB represented 5.5% of sample (n = 26); low HB, 17.0% (n = 87).
Conclusions
Long-term development may be adversely affected in infants with high HB who receive 12.7 mg/l iron-fortified formula. Optimal amounts of iron in infant formula warrant further study.
Keywords: iron fortification, infant formula, iron deficiency, children, development
INTRODUCTION
The high prevalence of iron deficiency in infancy has led to routine iron fortification of infant formula and foods in many countries. These interventions help reduce iron-deficiency anemia and iron deficiency without anemia. However, the optimal amount of iron in such products, especially infant formula, is debated.1,2 For instance, infant formula in Europe typically contains lower amounts of iron than in the US – about 4 – 7 mg/l compared to 12 – 13 mg/l.1,3 Concerns have been raised about providing iron to iron-sufficient infants, including poorer growth and increased morbidity.4 We have not observed such effects,5 but it is reasonable to wonder whether there might be risks to the developing brain. We had the opportunity to examine this question as part of a longitudinal study of the developmental and behavioral effects of preventing iron-deficiency anemia in infancy.
We report here a comparison of developmental outcome at 10 years in Chilean children who, as infants, received formula fortified at the level used in the US or low-iron formula in a double-blind randomized clinical trial (RCT).6 The low-iron group was reassessed for the first time at 10 years, making it possible to compare long-term developmental outcome in high- vs. low-iron formula. Given recent concern about giving iron to iron-sufficient infants, we also analyzed the 10-year results based on 6-month hemoglobin (HB), which was the only indicator of iron status upon enrollment in infancy available for the entire sample.
METHODS
Summary of infancy RCT
The RCT was undertaken in Chile in a period when infant iron deficiency was widespread and there was no national program of iron fortification. According to data available at the time, mixed feeding with powdered cow milk and breast milk was the norm, with weaning from the breast by around 6 months.6 The study was therefore designed to use infant formula as the supplementation vehicle, randomizing infants at 6 months to formula with or without iron. However, formula without iron was no longer commercially available, and the study started using low-iron instead of no-iron formula. Infants were randomly assigned to iron-fortified or low-iron formula during the initial period of subject enrollment (1991–1994). To avoid interference with breastfeeding, we enrolled infants taking at least 250 ml per day of cow milk or formula. (In the last years of subject enrollment [1994–1996], this criterion was dropped due to the success of breastfeeding campaigns in Chile, low-iron formula was no longer used, and a no-added-iron condition was included as originally planned.7)
All infants were born at term of uncomplicated vaginal births weighing ≥ 3.0 kg and were free of acute or chronic health problems. At about 6 months of age, infants received a screening capillary HB determination to avoid randomizing those with iron-deficiency anemia. Infants with low HB (< 103 g/l) and the next non-anemic infant received a venipuncture. The few infants with iron-deficiency anemia confirmed on a venous blood sample were excluded and given iron therapy.8–11 Anemia at 6 months was defined as venous HB ≤ 100 g/l. Iron deficiency was defined as 2 or more abnormal iron measures (mean corpuscular volume < 70 fl, free erythrocyte protoporphyrin ≥ 1.77 µmol/l red blood cells [100 µg/dl], serum ferritin < 12 µg/l).6 All other infants were randomized to receive the study-provided formula between 6 and 12 months; the only measure of iron status available for all infants prior to randomization was capillary HB. All infants received a venipuncture at 12 months to determine iron status at study conclusion. The cutoff for anemia was HB <110 g/l; the definition of iron deficiency was the same as at 6 months. See ref6 for a full description of the RCT of high- vs. low-iron formula.
Randomization
Infants were randomly assigned at 6 months to receive iron-fortified formula (mean 12.7 mg/l) or low-iron formula (mean 2.3 mg/l). Formulas were distributed in powdered form in identical cans that differed only in a number on the label (two numbers each for iron-fortified and low-iron formula). Study personnel gave participating infants the next available formula number on a predetermined randomly generated list (computer-generated by project statistician). Formula consumption and breastfeeding were recorded at weekly home visits. The RCT was double-blind, with families and project personnel unaware of whether the infant received iron-fortified or low-iron formula.
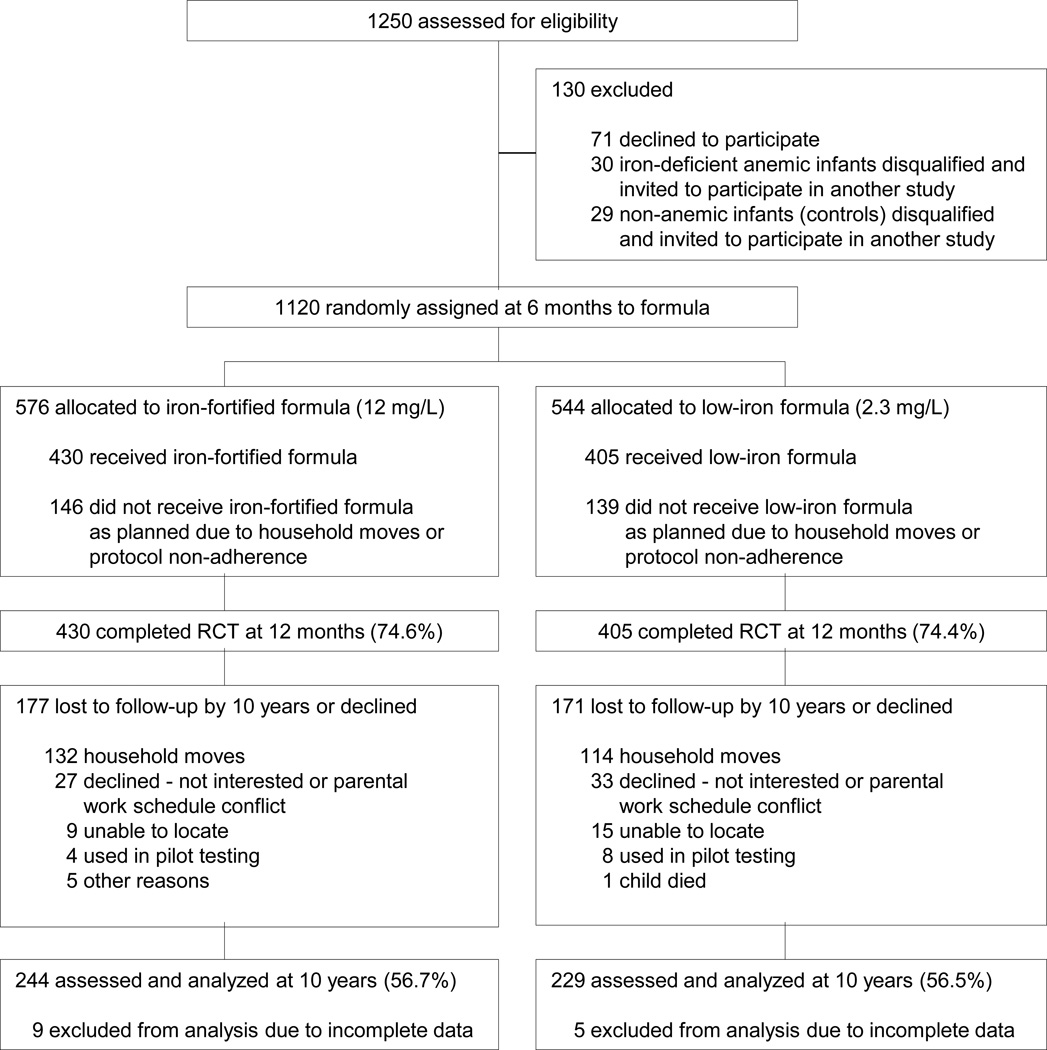
A total of 1120 infants were randomized; 835 completed the RCT and had a venous blood sample at 12 months: 405 in the low-iron group and 430 in the iron-fortified group (see Figure 1 for study design and flow chart of subject involvement). As reported, there were no statistically significant group differences in attrition, background characteristics, initial HB, formula intake, developmental outcome, or growth before, during, and at the conclusion of the RCT.6,7 Iron deficiency was more common among infants in the low-iron group (35% vs. 19% in the iron-fortified group, p < 0.001), but the prevalence of iron-deficiency anemia was similar in the two groups (3.8% vs. 2.8%, p = 0.35).6
Figure 1.
Flow chart of subject participation.
10-year follow-up
Written informed consent was obtained from the parents and assent from the children. The study was approved by Institutional Review Boards of the University of Michigan and University of Chile.
Iron status
At 10 years, cutoffs for age in NHANES II analyses, which included all our venous iron measures, were used to classify children as having iron deficiency (≥ 2 abnormal measures) or iron-deficiency anemia (low HB as well): HB < 118 g/l, mean cell volume < 76 fl, transferrin saturation < 14%, free erythrocyte protoporphyrin > 1.24 µmol/l red blood cells (70 µg/dl), and serum ferritin < 12 µg/l.12
Developmental outcomes
Measures with standardized scores in the 10-year follow-up included an abbreviated Wechsler Intelligence Scale for Children (WISC-R) as an overall measure of IQ,13 the spatial memory subtest of the Kaufman Assessment Battery for Children (KABC),14 the Wide Range Achievement Test-Revised (WRAT-R) as a screening measure of arithmetic achievement,15 the Beery-Buktenica Developmental Test of Visual-Motor Integration (VMI)16 along with supplemental tests of visual perception and motor coordination,16 and the Bruininks-Oseretsky Test of Motor Proficiency, short form, as a brief survey of general motor proficiency.17 All are widely used and normed, generally to mean = 100 with SD = 15. Scoring was according to test manuals.
Statistics
Background differences between groups (iron-fortified vs. low-iron formula) were tested with t-tests or χ2. Group differences in 10-year developmental outcome were tested using covaried regression analysis. To consider the role of initial HB (possibly a proxy for iron status), we used multiple regression to test for interactions between 6-month HB (venous where available, otherwise capillary) and formula group on developmental outcome at 10 years. Potential covariates were factors that correlated with outcome, namely gender, mother's IQ, gestational age, and HOME score in infancy. Forward procedures were used to remove non-significant covariates, although any that was significant for one outcome was included in all analyses. We examined suggestive (p < 0.10) as well as significant (p < 0.05) interactions to look for patterns across the various outcome measures. For those measures that showed a significant or suggestive interaction, we analyzed the pattern of differences using multiple cross-sectional comparisons. Since there was no prior literature to guide a choice of cutoffs for high and low HB, we used sensitivity analysis to identify empirically HB concentrations that showed diverging outcomes between groups, i.e., we analyzed test score differences for HB concentrations in 50 g/l intervals to determine where the estimated slopes differed significantly between formula groups (p < 0.05). The test for significance was a t-test on regression parameters for independent samples.18 For HB concentrations where the slopes diverged on a given test, we tested the significance of test-score differences using covaried regression analysis and calculated 95% confidence intervals. Post-hoc comparisons used the Bonferoni method to adjust the alpha level for multiple comparisons. JBS conducted the analyses using SPSS for WINDOWS 16.0 (SPSS Inc., Chicago, IL).
Role of the funding source
This work was supported by grants from the National Institutes of Health (HD14122 and HD33487). The National Institutes of Health had no role in study design; collection, analysis and interpretation of the data; in the writing of the manuscript; or in the decision to submit for publication. BL had full access to all the data and had final responsibility for the decision to submit for publication.
RESULTS
Sample at 10 years
At 10 years, 57% of each group was reassessed, ns = 244 and 229 in the iron-fortified and low-iron groups, respectively (Figure 1). There were no group differences in attrition (χ2 = 0.003, p = 0.95). The most common reason was moving outside the area (30.7%, n = 132, and 28.1%, n = 114, for the iron-fortified and low-iron groups, respectively), followed by refusal (6.3%, n = 27, and 8.1%, n = 33). Children who were assessed at 10 years were generally similar in infancy background characteristics to those not assessed. However, children with 10-year data had families with slightly higher socioeconomic status (mean social class index = 28.9 [0.3] vs. 27.7 [0.3], t1,816 = 2.67, p = 0.008) and higher developmental test scores at 12 months (Bayley mental scores, 104.4 [0.6] vs. 102.0 [0.7], t1,828 = −2.70, p = 0.007; Bayley motor scores, 97.7 [0.7] vs. 94.2 [0.8], t1,828 = −3.40, p = 0.001).
Children assessed at 10 years who received iron-fortified or low-iron formula as infants were similar in background characteristics, whether determined in infancy or at 10 years (Table 1). Developmental test scores were similar at the conclusion of the RCT. The only statistically significant differences were more daily formula intake in the low-iron group (about 54 ml more) and poorer iron status (Table 1). The differences in iron status had been observed in the complete RCT sample but higher formula intake had not.6
Table 1.
Sample characteristics*
| n † | Iron-fortified 244 |
Low-iron 229 |
|
|---|---|---|---|
| Infancy | |||
| Child characteristics | |||
| Male (n, %) | 125 (51.2) | 127 (55.5) | |
| Gestational age (wk) | 39.4 (0.07) | 39.5 (0.06) | |
| Birth weight (g) | 3511.3 (22.4) | 3524.7 (23.7) | |
| Weight-for-age z-score | 6 mo | 0.36 (0.06) | 0.38 (0.05) |
| 12 mo | −0.09 (0.06) | −0.15 (0.06) | |
| Height-for-age z-score | 6 mo | 0.10 (0.05) | 0.09 (0.05) |
| 12 mo | −0.14 (0.06) | −0.16 (0.05) | |
| Head circumference (cm) 6 mo | 43.8 (0.8) | 43.6 (0.7) | |
| 12 mo | 46.9 (0.9) | 46.7 (0.8) | |
| Hemoglobin (g/l) | 6 mo‡ | 111.9 (0.6) | 112.7 (0.6) |
| 12 mo | 124.3 (0.6) | 123.1 (0.6) | |
| Mean cell volume (fl) § | 12 mo | 74.7 (0.2) | 73.2 (0.3) |
| Ferritin (µg/l) § | 12 mo | 14.1 (0.6) | 10.3 (0.6) |
| Free erythrocyte protoporphyrin § 12 mo | |||
| (µmol/mol heme) | 83.5 (1.5) | 94.7 (2.2) | |
| [µg/dl red blood cells] | 94.3 (1.7) | 107.0 (2.5) | |
| Age at first bottle (mo) | 2.2 (0.1) | 2.2 (0.1) | |
| Still breastfed at 12 mo (n, %) | 45 (18.4) | 52 (22.7) | |
| Age at weaning if weaned (mo) | 4.6 (0.2) | 4.6 (0.2) | |
| Formula intake (ml/d) § | 609.9 (12.1) | 664.5 (12.3) | |
| 12-mo Bayley mental test scores | 105.2 (0.8) | 103.7 (0.8) | |
| 12-mo Bayley motor test scores | 97.9 (0.9) | 97.5 (1.0) | |
| Family characteristics | |||
| Maternal education (y) | 9.4 (0.2) | 9.2 (0.2) | |
| Paternal education (y) | 8.6 (0.2) | 8.4 (0.2) | |
| Father present (n, %) | 201 (83.1) | 184 (81.8) | |
| Number of children for mother | 2.2 (0.1) | 2.1 (0.1) | |
| Maternal IQ‖ | 84.4 (0.7) | 84.0 (0.7) | |
| Maternal depression¶ | 16.8 (0.8) | 16.3 (0.8) | |
| Maternal smoking in infancy (n, %) | 47, 20.9 | 42, 17.4 | |
| Social class index** | 27.3 (0.4) | 28.1 (0.5) | |
| Life stress†† | 4.5 (0.2) | 4.7 (0.2) | |
| Home environment‡‡ | 30.5 (0.3) | 30.7 (0.3) | |
| 10-year | |||
| Child characteristics | |||
| Age at testing (y) | 10.0 (0.0) | 10.0 (0.0) | |
| Male (n, %) | 129 (50.2) | 130 (53.7) | |
| Weight-for-age z-score | 0.42 (0.06) | 0.37 (0.06) | |
| Height-for-age z-score | −0.06 (0.06) | −0.12 (0.06) | |
| Body mass index | 19.3 (0.2) | 18.9 (0.2) | |
| Head circumference (cm) | 54.3 (0.1) | 54.2 (0.1) | |
| Hemoglobin (g/l) | 136.7 (0.5) | 137.7 (0.5) | |
| Mean cell volume (fl) | 82.0 (0.2) | 82.3 (0.2) | |
| Transferrin saturation (%) | 27.3 (9.0) | 25.9 (9.1) | |
| Ferritin (µg/l) | 30.1 (0.9) | 29.1 (0.9) | |
| Free erythrocyte protoporphyrin | |||
| (µmol/mol heme) | 50.0 (1.2) | 49.7 (1.0) | |
| [µg/dl red blood cells] | 56.5 (1.4) | 56.2 (1.1) | |
| Family characteristics | |||
| Maternal education (y) | 9.8 (0.2) | 9.5 (0.2) | |
| Paternal education (y) | 9.8 (0.2) | 9.8 (0.2) | |
| Father present (n, %) | 182 (71.4) | 170 (70.2) | |
| Maternal depression¶ | 18.6 (0.9) | 19.8 (0.9) | |
| Social class index** | 23.8 (0.4) | 24.7 (0.4) | |
| Life stress†† | 5.1 (0.2) | 5.1 (0.2) | |
| Home environment‡‡ | 36.8 (0.5) | 36.9 (0.5) | |
Values are means (SE) for continuous variables and percentages and n (%) for categorical variables.
ns vary slightly due to occasional missing data for some measures.
HemaCue at 6 mo.
The only statistically significant group differences were iron status measures in infancy by design and mean daily formula intake in infancy (low-iron group consumed about 54 ml more per day, t1,471 = 3.16, p = .002).
Measured by a short form of the Wechsler Adult Intelligence Scale-Revised.31
Measured by Center for Epidemiologic Studies Depression Scale.32
Measured by the Graffar scale, designed to differentiate families at the lower end of the socioeconomic spectrum;33 higher values indicate lower social class.
Measured by a scale modified from the Social Readjustment Rating Scale34
Measured by the Home Observation for Measurement of the Environment-Revised.35
Iron status at 10 years in iron-fortified vs. low-iron groups
There were no statistically significant group differences in iron status at 10 years (Table 1). Only one child had iron-deficiency anemia; 4.1% (n = 9) of the low-iron and 6.9% (n = 17) of the iron-fortified group met criteria for iron deficiency (χ2 = 1.79, p = 0.41).
Developmental outcomes in iron-fortified vs. low-iron groups
Table 2 shows the 10-year test scores results controlling for gender and gestational age – the only background factors that correlated with outcome and remained significant in models. Of the 7 tests, 2 showed statistically significant lower scores in the iron-fortified vs. low-iron group (spatial memory and VMI) and 4 showed suggestive trends (IQ, visual perception, motor coordination, and arithmetic). The test score differences ranged from 1.4 to 4.6 points, with effect sizes of .13 to .21.
Table 2.
Ten-year outcomes for children who received iron-fortified vs. low-iron formula in infancy *
| Iron-fortified | Low-iron | Effect size (CI) † | p-value | |
|---|---|---|---|---|
| N | 244 | 229 | ||
| IQ (WISC) | 91.5 ± 0.9 | 93.3 ± 0.9 | −0.13 (−0.25,− 0.01) | 0.057 |
| Spatial memory (KABC subtest) | 86.8 ± 1.0 | 91.4 ± 1.0 | −0.21 (−0.38, −0.04) | 0.022 |
| Arithmetic achievement (WRAT-R) ‡ | 87.0 ± 0.8 | 88.4 ± 0.8 | −0.10 (−0.19,− 0.01) | 0.066 |
| Visual-motor integration (VMI) | 97.2 ± 0.9 | 99.8 ± 1.0 | −0.21 (−0.40, −0.02) | 0.046 |
| Visual perception (VMI supplementary test) | 90.8 ± 1.0 | 93.0 ± 1.1 | −0.16 (−0.33, 0.01) | 0.056 |
| Motor coordination (VMI supplementary test) | 88.7 ± 0.8 | 90.4 ± 0.8 | −0.13 (−0.32, 0.05) | 0.101 |
| Motor proficiency (Bruininks-Oseretsky short form) | 44.2 ± 0.6 | 45.1 ± 0.7 | −0.08 (−0.25, 0.09) | 0.265 |
Values are standard scores (mean ± SE), controlling for gender and gestational age. The norm is 100 ± 15 (SD) for all tests except for motor proficiency where the norm is 50 ± 10 SD.
Effect size (CI, 95% confidence interval) calculated as score for iron-fortified group minus score for low-iron group divided by overall standard deviation.
We initially assessed reading using the WRAT, but due to the phonetic nature of Spanish, scores were extremely high with little variability, and the measure was dropped.
Outcomes depending on 6-month hemoglobin and formula group
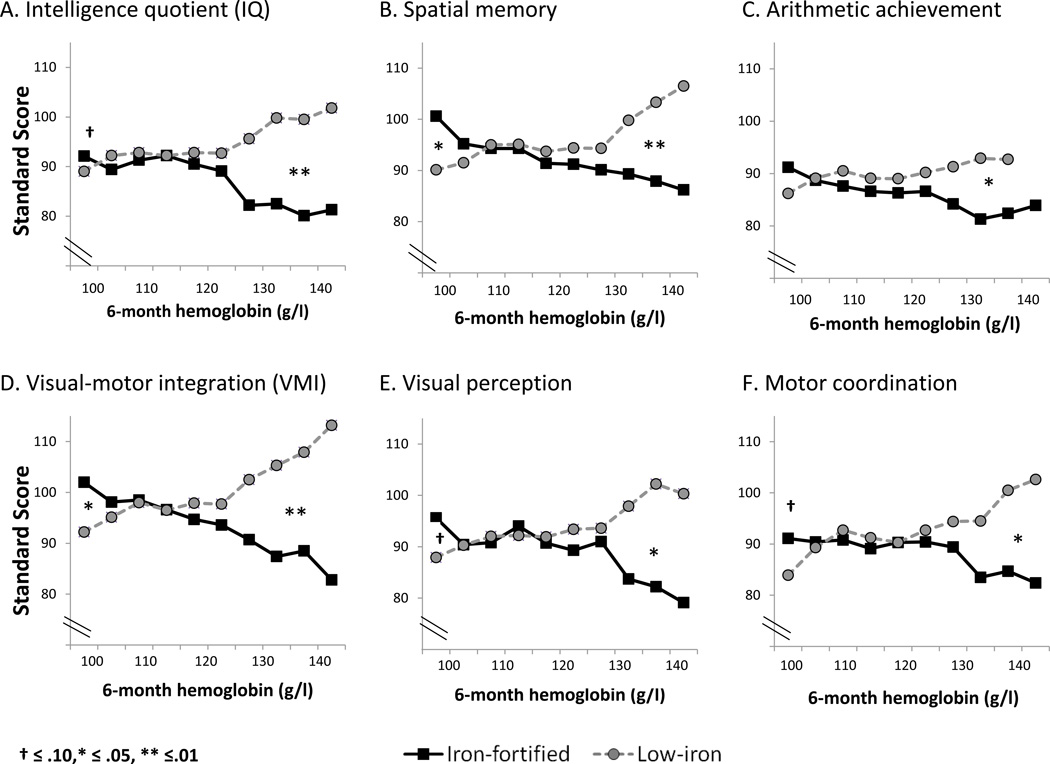
We further examined outcome in relation to HB at randomization and formula group, using multiple linear regression to test these main effects and their interaction, controlling for gender and gestational age. The interaction was statistically significant for IQ, spatial memory, VMI, and suggestive for motor coordination. Based on these interactions, we conducted subgroup analyses by HB level. The pattern was that children with the highest 6-month HB concentrations had lower 10-year scores if in the iron-fortified formula group, while those with the lowest 6-month HB had higher scores (Figure 2). Further sensitivity analyses (Table 3) showed a significant test-score disadvantage on all but one measure (visual perception) for iron-fortified formula at HB concentrations > 128 g/l, CI 127 – 129; 5.5% of the sample (n = 26) had HB above this level, which was 1.87 SD above the sample mean. HB ranged from 129 to 140 g/l in this subgroup (mean = 132.2, SD = 4.7). At HB concentrations < 105 g/l (CI 104 – 106), the advantage for iron-fortified formula was statistically significant for spatial memory and VMI and suggestive for IQ, visual perception, and motor coordination; 17.0% (n = 87) had HB below this level. Effect sizes were large (>.80),19 as were test score differences (10.7–19.3 points), for high HB and small (>.20 effect sizes, 2.6- to 4.5-point differences in test scores) for low HB (Table 3).
Figure 2.
Developmental outcomes at 10 years and the interaction between 6-month HB and iron-fortified vs. low-iron infant formula. The HB distribution is truncated at the low end by the criterion for anemia at 6 months; 34 capillary values ≤ 100 g/l are not shown since venous HB was higher. The pattern was better outcome with iron-fortified formula for children with the lowest HB and worse outcome for those with the highest HB. Cut points of about 105 g/l and 128 g/l were determined empirically by sensitivity analyses; the significance of test-score differences was based on covaried regression analysis controlling for gender and gestational age. Ns for the high and low HB cut points for each test are found in Table 3.
Table 3.
Differences in 10-year outcome depending on initial hemoglobin and iron-fortified vs. low-iron formula
| Low 6-month hemoglobin | High 6-month hemoglobin | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome* | HB cut point (g/l) |
n (%)† | Difference in score‡ (means) |
Effect size (CI)§ |
HB cut point (g/l) |
n (%) | Difference in score (means) |
Effect size (CI) |
| IQ (WISC) | 107 | 118 (24.0) | 4.5 (89.1, 93.6) | 0.34 (−0.01,0.68)‖ | 127 | 26 (5.5) | −12.9 (95.3, 82.4) | −0.96 (−1.60,−0.32) |
| Spatial memory (KABC subtest) | 105 | 87 (17.0) | 3.3 (91.2, 94.6) | 0.31 (0.05,0.56) | 127 | 26 (5.5) | −14.6 (104.1, 89.5) | −1.34 (−2.24,−0.44) |
| Arithmetic achievement (WRAT-R) | 104 | 47 (9.4) | 2.6 (88.1, 90.8) | 0.22 (−0.04,0.46) | 127 | 26 (5.5) | −10.7 (94.7, 84.0) | −0.85 (−1.38,−0.32) |
| Visual-motor integration (VMI) | 105 | 87 (17.0) | 4.1 (93.3, 97.4) | 0.36 (0.10,0.63) | 127 | 26 (5.5) | −19.3 (106.6, 87.3) | −1.36 (−2.00,−0.73) |
| Visual perception (VMI supplemental test) | 104 | 47 (9.4) | 3.4 (89.1, 92.5) | 0.26 (−0.03,0.56)‖ | 130 | 23 (4.9) | −17.8 (100.9, 83.1) | −1.08 (−1.85,−0.31) |
| Motor coordination (VMI supplemental test) | 104 | 47 (9.4) | 3.9 (87.2, 90.9) | 0.29 (−0.02,0.60)‖ | 127 | 26 (5.5) | −15.0 (100.2, 85.2) | −1.25 (−2.19,−0.32) |
There were no significant differences on motor proficiency (Bruininks-Oseretsky short form).
Cell sizes vary because the empirically-derived cut points vary by test. At the low end, a higher HB cut point results in a larger cell size (more children had HB up to that value). At the high end, a higher HB cut point results in a smaller cell size (fewer children had HB above the higher cut point).
Score for iron-fortified group minus score for low-iron group, expressed in points. Means adjusted for gestational age and gender for low-iron and iron-fortified groups are shown in ().
Effect size calculated as difference in score divided by overall standard deviation; CI, 95% confidence interval, shown in (). All differences for high HB and for low HB are statistically significant (p <0.05), i.e., CI does not include 0.
Suggestive differences for low HB (p < 0.10).
To illustrate the differences for high HB, IQ scores in the iron-fortified formula subgroup averaged 82.4 (4.1) vs. 95.3 (3.3) for those in the low-iron formula subgroup; for VMI, the corresponding values averaged 87.3 (3.5) vs.106.6 (4.4). Significance and pattern of results were unaffected by excluding outliers (5 highest and lowest HB values).
Factors related to high 6-month HB
We considered preexisting factors that differentiated infants who entered the RCT with high HB. They were disproportionately female (62%, n = 16, vs. 47%, n = 210, in the rest of the sample, χ2= 4.38, p = 0.036). A greater proportion of their mothers reported smoking (33%, n = 9, vs. 18%, n = 80, in the rest of the sample, χ2= 8.52, p = 0.004).
DISCUSSION
We found that children who received 12.7 mg/l iron-fortified formula as infants had lower cognitive and visual-motor scores at 10 years than those receiving low-iron formula. However, we observed differences only among children with the very highest or lowest HB upon entry into the RCT at 6 months. Children with high HB had lower 10-year test scores if they received iron-fortified formula, whereas those with low HB had higher scores. Although the cut-point for high HB (128 g/l) was 1.87 SD above the mean 6-mo HB for the Chile sample and only 26 children (5.5%) were affected, considerably higher proportions of iron-sufficient or iron-supplemented 6-month-old infants in North America and Europe have HB concentrations this high or higher.20–22
One possible explanation for poorer developmental outcome in children with high HB in infancy who received iron-fortified formula is that supplemental iron in iron-sufficient infants may have adverse effects on neurodevelopmental outcome. There is some supporting evidence in a rodent model,23 although the dose of iron, adjusted for body weight, was higher than in iron-fortified formula. This explanation presumes that children in our study with high HB in infancy were iron-sufficient. However, high HB can be due to other factors, such as chronic hypoxia. Without a panel of iron measures for all infants prior to randomization, the iron status of those with high HB in our study is uncertain. Another possible explanation is that some other factor(s) contributed both to high HB at 6 months and poorer developmental outcome. In our sample, there were more females and more maternal smoking among infants with high HB. The higher proportion of females seems consistent with numerous prior studies in which female infants had better iron status than males (cf.24) but appears unlikely to be a factor contributing to poorer developmental outcome, since there is no indication that females are at more developmental risk than males. In contrast, maternal smoking has been associated with poorer developmental outcome in some studies25,26 and can also elevate infant HB due to chronic mild hypoxia.27,28 However, a shared factor explanation requires that the infant brain exposed to that factor must be more vulnerable to iron. Animal studies confirm interactions between iron and hypoxia-ischemia at the level of both brain and behavior, but the model was not exposure to maternal smoking and iron deficiency preceded the hypoxic insult.29 Despite the uncertainty about explanation, iron is an essential nutrient where both too little and too much are problematic. If unneeded iron were absorbed, the brain might be vulnerable to adverse effects of excess iron.
In contrast to children with high HB in infancy, where their iron status was generally unknown, our study obtained iron measures on a venous blood sample for all those with very low capillary HB. To enter the preventive trial, venous HB had to be above 100 g/l and thus no infant in this report met study criteria for iron-deficiency anemia. However, most infants with low HB were iron-deficient, and some would have met criteria for iron-deficiency anemia with a less stringent cutoff for anemia at 6 months. Long-term developmental outcome was better when these children received iron-fortified formula, pointing to cognitive and visual-motor benefits of iron in iron-deficient infants.
It might appear paradoxical that we reported developmental benefits of iron supplementation in the full study in infancy7 but adverse outcomes at 10 years with iron-fortified formula. However, it was social-emotional outcomes that showed the biggest benefits of iron supplementation in the infancy trial. There were no global test score differences in the overall infancy study, and the cognitive and motor benefits were quite subtle, i.e., shorter looking time on a measure of information processing speed and a few days earlier in the age of crawling. As well, the comparison groups in the complicated full infant study are not the same as those reported here. This analysis focused on the simple RCT of iron-fortified vs. low-iron formulas in the early years of the infancy study.
Our findings cannot be compared to other studies, because there are none comparable. The results must be replicated, and no change in practice should result from a single study. Iron deficiency was widespread in Chile at the time, and results might not be the same in settings where maternal iron deficiency during pregnancy and iron deficiency in infancy are less widespread. Furthermore, many infants had been fed breast milk and unmodified cow milk before 6 months, but mixed feeding with infant formula, as in North America, Europe, and other areas, might have different effects. Our study cannot determine whether iron in different forms has different effects, since both formulas contained iron as ferrous sulfate.
A major study limitation is the small number of children with HB at the extreme high end, with comparisons involving only 11–13 children per formula group. Furthermore, there are cautions about subgroup analyses of RCTs,30 even if cell size is not a problem. The study is also limited by high attrition (25% between 6 and 12 months and 43% between 12 months and 10 years), although there was no differential attrition related to formula group and only minor differences comparing those lost to follow-up to those assessed. Other limitations are that HB was the only iron measure for all infants prior to randomization, and randomization was not stratified by iron status. We have no data on maternal smoking at 10 years or smoking habits of other household members at any point; exposure could affect long-term outcome.
If our results are replicated, there might be several implications. HB (and/or other measures of iron status) might need to be tested in early infancy before iron supplementation. The recommendations of universal iron supplementation might need reconsideration. In any case, the optimal level of iron in infant formula warrants further study to avoid giving more iron than infants need.
In conclusion, this study indicates poorer long-term developmental outcome in infants with high HB concentrations who received formula fortified with iron at levels currently used in the US. The large majority of infants showed no developmental effects of iron-fortified formula, and those with low HB in infancy had higher 10-year test scores if they received iron-fortified formula.
ACKNOWLEDGEMENTS
BL made substantial contributions to study conception and design, acquiring, analyzing and interpreting the data, and drafting and revising the manuscript for important intellectual content. MC made substantial contributions to study conception and design, acquiring data, and revising the article critically for important intellectual content. KMC made substantial contributions to analyzing and interpreting the data and drafting and revising the manuscript for important intellectual content. JBS made substantial contributions to analyzing and interpreting the data and drafting and revising the manuscript for important intellectual content. BL had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We thank study families and children for their continued participation and project psychologists for their dedicated effort and high degree of professionalism. Infant formulas (Similac®) were donated by Ross Laboratories. Supported by grants from the National Institutes of Health (HD14122 and HD33487). The National Institutes of Health had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
No author has a real or potential conflict of financial or personal interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The study was presented in platform format at the Pediatric Academic Societies meeting in Honolulu, Hawaii 2008.
References
- 1.Baker RD, Greer FR Committee on Nutrition. Clinical report- diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 months of age) Pediatrics. 2010;126:1. doi: 10.1542/peds.2010-2576. [DOI] [PubMed] [Google Scholar]
- 2.Koletzko B, Baker S, Cleghorn G, et al. Global standard for the composition of infant formula: Recommendations of an ESPGHAN coordinated international expert group. J Pediatr Gastroenterol Nut. 2005;41:584–599. doi: 10.1097/01.mpg.0000187817.38836.42. [DOI] [PubMed] [Google Scholar]
- 3.Moy RJD. Iron fortification of infant formula. Nutr Res Rev. 2000;13:215–227. doi: 10.1079/095442200108729070. [DOI] [PubMed] [Google Scholar]
- 4.Iannotti LL, Tielsch JM, Black MM, Black RE. Iron supplementation in early childhood: health benefits and risks. Am J Clin Nutr. 2006;84:1261–1276. doi: 10.1093/ajcn/84.6.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gahagan S, Yu S, Kaciroti N, Castillo M, Lozoff B. Linear and ponderal growth trajectories in well-nourished, iron-sufficient infants are unimpaired by iron supplementation. J Nutr. 2009;139:2106–2012. doi: 10.3945/jn.108.100735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walter T, Pino P, Pizarro F, Lozoff B. Prevention of iron-deficiency anemia: comparison of high- and low-iron formulas in term healthy infants after six months of life. J Pediatr. 1998;132:635–640. doi: 10.1016/s0022-3476(98)70352-x. [DOI] [PubMed] [Google Scholar]
- 7.Lozoff B, De Andraca I, Castillo M, Smith J, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics. 2003;112:846–854. [PubMed] [Google Scholar]
- 8.Roncagliolo M, Garrido M, Walter T, Peirano P, Lozoff B. Evidence of altered central nervous system development in infants with iron deficiency anemia at 6 mo: Delayed maturation of auditory brain stem responses. Am J Clin Nutr. 1998;68:683–690. doi: 10.1093/ajcn/68.3.683. [DOI] [PubMed] [Google Scholar]
- 9.Angulo-Kinzler RM, Peirano P, Lin E, Algarin C, Garrido M, Lozoff B. Twenty-four-hour motor activity in human infants with and without iron deficiency anemia. Early Hum Dev. 2002;70:85–101. doi: 10.1016/s0378-3782(02)00092-0. [DOI] [PubMed] [Google Scholar]
- 10.Angulo-Kinzler RM, Peirano P, Lin E, Garrido M, Lozoff B. Spontaneous motor activity in human infants with iron-deficiency anemia. Early Hum Dev. 2002;66:67–79. doi: 10.1016/s0378-3782(01)00238-9. [DOI] [PubMed] [Google Scholar]
- 11.Peirano P, Algarin C, Garrido M, Algarin D, Lozoff B. Iron-deficiency anemia is associated with altered characteristics of sleep spindles in NREM sleep in infancy. Neurochem Res. 2007;32:1665–1672. doi: 10.1007/s11064-007-9396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Life Sciences Research Office. Assessment of the Iron Nutrition Status of the U.S. Population Based on Data Collected in the Second National Health and Nutrition Survey, 1976–1980. Bethesda: Federation of American Societies for Experimental Biology; 1984. [Google Scholar]
- 13.Sattler JM. Assessment of Children. 3rd ed. San Diego: Jerome M. Sattler, Publisher, Inc.; 1992. Wechsler Intelligence Scale for Children--Revised (WISC-R): Description; pp. 119–143. [Google Scholar]
- 14.Kaufman AS, Kaufman NL. Kaufman Assessment Battery for Children: Administration and Scoring Manual. Circle Pines, MN: American Guidance Service, Inc.; 1983. [Google Scholar]
- 15.Jastak S, Wilkinson GS. Wide Range Achievement Test-Revised. Wilmington, Delaware: Jastak Associates, Inc.; 1984. [Google Scholar]
- 16.Beery KE. Administration, Scoring, and Teaching Manual for the Developmental Test of Visual-Motor Integration. 4th ed. Cleveland: Modern Curriculum Press; 1997. [Google Scholar]
- 17.Bruininks RH. Bruininks-Oseretsky Test of Motor Proficiency. Circle Pines, MN: American Guidance Service; 1978. [Google Scholar]
- 18.Cohen J, Cohen P, West SG, Aiken LS. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. New York: Routledge; 2002. [Google Scholar]
- 19.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York: Academic Press; 1977. [Google Scholar]
- 20.Dallman PR, Siimes MA. Percentile curves for hemoglobin and red cell volume in infancy and childhood. J Pediatr. 1979;94:26–31. doi: 10.1016/s0022-3476(79)80344-3. [DOI] [PubMed] [Google Scholar]
- 21.Ziegler EE, Nelson SE, Jeter JM. Iron status of breastfed infants is improved equally by medicinal iron and iron-fortified cereal. Am J Clin Nutr. 2009;90:76–87. doi: 10.3945/ajcn.2008.27350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Domellof M, Cohen RJ, Dewey KG, Hernell O, Rivera LL, Lonnerdal B. Iron supplementation of breast-fed Honduran and Swedish infants from 4 to 9 months of age. J Pediatr. 2001;138:679–687. doi: 10.1067/mpd.2001.112895. [DOI] [PubMed] [Google Scholar]
- 23.Kaur D, Peng J, Chinta SJ, et al. Increased murine neonatal iron intake results in Parkinson-like neurodegeneration with age. Neurobiol Aging. 2006;28:907–913. doi: 10.1016/j.neurobiolaging.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Domellof M, Lonnerdal B, Dewey KG, Cohen RJ, Rivera LL, Hernell O. Sex differences in iron status during infancy. Pediatrics. 2002;110:545–552. doi: 10.1542/peds.110.3.545. [DOI] [PubMed] [Google Scholar]
- 25.DiFranza JR, Aligne CA, Weizman R. Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics. 2004;113:1007–1015. [PubMed] [Google Scholar]
- 26.Shea AK, Steiner M. Cigarette smoking during pregnancy. Nicotine Tob Res. 2008;10:267–278. doi: 10.1080/14622200701825908. [DOI] [PubMed] [Google Scholar]
- 27.Varvarigou A, Beratis NG, Makri M, Vagenakis AG. Increased levels and positive correlation between erythropoletin and hemoglobin concentrations in newborn children of mothers who are smokers. J Pediatr. 1994;124:480–482. doi: 10.1016/s0022-3476(94)70380-9. [DOI] [PubMed] [Google Scholar]
- 28.Yeruchimovich M, Dollberg S, Green DW, Mimouni FB. Nucleated red blood cells in infants of smoking mothers. Obstet Gynecol. 1999;93:403–406. doi: 10.1016/s0029-7844(98)00442-6. [DOI] [PubMed] [Google Scholar]
- 29.Rao R, Tkac I, Townsend EL, Ennis K, Gruetter R, Gera T. Perinatal iron deficiency predisposes the developing rat hippocampus to greater injury from mild to moderate hypoxia-ischemia. J Cereb Blood Flow Metab. 2007;27:729–740. doi: 10.1038/sj.jcbfm.9600376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine-reporting of subgroup analyses in clinical trials. New Eng J Med. 2007;357:2189–2194. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 31.Wechsler D. Manual for the Wechsler Adult Intelligence Scale-Revised. San Antonio: The Psychological Corporation; 1981. [Google Scholar]
- 32.Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 33.Alvarez M, Muzzo S, Ivanovic D. Escala para la medicion del nivel socioeconomico en el area de la salud. Rev Med Chil. 1985;113:243–249. [PubMed] [Google Scholar]
- 34.Holmes TH, Rahe RH. The Social Readjustment Rating Scale. J Psychosom Med. 1967;11:213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- 35.Caldwell BM, Bradley RH. Home Observation for Measurement of the Environment (Revised Edition) Little Rock: University of Arkansas; 1984. [Google Scholar]