Abstract
It is well established that idiopathic generalized epilepsies (IGEs) show a polygenic origin and may arise from dysfunction of various types of voltage- and ligand-gated ion channels. There is an increasing body of literature implicating both high and low voltage-activated (HVA and LVA) calcium channels and their ancillary subunits in IGEs. Cav2.1 (P/Q-type) calcium channels control synaptic transmission at presynaptic nerve terminals, and mutations in the gene encoding the Cav2.1 α1 subunit (CACNA1A) have been linked to absence seizures in both humans and rodents. Similarly, mutations and loss of function mutations in ancillary HVA calcium channel subunits known to coassemble with Cav2.1 result in IGE phenotypes in mice. It is important to note that in all these mouse models with mutations in HVA subunits there is a compensatory increase in thalamic LVA currents, which likely leads to the seizure phenotype. In fact, gain of function mutations have been identified in Cav3.2 (an LVA or T-type calcium channel encoded by the CACNA1H gene) in patients with congenital forms of IGEs, consistent with increased excitability of neurons as a result of enhanced T-type channel function. Here we provide a broad overview of the roles of voltage-gated calcium channels, their mutations, and how they might contribute to the river that terminates in epilepsy.
Keywords: calcium channel, P/Q-type channels, T-type channels, epilepsy, seizures
Introduction
Epilepsy is a disorder that results from abnormal hyperexcitable and hypersynchronous activity of neurons [28]. Epileptic seizures can have a wide variety of origins, including brain damage and genetic causes [85]. Broadly, seizures can be classified into focal and generalized seizures. Focal seizures are typically localized to one brain hemisphere and may arise from insults such as localized brain lesions, or tumors. In contrast, generalized epilepsies are characterized by seizure activity over both brain hemispheres. They can be further classified into symptomatic and idiopathic epilepsy. Symptomatic generalized seizures can be caused by insults such as brain infection or oxygen deprivation. Idiopathic seizures often do not have a clear etiology, but may often include a genetic component [10, 59]. One of the key defining attributes of idiopathic generalized epilepsies (IGEs) is the occurrence of absence seizures [22]. Absence seizures are typically short in duration and manifest themselves as sudden behavioral arrest and impaired consciousness followed by sudden termination and return to normal behavior. In electroencephalogram (EEG) recordings, absence seizures are characterized by spike and wave discharges (SWDs) that arise from synchronous firing of thalamocortical networks [11].
The onset of IGE typically occurs in childhood, although adult onset may also occur [52]. Indeed, among epilepsies involving absence seizures, childhood absence epilepsy CAE) and juvenile absence epilepsy (JAE) are the most common forms of IGE. CAE has been extensively investigated at the cellular and network levels in both animals and humans, revealing a strong causal link to mutations in various types of voltage-gated and ligand-gated ion channels, including voltage-gated potassium, sodium, and calcium channels, as well as GABAA receptors [33, 50, 59, 82]. Here, we shall focus on the role of voltage-gated calcium channels in the development of IGEs.
Subtypes, subunit composition, and selected physiological roles of voltage gated calcium channels
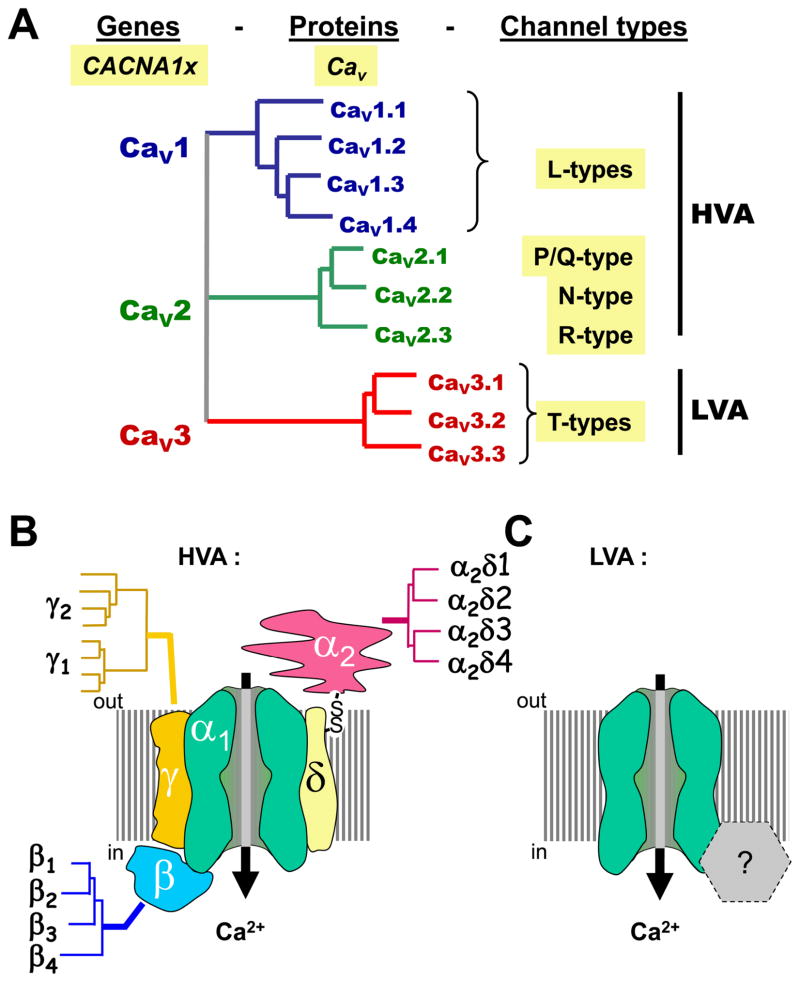
Voltage-gated calcium channels can be classified, based on their biophysical characteristics, into high voltage activated (HVA) and low voltage activated (LVA) channels, with HVA channels requiring larger membrane depolarizations for activation. HVA channels can be further subdivided into L-, N-, P-, Q- and R-types, by virtue of their distinct functional and pharmacological profiles (Fig. 1A). Members of the HVA channel family are heteromultimers of a pore forming α1 subunit that coassembles with ancillary β, α2δ (and in some cases a γ subunit) into a functional channel complex (Fig. 1B) [6, 19]. In contrast, LVA channels (also referred to as T-type) are thought to be α1 subunit monomers (Fig. 1C). The α1 subunit defines the channel subtype, whereas the ancillary subunits modulate α1 subunit function and surface expression. The α1 subunit is comprised of four homologous membrane repeats (each containing six transmembrane helices termed S1 through S6, plus a pore lining P-loop motif) that are connected via cytoplasmic linker regions and flanked by cytoplasmic N- and C-termini. Ten different α1 subunit subtypes belonging to three large families are expressed in the mammalian genome (Fig. 1A). The Cav1 family encodes L-type calcium channels and includes four different members (Cav1.1 through Cav1.4). The Cav2 family includes Cav2.1, Cav2.2 and Cav2.3, which encode P/Q-type, N-type and R-type channels, respectively. The Cav3 encompasses the family of T-type channels with three members (Cav3.1 through Cav3.3). Of particular note, P- and Q-type channels appear to arise from alternate splicing of Cav2.1 and possibly the coassembly with distinct β subunits [12, 67]. Vertebrates express four different types of calcium channel β subunits (β1 through β4), four different types of α2δ subunits (α2δ1 through α2δ4), and as many as 8 different γ subunits [6, 9] (Fig. 1B). The β subunits are cytoplasmic proteins that interact with the linker joining repeats I & II of the calcium channel α1 subunit and strongly modulate channel properties such as inactivation rates [6]. Moreover, these subunits play a major role in targeting the channels to the plasma membrane. The α2δ subunit is encoded by a single gene, and it is post-translationally cleaved into a transmembrane δ subunit that remains di-sulfide linked to an extracellular α2 subunit [25, 44]. The γ subunit includes four transmembrane helices and was first identified as part of the skeletal muscle L-type channel complex [3]. Since then, a number of neuronal γ subunits have been cloned; however, it is not entirely clear if they are bona fide calcium channel subunits. Functional effects on both HVA and LVA calcium channel activity have been reported in expression systems [68], but a much more pronounced role has been ascribed to γ subunits in the context of regulating AMPA receptor trafficking and function [78].
Figure 1. Voltage-gated calcium channels (VGCCs): naming and structure.
A. Dendrogram illustrating the three subfamilies of VGCCs: the Cav1 (L-types), the Cav2 (neuronal types), and the Cav3 (T-types). Cav1 and Cav2 channels are high-voltage activated (HVA), in contrast to Cav3 channels (T-types), which are low-voltage activated (LVA). B. The molecular structure of HVA channels comprises ancillary subunits α2δ, β, and γ, each encoded by several subunits. In contrast, the subunit composition of LVA/T-type channels is not yet resolved.
The different types of calcium channel α1 subunits are differentially distributed within neurons. In the context of this review, it is important to note that P/Q-type, N-type, and, to some extent, R-type channels are expressed highly at presynaptic nerve terminals where their activities evoke neurotransmitter release [80]. T-type calcium channels tend to be expressed at cell bodies and dendrites [55], where they contribute to the regulation of neuronal excitability [63]. Taken together, P/Q-type and T-type calcium channels show distinct functional properties, subunit composition, and subcellular distributions, and they serve distinct physiological roles, and yet, they both are major contributors to the development of absence seizures and IGE.
P/Q-type channels and absence seizures
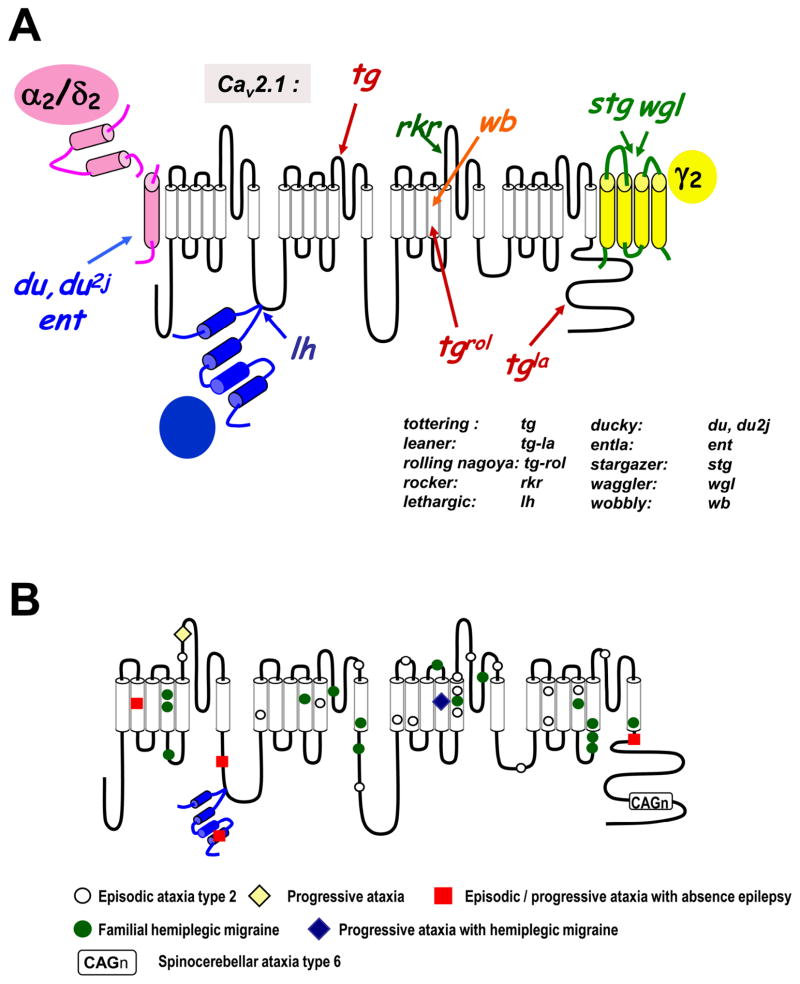
As noted above, Cav2.1 (P/Q-type) calcium channels are important mediators of synaptic transmission in central and peripheral neurons. As a result, changes in the functional properties of Cav2.1 channels may be expected to alter the behavior of neuronal networks. A possible role of P/Q-type channels in seizure disorders is readily apparent when considering the phenotypes of Cav2.1 channel KO mice. These mice show severe ataxia and die about four weeks after birth [42]. Moreover, they display absence seizures, indicating that P/Q-type channels may protect from the occurrence of such seizure activity during normal physiology. Additional evidence implicating Cav2.1 channels in absence seizures comes from several murine mouse models with P/Q-type channel mutations (Fig. 2A). In tottering (tg) mice, a proline residue in the repeat II S5–S6 region of Cav2.1 is substituted by a leucine residue [26, 32], leading to a reduction of P/Q-type channel activity as assayed in both neurons and in expression systems [88]. Consistent with their role in neurotransmitter release, the reduced P/Q-type channel function in these mice is paralleled by altered neurotransmitter release in cortical neurons [4], and reduced excitatory synaptic potentials evoked in thalamic neurons [17]. Similar to the Cav2.1 KO mice, tg mice show cerebellar ataxia and absence seizures with 5–7 Hz SWDs [32, 61]. The Cav2.1 calcium channel gene of leaner (tgla) mice exhibits a frameshift mutation in the C-terminal region, which results in a premature stop [26, 32]. This reportedly results in altered P/Q-type channel currents, as well as reduced neurotransmitter release at neocortical synapses [4]. The tgla mice display cortical SWDs that are consistent with absence seizures and, are, again, severely ataxic [32, 51]. Finally, in the Cav2.1 channels of Rocker (rkr) mice, a lysine residue in the repeat III S5–S6 region is replaced with a threonine [98]; however, it remains unclear what effects this mutation has on the biophysics of the channel. Nonetheless, these mice show spontaneous bilateral 6–7 Hz SWDs accompanied by behavioral arrest, typical of absence seizures [98].
Figure 2. Mutations in P/Q-type calcium channels in mouse (A) and humans (B).
A. Schematic representation of the multiple mutations that affect the various subunits of the P/Q-type calcium channels in mouse and the corresponding mouse phenotypes. b Location of mutations in human P/Q-type α1 subunits that related to episodic ataxia type 2 (EA2), familial hemiplegic migraine type 1 (FHM1), spinocerebellar ataxia type 6 (SCA6), as well as more complex clinical patterns such as progressive ataxia with absence epilepsy (red squares) are indicated.
A few other mutations associated with seizure activity have been mapped to the Cav2.1 subunit in mice, including rolling Nagoya (tgrol) and wobbly (wb; Fig. 2A). Most of these mouse genotypes and their associated phenotypes, except wobbly [91], show a recessive inheritance pattern and, at least in the tg and tgla mice, seem to parallel the phenotype of the Cav2.1 channel KO mouse. This may be due to the fact that both mutant mice show reduced Cav2.1 channel activity, which would be equivalent to a partial Cav2.1 knockdown. Importantly, all these mouse mutants also exhibit ataxia, possibly due to reduced P/Q-type channel activity and unbalanced excitatory/inhibitory neurotransmitter release in the cerebellum, since the P/Q-current accounts for ~90% of the total calcium current in Purkinje neurons [58].
In humans, mutations in P/Q-type calcium channels are more frequently associated with conditions such as episodic ataxia type 2 (EA-2) and familial hemiplegic migraine type 1 (FHM1) [reviewed in [64] and [65]], both of which are dominantly inherited (Fig. 2B). Ataxia is then a common phenotype in both mice and men with Cav2.1 mutations. Importantly, there are some instances in which patients with Cav2.1 mutations present with absence seizures (illustrated in Fig. 2B). For example, a truncation mutation in the C-terminal region of Cav2.1 that results in a non-functional channel has been associated with the occurrence of childhood episodes of absence epilepsy and primary generalized seizures in a patient with episodic ataxia type 2 [41]. Another mutation in the repeat I-S2 region is found in a different family of patients, which reduces P/Q-type channel activity and gives rise to an ataxic and epileptic phenotype [40]. An 11 year old girl with a missense mutation (I712V) in the Cav2.1 channel was recently described to have a range of symptoms, including seizures, headache, and ataxia [35], but the effect of the mutation on channel gating has not yet been determined. In patients with FHM associated with P/Q-type channel mutations, the occurrence of seizures is exceedingly rare and limited to only a few case reports [27]. This may be due to the notion that FHM appears to arise from a gain of function of Cav2.1, rather than a loss of function [18, 79]. Altogether, in both humans and rodents, mutations that give rise to diminished P/Q-type channel function have the propensity to cause absence seizures. As noted below, in these rodent models there is also a compensatory increase in T-currents of thalamic relay neurons [94], which likely underlies their seizure phenotype.
Ancillary calcium channel subunits and seizures
As noted above, HVA calcium channels are multi-subunit complexes where the ancillary subunits regulate channel function and/or membrane expression of the pore-forming α1 subunit. As such, one may expect loss of function mutations in ancillary subunits of Cav2.1 channels would reduce its channel activity and perhaps produce an epileptic phenotype in vivo. In mice, mutations associated with seizure activity have indeed been found in all major classes of ancillary calcium channel subunits (Fig. 2A). The lethargic (lh) mouse arises from a frameshift mutation in the calcium channel β4 subunit, thus resulting in an effective knockout of this subunit. These mice exhibit absence seizures, ataxia, and reduced excitatory neurotransmitter release [16]. It is interesting to note that similar mutations at splice junctions have been found in the β4 gene in two families displaying IGE [30].
There are also several mouse phenotypes associated with mutations in α2δ subunits, including two mutations which cause a loss of α2δ2 subunit protein and which give rise to two strains of ducky mice (du and du2J). These mice show 5–7 Hz SWD during seizure episodes, as well as behavioral arrest and ataxia [5]. In transient expression systems, the coexpression of the mutated recombinant subunits reduces the amount of Cav2.1 current [14], again, consistent with the idea that reduced Cav2.1 channel function may lead to the appearance of absence seizures. In the entla (ent) mouse, a duplication of an exon 3 interferes with the disulfide linkage of the α2 and δ2 subunits, thus rendering the subunits non-functional [13]. These mice also have reduced P/Q-type currents in the hippocampus, as well as 2–4 Hz SWDs in the cortex and hippocampus.
The stargazer (stg) and waggler (wgl) mice arise from dysfunctional calcium channel γ2 subunits (also referred to as stargazin). Their phenotypes are characterized by absence seizures and episodes of head tossing [45, 46]. Seizures are further exacerbated in waggler mice by knockout of the γ4 subunit [47]. In stg mice, there is a small reduction in P/Q-type channel activity due to shifts in the midpoint of the steady state inactivation curve of the channel, as assayed in Xenopus oocytes [reviewed in [9]]. In view of the known effects of stargazin on AMPA receptors, it is difficult to unequivocally attribute the physiological effects of truncation mutations in stargazin to an alteration of calcium channel function.
Role of T-type channels in neuronal excitability
Entry of Ca2+ ions through T-channels leads to depolarization of the membrane, allowing T-currents to generate low threshold spikes (LTS) that trigger bursts of Na-dependent action potentials [49]. This role is especially prominent in thalamic neurons, which express T-currents at very high densities (reviewed in [63]). Thalamic neurons form a reciprocally connected circuit that oscillates during natural processes like sleep, but can also oscillate at inappropriate times, as during a generalized seizure [53]. This circuit is composed of thalamic reticular neurons (nRT; GABAergic), thalamocortical neurons that reside in the relay nuclei (TC, glutamatergic), and cerebral cortical neurons (glutamatergic). The unique voltage dependence of T-channels allows them to generate LTS after either an inhibitory or excitatory post-synaptic potential (IPSP, EPSP). Rebound firing after an IPSP occurs because T-channels are inactivated at the resting membrane potential of many neurons, then recover from inactivation during an IPSP. As the IPSP decays and the resting membrane potential is restored, T-channels can open and create a LTS. Another very interesting property of T-channels is their ability to generate “window currents.” Window currents arise when a channel is available to open at a given potential and not totally inactivated. They are operationally defined by the overlap of the steady-state activation and inactivation curves. Notably, T-window currents occur at the resting membrane potential of most neurons, allowing them to contribute to membrane bistability; e.g. thalamic neurons have two “resting” membrane potentials, one around −77 mV, and a second around −60 mV [23]. Cav3.2 channels have been shown to generate window currents and increase resting basal Ca2+ concentrations [20].
T-channels open and close at negative membrane potentials, where there is a large driving force for Ca2+ entry. Their opening can lead to robust increases in intracellular Ca2+, especially in small compartments such as dendrites [60, 97]. Ca2+ entry via T-channels has also been shown to cause Ca-induced Ca-release (CICR) from internal stores, which can trigger a form of long-term depression in hippocampal neurons [62, 89]. In some neurons, Ca2+ influx via T-channels dampens excitability by opening Ca-activated K+ channels, which contributes to spike repolarization and after-hyperpolarizations [24, 48, 83, 90]. In summary, T-channels play important physiological roles by controlling membrane potential and by controlling intracellular Ca2+ concentrations.
Upregulation of T-type channels in epilepsy
There is significant evidence that T-channels play a role in epilepsy and pain. In particular, T-channels are thought to play an important role in idiopathic generalized epilepsies (IGE), such as absence epilepsy, due to their high expression in the thalamus. In addition to being supported by pharmacology studies, this hypothesis is further supported by studies in the GAERS model of absence epilepsy. Reticular thalamic neurons isolated from these rats have 55% larger T-currents than the control strain and show a 16% increase in Cav3.2 mRNA [75, 81]. Similar results were found in the WAG/Rij model of absence epilepsy [15]. It is interesting to note that increased T-type calcium channel activity was first implicated in studies of mice with absence seizure phenotypes, where the underlying mutation is in high voltage-activated channels [94]. This result provides a clear example of how misleading the correlation between phenotype and gene knockout can be. Examples of this phenomenon include: tottering mice with Cav2.1 mutations; stargazer mice with mutations in γ2; and lethargic mice with mutations in β4. Mutations in non-calcium channel genes also lead to absence epilepsy in the mouse model Coloboma, and increases in T-currents increase prior to the onset of epilepsy [95]. The hypothesis that increased T-currents play a direct role in epileptogenesis is provided by inducing epilepsy by forced overexpression of Cav3.1 currents [29], and by “curing” tottering, stargazer, and lethargic mice by crossing with Cav3.1 knockout mice [71]. It remains to be determined whether the absence seizures arise from the ability of increased T-currents to alter membrane excitability or intracellular calcium concentrations, although it should be noted that early modeling studies suggested a 2-fold increase in T-currents would be sufficient to induce oscillations of thalamocortical circuitry [54]. Similarly, a recent modeling study concluded that small changes (2–3 mV, which would be virtually undetectable) in the voltage dependence of activation of Na+ or Ca2+ channels could underlie seizures [77]. Indeed, over-activity of T-channels appears critical, as demonstrated by the observation that transgenic mice overexpressing Cav3.1 channels display spike-and wave-discharges, the EEG signature of absence epilepsy [29]. This provided strong evidence that primary elevation of T-type channel activity is sufficient to induce a pure absence epilepsy phenotype. Conversely, a small reduction in T-currents by the anti-absence seizure medication, ethosuximide, may be sufficient to reduce oscillation of thalamocortical circuits in vitro [39]. Indeed, a recent study has documented that a selective T-channel blocker exhibits strong efficacy in suppression of absence seizures in the genetic rat model WAG/Rij [93].
T-currents are also upregulated in CA1 hippocampal neurons in the pilocarpine model of temporal lobe epilepsy (TLE) [7, 74]. The original observation was that pilocarpine-induced status epilepticus promoted spontaneous firing of CA1 neurons [74]. A subsequent study using focal application of Ni2+ and amiloride implicated upregulation of T-currents in apical dendrites [92]. A recent study established the role of Cav3.2 channels in this form of epilepsy using Cav3.2 (−/−) mice [7]. Interestingly, the strength of synaptic inputs into CA1 neurons is altered in this model of TLE with a dramatic increase in the entorhinal cortex (EC) input via the temporoammonic pathway [1]. These inputs terminate in the most distal dendrites in the stratum lacunosum-moleculare [56]. Although other factors may be involved, a plausible explanation is that upregulation of Cav3.2 channels boosts EPSPs triggered in distal dendrites. Hippocampal T-current density is also increased after electrical kindling [31]. These animal models mimic human temporal lobe and focal epilepsies, implicating possible upregulation of T-currents in many forms of epilepsy.
CACNA1H is an epilepsy susceptibility gene
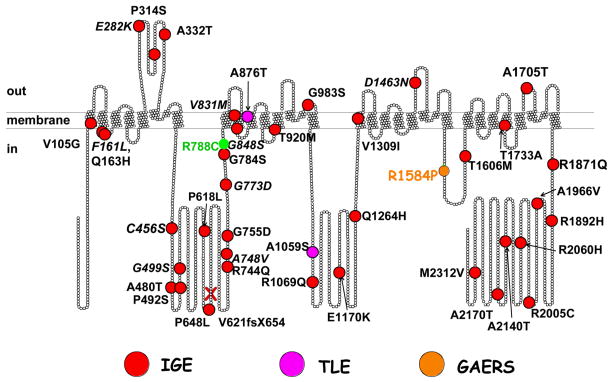
The word mutation has two connotations. To a molecular biologist, a mutation is any change in the nucleotide sequence that differs from a standard reference sequence (e.g. the GenBank). To a geneticist, a mutation is a change in nucleotide sequence that has been shown to cause disease. This strict interpretation requires that the term mutation only applies to monogenic autosomal dominant disorders. Monogenic disorders that cause epilepsy are relatively rare [57]. In contrast, idiopathic generalized epilepsy (IGE) is a polygenic disorder [34, 38, 69, 76, 84]. The first evidence that IGE was a polygenic disorder came from Dr. Lennox’s study of epilepsy in twins [84]. He found that even in monozygotic twins there was only a 75% concordance for absence epilepsy, with a 0% concordance in dizygotic twins. Other characteristics of polygenic disorders found in absence epilepsy include a variable age of onset, variable age of recovery, and periodicity [34]. Furthermore, generalized spike-and-wave discharges have been observed in family members who do not have seizures [69]. Finally, the conclusion from many linkage studies is that the complex pattern of IGE inheritance is not due to a single gene, but that “each gene contributes a small or modest effect to the epilepsy phenotype, and by itself is insufficient to cause epilepsy. These are ‘susceptibility genes’” [76]. Therefore, common single nucleotide polymorphisms (SNPs) can contribute to epilepsy susceptibility. More importantly, a large number of SNPs have been found only in epilepsy patients (Fig. 3). We will refer to these as “variants.” The first of these studies was performed on Childhood Absence Epilepsy (CAE) patients in China, leading to the identification of 12 specific variants in CACNA1H associated with CAE [21]. Interestingly, patients from 3 distinct families harbored both the G773D variant and “common” SNP R788C, which was found in 20% of CAE patients and in 10% of the general population [87]. A second study extended the finding of CACNA1H variants to many related epilepsies, such as juvenile absence, juvenile myoclonic epilepsy (JME), febrile seizures, and temporal lobe epilepsy (TLE) [36]. Sequence variations in the Cav3.2 gene, CACNA1H, have also been linked to Autism Spectrum Disorders (ASD) [72]. In addition, variants were detected in the Cav3.1 gene, CACNA1G, in IGE patients, although no phenotypic differences were noted in channel behavior [70]. Studies on the Generalized Absence Epilepsy Rat of Strasbourg (GAERS) confirmed this conclusion, as a variant in the III–IV loop (Fig. 1) accounts for some, but not all, of the seizure phenotype [66]. Notably, this variant, R1584P, alters the ratio of III–IV loop splice variants, favoring the variant with faster recovery from inactivation. Similarly, IGE of the human CACNA1H gene, supporting the hypothesis that variants simply alter the ratio of splice variants with unique properties, but do not themselves induce novel phenotypes [96]. In crosses of GAERS with control rats, the R1584P mutation segregated with seizures (percentage time and total number of seizures). Interestingly, a subset of rats did not inherit the mutation, yet were still prone to seizures, and vice versa. Taken together, these studies on both humans and animal models clearly establish that IGE is a polygenic disorder, and that sequence variations in CACNA1H can contribute to seizure susceptibility.
Figure 3. Variants of Cav3.2.
Location of IGE variants are mapped on a scaled model of Cav3.2 (each ball represents an amino acid). Key: red balls, IGE variants reported by Chen et al., [21] and Heron et al., [37]; orange ball, the GAERS variant [66]; pink balls, TLE variants [37]; and green, the common SNP, R788C. A frame-shift mutation, V621fsX654, was found in patients with febrile seizures, which leads to premature truncation of the protein at a.a. residue 654 [37].
Despite considerable progress in defining the role of calcium channels in epilepsy, there remain many unanswered questions. How do these Cav3 variants increase seizure susceptibility? In the most simplistic sense, epilepsy is a disorder in which the balance between excitatory and inhibitory neurotransmission is tipped towards excitability. Therefore, one can predict that T-channel variants might cause changes in gating that would increase seizure susceptibility, such as: 1) shifting the voltage-dependence of activation; 2) shifting the voltage dependence of inactivation; 3) accelerating channel opening; slowing channel inactivation; 4) slowing deactivation (open to close transitions); 5) accelerating recovery; 6) increasing the probability of channel opening (Po); and 7) increasing single channel conductance. Notably, variants associated with IGE do affect many of these properties [43, 87]. Surprisingly, some of the Cav3.2 variants found in IGE patients had no detectable effect on channel biophysics. During a structure-function study it was discovered that the loop connecting repeat I to II (I–II loop; Fig. 1) played a role in channel expression [2]. This study went on to show that all CAE variants located in this loop increased surface expression, thereby providing a unifying mechanism for their role in seizure susceptibility [86]. Mechanisms by which this might occur include: enhanced trafficking out of ER/Golgi to plasma membrane (PM); slower retrieval from PM; altered fate of internalized early endosomes from degradation to PM recycling; and altered distribution of T-channels in neurons, e.g. enhanced trafficking into dendrites or axon hillock. Another unresolved question is: Do the Cav3 variants alter trafficking to dendrites? Based on their known roles in dendritic physiology [73], this would likely have significant effects on burst firing and synaptic integration. Finally, we would like to emphasize the difficulty in demonstrating that a channel variant is the root cause of epilepsy, because IGE is a polygenic disorder and by definition, requires the co-inheritance of variants in other genes (nature), as well as contributions from the environment (nurture) [8, 77].
Acknowledgments
GWZ is a Scientist of the Alberta Heritage Foundation for Medical Research and a Canada Research Chair in Molecular Neurobiology. PL is supported by CNRS and grants from ANR (ANR-2006-Neuro35) and Fédération pour la Recherche sur le Cerveau. EPR is supported by grants from NIH (NS067456).
References
- 1.Ang CW, Carlson GC, Coulter DA. Massive and specific dysregulation of direct cortical input to the hippocampus in temporal lobe epilepsy. J Neurosci. 2006;26:11850–11856. doi: 10.1523/JNEUROSCI.2354-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arias JM, Murbartián J, Vitko I, et al. Transfer of β subunit regulation from high to low voltage-gated Ca2+ channels. FEBS Lett. 2005;579:3907–3912. doi: 10.1016/j.febslet.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol. 2003;13:298–307. doi: 10.1016/s0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 4.Ayata C, Shimizu-Sasamata M, Lo EH, et al. Impaired neurotransmitter release and elevated threshold for cortical spreading depression in mice with mutations in the α1A subunit of P/Q type calcium channels. Neurosci. 1999;95:639–645. doi: 10.1016/s0306-4522(99)00446-7. [DOI] [PubMed] [Google Scholar]
- 5.Barclay J, Balaguero N, Mione M, et al. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J Neurosci. 2001;21:6095–6104. doi: 10.1523/JNEUROSCI.21-16-06095.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumgart JP, Perez–Reyes E. Voltage-gated Ca2+ channels. In: Kew JN, Davies C, editors. Ion channels: from structure to function. Oxford University Press; London: 2009. [Google Scholar]
- 7.Becker AJ, Pitsch J, Sochivko D, et al. Transcriptional upregulation of Cav3.2 mediates epileptogenesis in the pilocarpine model of epilepsy. J Neurosci. 2008;28:13341–13353. doi: 10.1523/JNEUROSCI.1421-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berkovic SF, Mulley JC, Scheffer IE, et al. Human epilepsies: interaction of genetic and acquired factors. Trends Neurosci. 2006;29:391–397. doi: 10.1016/j.tins.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Black JL., 3rd The voltage-gated calcium channel γ subunits: a review of the literature. J Bioenerg Biomembr. 2003;35:649–660. doi: 10.1023/b:jobb.0000008029.22650.c5. [DOI] [PubMed] [Google Scholar]
- 10.Blumenfeld H. From molecules to networks: cortical/subcortical interactions in the pathophysiology of idiopathic generalized epilepsy. Epilepsia. 2003;44(Suppl 2):7–15. doi: 10.1046/j.1528-1157.44.s.2.2.x. [DOI] [PubMed] [Google Scholar]
- 11.Blumenfeld H. Cellular and network mechanisms of spike-wave seizures. Epilepsia. 2005;46(Suppl 9):21–33. doi: 10.1111/j.1528-1167.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- 12.Bourinet E, Soong TW, Sutton K, et al. Splicing of α1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nat Neurosci. 1999;2:407–415. doi: 10.1038/8070. [DOI] [PubMed] [Google Scholar]
- 13.Brill J, Klocke R, Paul D, et al. entla, a novel epileptic and ataxic Cacna2d2 mutant of the mouse. J Biol Chem. 2004;279:7322–7330. doi: 10.1074/jbc.M308778200. [DOI] [PubMed] [Google Scholar]
- 14.Brodbeck J, Davies A, Courtney JM, et al. The ducky mutation in cacna2d2 results in altered Purkinje cell morphology and is associated with the expression of a truncated α2δ-2 protein with abnormal function. J Biol Chem. 2002;277:7684–7693. doi: 10.1074/jbc.M109404200. [DOI] [PubMed] [Google Scholar]
- 15.Broicher T, Kanyshkova T, Meuth P, et al. Correlation of T-channel coding gene expression, I(T), and the low threshold Ca(2+) spike in the thalamus of a rat model of absence epilepsy. Mol Cell Neurosci. 2008 doi: 10.1016/j.mcn.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Burgess DL, Jones JM, Meisler MH, et al. Mutation of the Ca2+ channel subunit gene Cchb4 is associated with ataxia and seizures in the lethargic (lh) mouse. Cell. 1997;88:385–392. doi: 10.1016/s0092-8674(00)81877-2. [DOI] [PubMed] [Google Scholar]
- 17.Caddick SJ, Wang C, Fletcher CF, et al. Excitatory but not inhibitory synaptic transmission is reduced in lethargic (Cacnb4lh) and tottering (Cacna1atg) mouse thalami. J Neurophysiol. 1999;81:2066. doi: 10.1152/jn.1999.81.5.2066. [DOI] [PubMed] [Google Scholar]
- 18.Catterall WA, Dib-Hajj S, Meisler MH, et al. Inherited neuronal ion channelopathies: new windows on complex neurological diseases. J Neurosci. 2008;28:11768–11777. doi: 10.1523/JNEUROSCI.3901-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Catterall WA, Perez-Reyes E, Snutch TP, et al. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 20.Chemin J, Monteil A, Briquaire C, et al. Overexpression of T-type calcium channels in HEK-293 cells increases intracellular calcium without affecting cellular proliferation. FEBS Lett. 2000;478:166–172. doi: 10.1016/s0014-5793(00)01832-9. [DOI] [PubMed] [Google Scholar]
- 21.Chen YC, Lu JJ, Pan H, et al. Association between genetic variation of CACNA1H and childhood absence epilepsy. Ann Neurol. 2003;54:239–243. doi: 10.1002/ana.10607. [DOI] [PubMed] [Google Scholar]
- 22.Commission on classification and terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 23.Crunelli V, Toth TI, Cope DW, et al. The T-type calcium current in brain dynamics of different behavioural states. J Physiol (Lond) 2005;562:121–129. doi: 10.1113/jphysiol.2004.076273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cueni L, Canepari M, Lujan R, et al. T-type Ca2+ channels, SK2 channels and SERCAs gate sleep-related oscillations in thalamic dendrites. Nat Neurosci. 2008;11:683–692. doi: 10.1038/nn.2124. [DOI] [PubMed] [Google Scholar]
- 25.De Jongh KS, Warner C, Catterall WA. Subunits of purified calcium channels. α2 and δ are encoded by the same gene. J Biol Chem. 1990;265:14738–14741. [PubMed] [Google Scholar]
- 26.Doyle J, Ren X, Lennon G, et al. Mutations in the Cacnl1a4 calcium channel gene are associated with seizures, cerebellar degeneration, and ataxia in tottering and leaner mutant mice. Mammalian Genome. 1997;8:113–120. doi: 10.1007/s003359900369. [DOI] [PubMed] [Google Scholar]
- 27.Ducros A, Denier C, Joutel A, et al. The clinical spectrum of familial hemiplegic migraine associated with mutations in a neuronal calcium channel. N Engl J Med. 2001;345:17–24. doi: 10.1056/NEJM200107053450103. [DOI] [PubMed] [Google Scholar]
- 28.Engel J, Pedley TA, Aicardi J, et al., editors. Epilepsy: A comprehensive textbook. Lippincott Williams & Wilkins; Philadelphia: [Google Scholar]
- 29.Ernst WL, Zhang Y, Yoo JW, et al. Genetic enhancement of thalamocortical network activity by elevating α1G-mediated low-voltage-activated calcium current induces pure absence epilepsy. J Neurosci. 2009;29:1615–1625. doi: 10.1523/JNEUROSCI.2081-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Escayg A, De Waard M, Lee DD, et al. Coding and noncoding variation of the human calcium-channel β4-subunit gene CACNB4 in patients with idiopathic generalized epilepsy and episodic ataxia. Am J Hum Genet. 2000;66:1531–1539. doi: 10.1086/302909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faas GC, Vreugdenhil M, Wadman WJ. Calcium currents in pyramidal CA1 neurons in vitro after kindling epileptogenesis in the hippocampus of the rat. Neurosci. 1996;75:57–67. doi: 10.1016/0306-4522(96)00254-0. [DOI] [PubMed] [Google Scholar]
- 32.Fletcher CF, Lutz CM, O’xSullivan TN, et al. Absence epilepsy in tottering mutant mice is associated with calcium channel defects. Cell. 1996;87:607–617. doi: 10.1016/s0092-8674(00)81381-1. [DOI] [PubMed] [Google Scholar]
- 33.Gardiner RM. Genetic basis of the human epilepsies. Epilepsy Res. 1999;36:91–95. doi: 10.1016/s0920-1211(99)00043-1. [DOI] [PubMed] [Google Scholar]
- 34.Gargus JJ. Unraveling monogenic channelopathies and their implications for complex polygenic disease. Am J Hum Genet. 2003;72:785–803. doi: 10.1086/374317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guerin AA, Feigenbaum A, Donner EJ, et al. Stepwise developmental regression associated with novel CACNA1A mutation. Pediatr Neurol. 2008;39:363–364. doi: 10.1016/j.pediatrneurol.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 36.Heron SE, Khosravani H, Varela D, et al. Extended spectrum of idiopathic generalized epilepsies associated with CACNA1H functional variants. Ann Neurol. 2007;62:560–568. doi: 10.1002/ana.21169. [DOI] [PubMed] [Google Scholar]
- 37.Heron SE, Phillips HA, Mulley JC, et al. Genetic variation of CACNA1H in idiopathic generalized epilepsy. Ann Neurol. 2004;55:595–596. doi: 10.1002/ana.20028. [DOI] [PubMed] [Google Scholar]
- 38.Heron SE, Scheffer IE, Berkovic SF, et al. Channelopathies in idiopathic epilepsy. Neurotherapeutics. 2007;4:295–304. doi: 10.1016/j.nurt.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Huguenard JR, Prince DA. Intrathalamic rhythmicity studied in vitro: nominal T-current modulation causes robust antioscillatory effects. J Neurosci. 1994;14:5485–5502. doi: 10.1523/JNEUROSCI.14-09-05485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imbrici P, Jaffe SL, Eunson LH, et al. Dysfunction of the brain calcium channel CaV2.1 in absence epilepsy and episodic ataxia. Brain. 2004;127:2682–2692. doi: 10.1093/brain/awh301. [DOI] [PubMed] [Google Scholar]
- 41.Jouvenceau A, Eunson LH, Spauschus A, et al. Human epilepsy associated with dysfunction of the brain P/Q-type calcium channel. The Lancet. 2001;358:801–807. doi: 10.1016/S0140-6736(01)05971-2. [DOI] [PubMed] [Google Scholar]
- 42.Jun K, Piedras-Renteria ES, Smith SM, et al. Ablation of P/Q-type Ca2+ channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the alpha1A-subunit. Proc Natl Acad Sci USA. 1999;96:15245–15250. doi: 10.1073/pnas.96.26.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khosravani H, Zamponi GW. Voltage-gated calcium channels and idiopathic generalized epilepsies. Physiol Rev. 2006;86:941–966. doi: 10.1152/physrev.00002.2006. [DOI] [PubMed] [Google Scholar]
- 44.Klugbauer N, Lacinová L, Marais E, et al. Molecular diversity of the calcium channel α2δ subunit. J Neurosci. 1999;19:684–691. doi: 10.1523/JNEUROSCI.19-02-00684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Letts VA, Felix R, Biddlecome GH, et al. The mouse stargazer gene encodes a neuronal Ca2+-channel γ subunit. Nat Genet. 1998;19:340–347. doi: 10.1038/1228. [DOI] [PubMed] [Google Scholar]
- 46.Letts VA, Kang MG, Mahaffey CL, et al. Phenotypic heterogeneity in the stargazin allelic series. Mamm Genome. 2003;14:506–513. doi: 10.1007/s00335-003-2268-x. [DOI] [PubMed] [Google Scholar]
- 47.Letts VA, Mahaffey CL, Beyer B, et al. A targeted mutation in Cacng4 exacerbates spike-wave seizures in stargazer (Cacng2) mice. Proc Natl Acad Sci USA. 2005;102:2123–2128. doi: 10.1073/pnas.0409527102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Llinás R, Yarom Y. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J Physiol (Lond) 1981;315:569–584. doi: 10.1113/jphysiol.1981.sp013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Llinás RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242:1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- 50.Lorenzon NM, Beam KG. Channels. Austin, Tex: 2008. Disease causing mutations of calcium channels; p. 2. [DOI] [PubMed] [Google Scholar]
- 51.Lorenzon NM, Lutz CM, Frankel WN, et al. Altered calcium channel currents in Purkinje cells of the neurological mutant mouse leaner. J Neurosci. 1998;18:4482–4489. doi: 10.1523/JNEUROSCI.18-12-04482.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marini C, King MA, Archer JS, et al. Idiopathic generalised epilepsy of adult onset: clinical syndromes and genetics. J Neurol Neurosurg Psychiatry. 2003;74:192–196. doi: 10.1136/jnnp.74.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCormick DA, Contreras D. On the cellular and network bases of epileptic seizures. Annu Rev Physiol. 2001;63:815–846. doi: 10.1146/annurev.physiol.63.1.815. [DOI] [PubMed] [Google Scholar]
- 54.McCormick DA, Huguenard JR. A model of the electrophysiological properties of thalamocortical relay neurons. J Neurophysiol. 1992;68:1384–1400. doi: 10.1152/jn.1992.68.4.1384. [DOI] [PubMed] [Google Scholar]
- 55.McKay BE, McRory JE, Molineux ML, et al. Cav3 T-type calcium channel isoforms differentially distribute to somatic and dendritic compartments in rat central neurons. European Journal of Neuroscience. 2006;24:2581–2594. doi: 10.1111/j.1460-9568.2006.05136.x. [DOI] [PubMed] [Google Scholar]
- 56.Megías M, Emri Z, Freund TF, et al. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neurosci. 2001;102:527–540. doi: 10.1016/s0306-4522(00)00496-6. [DOI] [PubMed] [Google Scholar]
- 57.Meisler MH, Kearney J, Ottman R, et al. Identification of epilepsy genes in human and mouse. Annu Rev Genet. 2001;35:567–588. doi: 10.1146/annurev.genet.35.102401.091142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mintz IM, Adams ME, Bean BP. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992;9:85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- 59.Mulley JC, Scheffer IE, Harkin LA, et al. Susceptibility genes for complex epilepsy. Hum Mol Genet. 2005;14(Spec No 2):R243–249. doi: 10.1093/hmg/ddi355. [DOI] [PubMed] [Google Scholar]
- 60.Munsch T, Budde T, Pape HC. Voltage-activated intracellular calcium transients in thalamic relay cells and interneurons. Neuroreport. 1997;8:2411–2418. doi: 10.1097/00001756-199707280-00001. [DOI] [PubMed] [Google Scholar]
- 61.Noebels JL, Sidman RL. Inherited epilepsy: spike-wave and focal motor seizures in the mutant mouse tottering. Science. 1979;204:1334–1336. doi: 10.1126/science.572084. [DOI] [PubMed] [Google Scholar]
- 62.Oliet SH, Malenka RC, Nicoll RA. Two distinct forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neuron. 1997;18:969–982. doi: 10.1016/s0896-6273(00)80336-0. [DOI] [PubMed] [Google Scholar]
- 63.Perez-Reyes E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 64.Pietrobon D. Calcium channels and channelopathies of the central nervous system. Mol Neurobiol. 2002;25:31–50. doi: 10.1385/MN:25:1:031. [DOI] [PubMed] [Google Scholar]
- 65.Pietrobon D, Striessnig J. Neurobiology of migraine. Nature Reviews Neuroscience. 2003;4:386–398. doi: 10.1038/nrn1102. [DOI] [PubMed] [Google Scholar]
- 66.Powell KL, Cain SM, Ng C, et al. A Cav3.2 T-type calcium channel point mutation has splice-variant-specific effects on function and segregates with seizure expression in a polygenic rat model of absence epilepsy. J Neurosci. 2009;29:371–380. doi: 10.1523/JNEUROSCI.5295-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Richards KS, Swensen AM, Lipscombe D, et al. Novel CaV2.1 clone replicates many properties of Purkinje cell CaV2.1 current. Eur J Neurosci. 2007;26:2950–2961. doi: 10.1111/j.1460-9568.2007.05912.x. [DOI] [PubMed] [Google Scholar]
- 68.Rousset M, Cens T, Restituito S, et al. Functional roles of γ2, γ3, and γ4, three new Ca2+ channel subunits, in P/Q-type Ca2+ channel expressed in Xenopus oocyte. J Physiol (Lond) 2001;532:583–593. doi: 10.1111/j.1469-7793.2001.0583e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sander T. The genetics of idiopathic generalized epilepsy: implications for the understanding of its aetiology. Mol Med Today. 1996;2:173–180. doi: 10.1016/1357-4310(96)88793-4. [DOI] [PubMed] [Google Scholar]
- 70.Singh B, Monteil A, Bidaud I, et al. Mutational analysis of CACNA1G in idiopathic generalized epilepsy. Hum Mutat. 2007;28:524–525. doi: 10.1002/humu.9491. [DOI] [PubMed] [Google Scholar]
- 71.Song I, Kim D, Choi S, et al. Role of the α1G T-type calcium channel in spontaneous absence seizures in mutant mice. J Neurosci. 2004;24:5249–5257. doi: 10.1523/JNEUROSCI.5546-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Splawski I, Yoo DS, Stotz SC, et al. CACNA1H mutations in autism spectrum disorders. J Biol Chem. 2006;281:22085–22091. doi: 10.1074/jbc.M603316200. [DOI] [PubMed] [Google Scholar]
- 73.Stuart G, Spruston N, Häusser M. Dendrites. Oxford University Press; Oxford: 2008. [Google Scholar]
- 74.Su H, Sochivko D, Becker A, et al. Upregulation of a T-type Ca2+ channel causes a long-lasting modification of neuronal firing mode after status epilepticus. J Neurosci. 2002;22:3645–3655. doi: 10.1523/JNEUROSCI.22-09-03645.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Talley EM, Solórzano G, Depaulis A, et al. Low-voltage-activated calcium channel subunit expression in a genetic model of absence epilepsy in the rat. Mol Brain Res. 2000;75:159–165. doi: 10.1016/s0169-328x(99)00307-1. [DOI] [PubMed] [Google Scholar]
- 76.Tan NC, Mulley JC, Berkovic SF. Genetic association studies in epilepsy: “the truth is out there. Epilepsia. 2004;45:1429–1442. doi: 10.1111/j.0013-9580.2004.22904.x. [DOI] [PubMed] [Google Scholar]
- 77.Thomas EA, Reid CA, Berkovic SF, et al. Prediction by modeling that epilepsy may be caused by very small functional changes in ion channels. Arch Neurol. 2009;66:1225–1232. doi: 10.1001/archneurol.2009.219. [DOI] [PubMed] [Google Scholar]
- 78.Tomita S, Chen L, Kawasaki Y, et al. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J Cell Biol. 2003;161:805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tottene A, Conti R, Fabbro A, et al. Enhanced excitatory transmission at cortical synapses as the basis for facilitated spreading depression in CaV2. 1 knockin migraine mice. Neuron. 2009;61:762–773. doi: 10.1016/j.neuron.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 80.Trimmer JS, Rhodes KJ. Localization of voltage-gated ion channels in mammalian brain. Annu Rev Physiol. 2004;66:477–519. doi: 10.1146/annurev.physiol.66.032102.113328. [DOI] [PubMed] [Google Scholar]
- 81.Tsakiridou E, Bertollini L, de Curtis M, et al. Selective increase in T-type calcium conductance of reticular thalamic neurons in a rat model of absence epilepsy. J Neurosci. 1995;15:3110–3117. doi: 10.1523/JNEUROSCI.15-04-03110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Turnbull J, Lohi H, Kearney JA, et al. Sacred disease secrets revealed: the genetics of human epilepsy. Hum Mol Genet. 2005;14:2491–2500. doi: 10.1093/hmg/ddi250. [DOI] [PubMed] [Google Scholar]
- 83.Umemiya M, Berger AJ. Properties and function of low- and high-voltage-activated Ca2+ channels in hypoglossal motoneurons. J Neurosci. 1994;14:5652–5660. doi: 10.1523/JNEUROSCI.14-09-05652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vadlamudi L, Andermann E, Lombroso CT, et al. Epilepsy in twins: insights from unique historical data of William Lennox. Neurology. 2004;62:1127–1133. doi: 10.1212/01.wnl.0000118201.89498.48. [DOI] [PubMed] [Google Scholar]
- 85.Vadlamudi L, Scheffer IE, Berkovic SF. Genetics of temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2003;74:1359–1361. doi: 10.1136/jnnp.74.10.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vitko I, Bidaud I, Arias JM, et al. The I–II loop controls plasma membrane expression and gating of Cav3.2 T-type Ca2+ channels: a paradigm for Childhood Absence Epilepsy. J Neurosci. 2007;27:322–330. doi: 10.1523/JNEUROSCI.1817-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vitko I, Chen Y, Arias JM, et al. Functional characterization and neuronal modeling of the effects of Childhood Absence Epilepsy variants of CACNA1H, a T-type calcium channel. J Neurosci. 2005;25:4844–4855. doi: 10.1523/JNEUROSCI.0847-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wakamori M, Yamazaki K, Matsunodaira H, et al. Single tottering mutations responsible for the neuropathic phenotype of the P-type calcium channel. J Biol Chem. 1998;273:34857–34867. doi: 10.1074/jbc.273.52.34857. [DOI] [PubMed] [Google Scholar]
- 89.Wang Y, Rowan MJ, Anwyl R. Induction of LTD in the dentate gyrus in vitro is NMDA receptor independent, but dependent on Ca2+ influx via low-voltage-activated Ca2+ channels and release of Ca2+ from intracellular stores. J Neurophysiol. 1997;77:812–825. doi: 10.1152/jn.1997.77.2.812. [DOI] [PubMed] [Google Scholar]
- 90.Wolfart J, Roeper J. Selective coupling of T-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons. J Neurosci. 2002;22:3404–3413. doi: 10.1523/JNEUROSCI.22-09-03404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xie G, Clapcote SJ, Nieman BJ, et al. Forward genetic screen of mouse reveals dominant missense mutation in the P/Q-type voltage-dependent calcium channel, CACNA1A. Genes Brain Behav. 2007;6:717–727. doi: 10.1111/j.1601-183X.2007.00302.x. [DOI] [PubMed] [Google Scholar]
- 92.Yaari Y, Yue C, Su H. Recruitment of apical dendritic T-type Ca2+ channels by backpropagating spikes underlies de novo intrinsic bursting in hippocampal epileptogenesis. J Physiol (Lond) 2007;580:435–450. doi: 10.1113/jphysiol.2007.127670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang Z-Q, Barrow JC, Shipe WD, et al. Discovery of 1,4-substituted piperidines as potent and selective inhibitors of T-type calcium channels. Journal of Medicinal Chemistry. 2008;51:6471–6477. doi: 10.1021/jm800830n. [DOI] [PubMed] [Google Scholar]
- 94.Zhang Y, Mori M, Burgess DL, et al. Mutations in high-voltage-activated calcium channel genes stimulate low-voltage-activated currents in mouse thalamic relay neurons. J Neurosci. 2002;22:6362–6371. doi: 10.1523/JNEUROSCI.22-15-06362.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Y, Vilaythong AP, Yoshor D, et al. Elevated thalamic low-voltage-activated currents precede the onset of absence epilepsy in the SNAP25-deficient mouse mutant Coloboma. J Neurosci. 2004;24:5239–5248. doi: 10.1523/JNEUROSCI.0992-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhong XL, Liu JRR, Kyle JW, et al. A profile of alternative RNA splicing and transcript variation of CACNA1H, a human T-channel gene candidate for idiopathic generalized epilepsies. Hum Mol Gen. 2006;15:1497–1512. doi: 10.1093/hmg/ddl068. [DOI] [PubMed] [Google Scholar]
- 97.Zhou Q, Godwin DW, O’Malley DM, et al. Visualization of calcium influx through channels that shape the burst and tonic firing modes of thalamic relay cells. J Neurophysiol. 1997;77:2816–2825. doi: 10.1152/jn.1997.77.5.2816. [DOI] [PubMed] [Google Scholar]
- 98.Zwingman TA, Neumann PE, Noebels JL, et al. Rocker is a new variant of the voltage-dependent calcium channel gene Cacna1a. J Neurosci. 2001;21:1169–1178. doi: 10.1523/JNEUROSCI.21-04-01169.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]